Epigenetic Aberrations in Major Psychiatric Diseases Related to Diet and Gut Microbiome Alterations
Highlights
- We first review the association between alterations in the microbial structure and composition resulting from gut dysbiosis and the emergence of specific epigenetic aberrations in the brain, along with their role in the pathophysiology of major mental disorders through the induction of oxidative stress and neuroinflammation.
- We next discuss how specific diets—particularly maternal diets—and microbiome-based therapies, including probiotics and fecal microbiota transplantation, can mitigate epigenetic aberrations and either prevent or improve outcomes in major mental disorders.
- This knowledge is essential for advancing safe, novel, and adjunctive treatments that utilize specific diets and microbiome-based therapies to enhance outcomes for patients with major mental disorders by normalizing epigenetic abnormalities.
Abstract
1. Introduction
2. Epigenetic Aberrations and Mental Disorders
3. Microbiome–Gut–Brain Axis Influence on Brain Functions Mediated by Epigenetic Modification
4. Maternal Diet, Offspring Gut Microbiome, and Brain Functions
5. Gut Microbiome, Oxidative Stress, Inflammation, and Epigenetic Changes
6. Roles of Gut-Blood Barrier, Microbiome-Derived Metabolites, and Diets in the Progression or Therapy of Mental Disorders
6.1. Gut Microbiome-Derived Metabolites May Alleviate Mental Disorders via Epigenetic Alterations
6.2. Ketogenic Diet for the Treatment of Mental Disorders via Epigenetic Changes
6.3. Probiotic Therapy of Mental Disorders via Epigenetic Changes
6.4. Fecal Microbiota Transplantation for Improving Mental Health via Epigenetic Changes
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ginn, S.; Horder, J. “One in four” with a mental health problem: The anatomy of a statistic. BMJ 2012, 344, e1302. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Myers, J.M.; Maes, H.H.; Keyes, C.L. The relationship between the genetic and environmental influences on common internalizing psychiatric disorders and mental well-being. Behav. Genet. 2011, 41, 641–650. [Google Scholar] [CrossRef]
- Legge, S.E.; Santoro, M.L.; Periyasamy, S.; Okewole, A.; Arsalan, A.; Kowalec, K. Genetic architecture of schizophrenia: A review of major advancements. Psychol. Med. 2021, 51, 2168–2177. [Google Scholar] [CrossRef]
- Kendall, K.; Van Assche, E.; Andlauer, T.; Choi, K.; Luykx, J.; Schulte, E.; Lu, Y. The genetic basis of major depression. Psychol. Med. 2021, 51, 2217–2230. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Zhou, J.-R.; Thiagalingam, S. Cataloging recent advances in epigenetic alterations in major mental disorders and autism. Epigenomics 2021, 13, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleky, H.M.; Martin, M.; Zhou, J.-R.; Thiagalingam, S. Epigenetic Alterations of Brain Non-Neuronal Cells in Major Mental Diseases. Genes 2023, 14, 896. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.W.; Morris, B.J. Molecular Biology of the Neuron; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Lodato, M.A.; Woodworth, M.B.; Lee, S.; Evrony, G.D.; Mehta, B.K.; Karger, A.; Lee, S.; Chittenden, T.W.; D’Gama, A.M.; Cai, X. Somatic mutation in single human neurons tracks developmental and transcriptional history. Science 2015, 350, 94–98. [Google Scholar] [CrossRef]
- Løkhammer, S.; Stavrum, A.-K.; Polushina, T.; Aas, M.; Ottesen, A.A.; Andreassen, O.A.; Melle, I.; Le Hellard, S. An epigenetic association analysis of childhood trauma in psychosis reveals possible overlap with methylation changes associated with PTSD. Transl. Psychiatry 2022, 12, 177. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Zhou, J.-R.; Thiagalingam, S. An update on the epigenetics of psychotic diseases and autism. Epigenomics 2015, 7, 427–449. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. The link among microbiota, epigenetics, and disease development. Environ. Sci. Pollut. Res. 2021, 28, 28926–28964. [Google Scholar] [CrossRef]
- Corley, J.; Cox, S.R.; Harris, S.E.; Hernandez, M.V.; Maniega, S.M.; Bastin, M.E.; Wardlaw, J.M.; Starr, J.M.; Marioni, R.E.; Deary, I.J. Epigenetic signatures of smoking associate with cognitive function, brain structure, and mental and physical health outcomes in the Lothian Birth Cohort 1936. Transl. Psychiatry 2019, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.I.; Kapila, R. Dietary metabolites derived from gut microbiota: Critical modulators of epigenetic changes in mammals. Nutr. Rev. 2017, 75, 374–389. [Google Scholar] [CrossRef]
- Yousefi, B.; Kokhaei, P.; Mehranfar, F.; Bahar, A.; Abdolshahi, A.; Emadi, A.; Eslami, M. The role of the host microbiome in autism and neurodegenerative disorders and effect of epigenetic procedures in the brain functions. Neurosci. Biobehav. Rev. 2022, 132, 998–1009. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, J.; Li, H. Mechanistic and therapeutic advances in non-alcoholic fatty liver disease by targeting the gut microbiota. Front. Med. 2018, 12, 645–657. [Google Scholar] [CrossRef]
- Li, Y.; He, P.; Ahmed, A.; Liu, Y.; Ahmed, W.; Wu, Y.; He, Y.; He, P.; Munir, S. Endophyte mediated restoration of citrus microbiome and modulation of host defense genes against Candidatus Liberibacter asiaticus. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Heddes, M.; Altaha, B.; Niu, Y.; Reitmeier, S.; Kleigrewe, K.; Haller, D.; Kiessling, S. The intestinal clock drives the microbiome to maintain gastrointestinal homeostasis. Nat. Commun. 2022, 13, 6068. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Kosciolek, T.; Daly, R.E.; Vázquez-Baeza, Y.; Swafford, A.; Knight, R.; Jeste, D.V. Gut microbiome in Schizophrenia: Altered functional pathways related to immune modulation and atherosclerotic risk. Brain Behav. Immun. 2021, 91, 245–256. [Google Scholar] [CrossRef]
- Liu, C.; Sun, C.; Cheng, Y. β-Glucan alleviates mice with ulcerative colitis through interactions between gut microbes and amino acids metabolism. J. Sci. Food Agric. 2023, 103, 4006–4016. [Google Scholar] [CrossRef]
- Gu, F.; Zhu, S.; Hou, J.; Tang, Y.; Liu, J.-X.; Xu, Q.; Sun, H.-Z. The hindgut microbiome contributes to host oxidative stress in postpartum dairy cows by affecting glutathione synthesis process. Microbiome 2023, 11, 87. [Google Scholar] [CrossRef]
- Choi, B.S.-Y.; Daoust, L.; Pilon, G.; Marette, A.; Tremblay, A. Potential therapeutic applications of the gut microbiome in obesity: From brain function to body detoxification. Int. J. Obes. 2020, 44, 1818–1831. [Google Scholar] [CrossRef]
- Rudzki, L.; Stone, T.W.; Maes, M.; Misiak, B.; Samochowiec, J.; Szulc, A. Gut microbiota-derived vitamins–underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110240. [Google Scholar] [CrossRef] [PubMed]
- Fock, E.; Parnova, R. Mechanisms of blood–brain barrier protection by microbiota-derived short-chain fatty acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Leigh, S.-J.; Morris, M.J. Diet, inflammation and the gut microbiome: Mechanisms for obesity-associated cognitive impairment. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165767. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.; Bolte, L.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; McVey Neufeld, K.A.; Waworuntu, R.V.; Pusceddu, M.M.; Manurung, S.; Murphy, K.; Strain, C.; Laguna, M.C.; Peterson, V.L.; Stanton, C. The enduring effects of early-life stress on the microbiota–gut–brain axis are buffered by dietary supplementation with milk fat globule membrane and a prebiotic blend. Eur. J. Neurosci. 2020, 51, 1042–1058. [Google Scholar] [CrossRef]
- Bistoletti, M.; Caputi, V.; Baranzini, N.; Marchesi, N.; Filpa, V.; Marsilio, I.; Cerantola, S.; Terova, G.; Baj, A.; Grimaldi, A. Antibiotic treatment-induced dysbiosis differently affects BDNF and TrkB expression in the brain and in the gut of juvenile mice. PLoS ONE 2019, 14, e0212856. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, Y.; Meng, R.; Tang, J.; Zhu, J.; Aldrich, M.C.; Cox, N.J.; Zhu, Y.; Li, Y.; Zhou, D. Cross-talks between gut microbiota and tobacco smoking: A two-sample Mendelian randomization study. BMC Med. 2023, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Le Roy, T.; Furgiuele, S.; Coste, V.; Bindels, L.B.; Leyrolle, Q.; Neyrinck, A.M.; Quoilin, C.; Amadieu, C.; Petit, G. Gut microbiota-induced changes in β-hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell Rep. 2020, 33, 108238. [Google Scholar] [CrossRef]
- Qamar, N.; Castano, D.; Patt, C.; Chu, T.; Cottrell, J.; Chang, S.L. Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact. Behav. Brain Res. 2019, 376, 112196. [Google Scholar] [CrossRef]
- Alam, R.; Abdolmaleky, H.M.; Zhou, J.R. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The microbiota–gut–brain Axis in psychiatric disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-F.; Huang, W.-C.; Wu, C.W.; Huang, C.-Y.; Yang, Y.-C.S.; Tung, Y.-T. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol. Res. 2023, 268, 127292. [Google Scholar] [CrossRef]
- Panariello, F.; Fanelli, G.; Fabbri, C.; Atti, A.R.; De Ronchi, D.; Serretti, A. Epigenetic basis of psychiatric disorders: A narrative review. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2022, 21, 302–315. [Google Scholar] [CrossRef]
- Meng, Y.; Du, J.; Liu, N.; Qiang, Y.; Xiao, L.; Lan, X.; Ma, L.; Yang, J.; Yu, J.; Lu, G. Epigenetic modulation: Research progress on histone acetylation levels in major depressive disorders. J. Drug Target. 2023, 31, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Di Bartolomeo, M.; Di Martino, S.; Stark, T.; Dell’Osso, B.; Drago, F.; D’Addario, C. Are the epigenetic changes predictive of therapeutic efficacy for psychiatric disorders? A translational approach towards novel drug targets. Pharmacol. Ther. 2022, 241, 108279. [Google Scholar] [CrossRef] [PubMed]
- Demaili, A.; Portugalov, A.; Dudai, M.; Maroun, M.; Akirav, I.; Braun, K.; Bock, J. Epigenetic (re) programming of gene expression changes of CB1R and FAAH in the medial prefrontal cortex in response to early life and adolescence stress exposure. Front. Cell. Neurosci. 2023, 17, 1129946. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Shields, C.E.; Haynes, K.A. Beyond the marks: Reader-effectors as drivers of epigenetics and chromatin engineering. Trends Biochem. Sci. 2022, 47, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Lu, G.; Yan, Z.; Jiang, R.; Sun, Y.; Zhang, P. Epigenetic mechanisms of DNA methylation in the transgenerational effect of ethylhexyl salicylate on zebrafish. Chemosphere 2022, 295, 133926. [Google Scholar] [CrossRef] [PubMed]
- Comb, M.; Goodman, H.M. CpG methylation inhibits proenkephalin gene expression and binding of the transcription factor AP-2. Nucleic Acids Res. 1990, 18, 3975–3982. [Google Scholar] [CrossRef]
- Inamdar, N.M.; Ehrlich, K.C.; Ehrlich, M. CpG methylation inhibits binding of several sequence-specific DNA-binding proteins from pea, wheat, soybean and cauliflower. Plant Mol. Biol. 1991, 17, 111–123. [Google Scholar] [CrossRef]
- Ng, H.-H.; Zhang, Y.; Hendrich, B.; Johnson, C.A.; Turner, B.M.; Erdjument-Bromage, H.; Tempst, P.; Reinberg, D.; Bird, A. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat. Genet. 1999, 23, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Takebayashi, S.-i.; Okumura, K.; Kudo, S.; Chiba, T.; Saya, H.; Nakao, M. Methylation-mediated transcriptional silencing in euchromatin by methyl-CpG binding protein MBD1 isoforms. Mol. Cell. Biol. 1999, 19, 6415–6426. [Google Scholar] [CrossRef] [PubMed]
- Corneo, E.; Michels, M.; Abatti, M.; Vieira, A.; Gonçalves, R.C.; Gabriel, F.F.; Borges, H.; Goulart, A.; da Silva Matos, N.; Dominguini, D. Enriched environment causes epigenetic changes in hippocampus and improves long-term cognitive function in sepsis. Sci. Rep. 2022, 12, 11529. [Google Scholar] [CrossRef]
- Rahman, M.F.; McGowan, P.O. Cell-type-specific epigenetic effects of early life stress on the brain. Transl. Psychiatry 2022, 12, 326. [Google Scholar] [CrossRef]
- Penner-Goeke, S.; Binder, E.B. Epigenetics and depression. Dialogues Clin. Neurosci. 2019, 21, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.M.; Adams, N.M.; Geary, C.D.; Weizman, O.-E.; Rapp, M.; Pritykin, Y.; Leslie, C.S.; Sun, J.C. Epigenetic control of innate and adaptive immune memory. Nat. Immunol. 2018, 19, 963–972. [Google Scholar] [CrossRef]
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, epigenetics and depression: A systematic review. Neurosci. Biobehav. Rev. 2019, 102, 139–152. [Google Scholar] [CrossRef]
- Clark, S.L.; Hattab, M.W.; Chan, R.F.; Shabalin, A.A.; Han, L.K.; Zhao, M.; Smit, J.H.; Jansen, R.; Milaneschi, Y.; Xie, L.Y. A methylation study of long-term depression risk. Mol. Psychiatry 2020, 25, 1334–1343. [Google Scholar] [CrossRef]
- Li, S.; Zong, L.; Hou, Y.; Zhang, W.; Zhou, L.; Yang, Q.; Wang, L.; Jiang, W.; Li, Q.; Huang, X. Altered DNA methylation of the Alu y subfamily in schizophrenia and bipolar disorder. Epigenomics 2019, 11, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Lv, X.; Huang, H.; Li, M.; Huai, C.; Wu, X.; Wu, H.; Ma, J.; Chen, L.; Wang, T. Genome-wide analysis of DNA methylation in 106 schizophrenia family trios in Han Chinese. eBioMedicine 2021, 72, 103609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, K.; Lyu, N.; Zhang, Y.; Liang, G.; Zhang, W.; Wang, X.; Wen, H.; Wen, L.; Ma, H. Genome-wide DNA methylation analysis in families with multiple individuals diagnosed with schizophrenia and intellectual disability. World J. Biol. Psychiatry, 2023; ahead of print. [Google Scholar]
- Yang, Y.; Long, J.; Shrubsole, M.J.; Cai, Q.; Zhao, Z.; Ye, F.; Li, Z.; Guo, X.; Li, B.; Bordenstein, S.R. Commensal microbiota, host DNA methylation and gene expression: A pilot study in colorectal adenomas. Cancer Res. 2022, 82, 3051. [Google Scholar] [CrossRef]
- Takahashi, K.; Sugi, Y.; Nakano, K.; Kobayakawa, T.; Nakanishi, Y.; Tsuda, M.; Hosono, A.; Kaminogawa, S. Regulation of gene expression through gut microbiota-dependent DNA methylation in colonic epithelial cells. ImmunoHorizons 2020, 4, 178–190. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Thiagalingam, S. Pathogenic histone modifications in schizophrenia are targets for therapy. In Epigenetics in Psychiatry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 309–319. [Google Scholar]
- Fuchikami, M.; Yamamoto, S.; Morinobu, S.; Okada, S.; Yamawaki, Y.; Yamawaki, S. The potential use of histone deacetylase inhibitors in the treatment of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 320–324. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Shaik, S.M.; Cao, Y.; Dodiya, H.B.; Zhang, X.; Boutej, H.; Han, W.; Kriz, J.; Sisodia, S.S. Translational profiling identifies sex-specific metabolic and epigenetic reprogramming of microglia in cerebral amyloidosis models with an antibiotic-altered gut microbiome. Alzheimers Dement. 2022, 18, e059695. [Google Scholar] [CrossRef]
- Yu, L.W.; Agirman, G.; Hsiao, E.Y. The gut microbiome as a regulator of the neuroimmune landscape. Annu. Rev. Immunol. 2022, 40, 143–167. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Raybould, H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010, 153, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Gui, S.; Zeng, B.; Pu, J.; Zheng, P.; Zeng, L.; Luo, Y.; Wu, Y.; Zhou, C. Proteomics analysis of the gut–brain axis in a gut microbiota-dysbiosis model of depression. Transl. Psychiatry 2021, 11, 568. [Google Scholar] [CrossRef]
- Ettinger, S. Diet, gut microbiome, and cognitive decline. Curr. Nutr. Rep. 2022, 11, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhu, X.; Zeng, M.; Qi, L.; Tang, X.; Wang, D.; Zhang, M.; Xie, Y.; Li, H.; Yang, X. A diet high in sugar and fat influences neurotransmitter metabolism and then affects brain function by altering the gut microbiota. Transl. Psychiatry 2021, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Littmann, E.R.; Fontana, E.; Moody, T.U.; Kohout, C.E.; Gjonbalaj, M.; Eaton, V.; Seok, R.; Leiner, I.M.; Pamer, E.G. Functional and genomic variation between human-derived isolates of Lachnospiraceae reveals inter-and intra-species diversity. Cell Host Microbe 2020, 28, 134–146.e4. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the intestinal microbiome, intestinal barrier and psychobiotics in depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky gut: Effect of dietary fiber and fats on microbiome and intestinal barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- DeCastro, M.; Nankova, B.B.; Shah, P.; Patel, P.; Mally, P.V.; Mishra, R.; La Gamma, E.F. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Mol. Brain Res. 2005, 142, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.K.; Schneider, J.S. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010, 1354, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, ra158–ra263. [Google Scholar] [CrossRef]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: Assessment, treatment and classification implications. Curr. Top. Med. Chem. 2020, 20, 524–539. [Google Scholar] [CrossRef]
- Li, X.; Yuan, X.; Pang, L.; Zhang, S.; Li, Y.; Huang, X.; Fan, X.; Song, X. The effect of serum lipids and short-chain fatty acids on cognitive functioning in drug-naïve, first episode schizophrenia patients. Psychiatry Res. 2022, 313, 114582. [Google Scholar] [CrossRef]
- Peng, H.; Ouyang, L.; Li, D.; Li, Z.; Yuan, L.; Fan, L.; Liao, A.; Li, J.; Wei, Y.; Yang, Z. Short-chain fatty acids in patients with schizophrenia and ultra-high risk population. Front. Psychiatry 2022, 13, 977538. [Google Scholar] [CrossRef]
- He, Y.; Kosciolek, T.; Tang, J.; Zhou, Y.; Li, Z.; Ma, X.; Zhu, Q.; Yuan, N.; Yuan, L.; Li, C. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur. Psychiatry 2018, 53, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Martinez-del Campo, A.; Kasahara, K.; Chittim, C.L.; Vivas, E.I.; Amador-Noguez, D.; Balskus, E.P.; Rey, F.E. Metabolic, epigenetic, and transgenerational effects of gut bacterial choline consumption. Cell Host Microbe 2017, 22, 279–290.e7. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Dilworth, M.; Woods, D. Cobalamin and the synthesis of methionine by Escherichia coli. Nature 1964, 201, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhao, H. Epigenetic factors in atherosclerosis: DNA methylation, folic acid metabolism, and intestinal microbiota. Clin. Chim. Acta 2021, 512, 7–11. [Google Scholar] [CrossRef]
- Sterrett, J.D.; Andersen, N.D.; Lowry, C.A. The influence of the microbiota on brain structure and function: Implications for stress-related neuropsychiatric disorders. In Evolution, Biodiversity and a Reassessment of the Hygiene Hypothesis; Springer: Berlin/Heidelberg, Germany, 2022; pp. 267–337. [Google Scholar]
- Cheng, L.; Wu, H.; Chen, Z.; Hao, H.; Zheng, X. Gut microbiome at the crossroad of genetic variants and behavior disorders. Gut Microbes 2023, 15, 2201156. [Google Scholar] [CrossRef]
- Lai, W.-T.; Zhao, J.; Xu, S.-X.; Deng, W.-F.; Xu, D.; Wang, M.-B.; He, F.-S.; Liu, Y.-H.; Guo, Y.-Y.; Ye, S.-W. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in bipolar disorder with current major depressive episode patients. J. Affect. Disord. 2021, 278, 311–319. [Google Scholar] [CrossRef]
- Flowers, S.A.; Baxter, N.T.; Ward, K.M.; Kraal, A.Z.; McInnis, M.G.; Schmidt, T.M.; Ellingrod, V.L. Effects of atypical antipsychotic treatment and resistant starch supplementation on gut microbiome composition in a cohort of patients with bipolar disorder or schizophrenia. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 161–170. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, Y.; Chen, F.; Yan, X.; Liu, X.; Shao, L.; Jin, G.; Zhou, D.; Jiang, G.; Li, H. Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: A case-control study. Front. Immunol. 2022, 13, 964910. [Google Scholar] [CrossRef]
- Olde Loohuis, L.M.; Mangul, S.; Ori, A.P.; Jospin, G.; Koslicki, D.; Yang, H.T.; Wu, T.; Boks, M.P.; Lomen-Hoerth, C.; Wiedau-Pazos, M. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl. Psychiatry 2018, 8, 96. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Yuan, X.; Pang, L.; Hu, S.; Wang, Y.; Huang, X.; Song, X. The role of butyric acid in treatment response in drug-naive first episode schizophrenia. Front. Psychiatry 2021, 12, 724664. [Google Scholar] [CrossRef]
- Li, Z.; Qing, Y.; Cui, G.; Li, M.; Liu, T.; Zeng, Y.; Zhou, C.; Hu, X.; Jiang, J.; Wang, D. Shotgun metagenomics reveals abnormal short-chain fatty acid-producing bacteria and glucose and lipid metabolism of the gut microbiota in patients with schizophrenia. Schizophr. Res. 2023, 255, 59–66. [Google Scholar] [CrossRef]
- Becerra, C.Y.; Wells, R.K.; Kunihiro, B.P.; Lee, R.H.; Umeda, L.; Allan, N.P.; Rubas, N.C.; McCracken, T.A.; Nunokawa, C.K.; Lee, M.-H. Examining the immunoepigenetic-gut microbiome axis in the context of self-esteem among Native Hawaiians and other Pacific Islanders. Front. Genet. 2023, 14, 1125217. [Google Scholar] [CrossRef]
- Strachan, E.; Zhao, J.; Roy-Byrne, P.P.; Fowler, E.; Bacus, T. Study design and rationale for the mood and methylation study: A platform for multi-omics investigation of depression in twins. Twin Res. Hum. Genet. 2018, 21, 507–513. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Rao, X.; Liu, L.; Zheng, P.; Li, W.; Zhou, W.; Chai, T.; Ji, P.; Song, J. Proteomic profiling of lysine acetylation indicates mitochondrial dysfunction in the hippocampus of gut microbiota-absent mice. Front. Mol. Neurosci. 2021, 14, 594332. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Z.; Chen, M.; Chen, G.; Huang, Q.; Yang, X.; Yin, H.; Chen, L.; Zhang, W.; Lin, H. Reduced stress-associated FKBP5 DNA methylation together with gut microbiota dysbiosis is linked with the progression of obese PCOS patients. npj Biofilms Microbiomes 2021, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Rao, X.; Yu, Y.; Li, W.; Zheng, P.; Zhao, L.; Zhou, C.; Pu, J.; Yang, D. Comprehensive analysis of the lysine acetylome and succinylome in the hippocampus of gut microbiota-dysbiosis mice. J. Adv. Res. 2021, 30, 27–38. [Google Scholar] [CrossRef]
- Yap, C.X.; Henders, A.K.; Alvares, G.A.; Wood, D.L.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021, 184, 5916–5931.e7. [Google Scholar] [CrossRef] [PubMed]
- Belardo, A.; Gevi, F.; Zolla, L. The concomitant lower concentrations of vitamins B6, B9 and B12 may cause methylation deficiency in autistic children. J. Nutr. Biochem. 2019, 70, 38–46. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Hua, X.; Zhu, J.; Yang, T.; Guo, M.; Li, Q.; Chen, J.; Li, T. The gut microbiota and associated metabolites are altered in sleep disorder of children with autism spectrum disorders. Front. Psychiatry 2020, 11, 855. [Google Scholar] [CrossRef]
- Lin, C.-W.; Septyaningtrias, D.E.; Chao, H.-W.; Konda, M.; Atarashi, K.; Takeshita, K.; Tamada, K.; Nomura, J.; Sasagawa, Y.; Tanaka, K. A common epigenetic mechanism across different cellular origins underlies systemic immune dysregulation in an idiopathic autism mouse model. Mol. Psychiatry 2022, 27, 3343–3354. [Google Scholar] [CrossRef] [PubMed]
- Bengesser, S.; Mörkl, S.; Painold, A.; Dalkner, N.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; Hamm, C.; Maget, A. Epigenetics of the molecular clock and bacterial diversity in bipolar disorder. Psychoneuroendocrinology 2019, 101, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, J.; Zhang, J.; Liang, C.; Wang, Y.; Chen, B.; Zhao, C.; Wang, J.; Zhang, G.; Zhao, D. Correlation of gut microbiome between ASD children and mothers and potential biomarkers for risk assessment. Genom. Proteom. Bioinform. 2019, 17, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Liu, Y.; Wang, S.; Ma, J.; Yang, H. Distribution characteristics of intestinal microbiota during pregnancy and postpartum in healthy women. J. Matern. Fetal Neonatal Med. 2022, 35, 2915–2922. [Google Scholar] [CrossRef]
- Codagnone, M.G.; Stanton, C.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental trajectories: Role of maternal and early-life nutrition. Ann. Nutr. Metab. 2019, 74, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Gawliński, D.; Gawlińska, K.; Smaga, I. Maternal high-fat diet modulates Cnr1 gene expression in male rat offspring. Nutrients 2021, 13, 2885. [Google Scholar] [CrossRef]
- Kim, S.-A.; Chai, J.-H.; Jang, E.-H. Prenatal Trimethyltin Exposure Induces Long-Term DNA Methylation Changes in the Male Mouse Hippocampus. Int. J. Mol. Sci. 2021, 22, 8009. [Google Scholar] [CrossRef]
- Ondičová, M.; Irwin, R.E.; Thursby, S.-J.; Hilman, L.; Caffrey, A.; Cassidy, T.; McLaughlin, M.; Lees-Murdock, D.J.; Ward, M.; Murphy, M. Folic acid intervention during pregnancy alters DNA methylation, affecting neural target genes through two distinct mechanisms. Clin. Epigenet. 2022, 14, 63. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Besson, M.; Guérin, S.; Coquery, N.; Randuineau, G.; Kanzari, A.; Quesnel, H.; Bonhomme, N.; Bolhuis, J.E.; Kemp, B. A maternal Western diet during gestation and lactation modifies offspring’s microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in Yucatan pigs. FASEB J. 2017, 31, 2037–2049. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ali, M.M. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef]
- Mews, P.; Egervari, G.; Nativio, R.; Sidoli, S.; Donahue, G.; Lombroso, S.; Alexander, D.; Riesche, S.; Heller, E.; Nestler, E. Alcohol metabolism contributes to brain histone acetylation. Nature 2019, 574, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Hofford, R.S.; Mervosh, N.L.; Euston, T.J.; Meckel, K.R.; Orr, A.T.; Kiraly, D.D. Alterations in microbiome composition and metabolic byproducts drive behavioral and transcriptional responses to morphine. Neuropsychopharmacology 2021, 46, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wang, J.; Wang, B.; Wang, R.; Li, G.; Jia, Y.; Chen, T.; Chen, Y. Alterations in gut microbiota affect behavioral and inflammatory responses to methamphetamine in mice. Psychopharmacology 2022, 239, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, S.; Zhang, M.; Bai, S.; Ni, Y.; Xu, Q.; Fan, Y.; Lu, C.; Xu, Z.; Ji, C. Early-life bisphenol AP exposure impacted neurobehaviors in adulthood through microglial activation in mice. Chemosphere 2023, 317, 137935. [Google Scholar] [CrossRef]
- Yi, W.; Ji, Y.; Gao, H.; Luo, S.; Pan, R.; Song, J.; He, Y.; Li, Y.; Wu, Y.; Yan, S. Effects of urban particulate matter on gut microbiome and partial schizophrenia-like symptoms in mice: Evidence from shotgun metagenomic and metabolomic profiling. Sci. Total Environ. 2023, 857, 159305. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Pal, N.; Kumawat, M.; Shubham, S.; Sarma, D.K.; Tiwari, R.R.; Kumar, M.; Nagpal, R. Impact of Environmental Pollutants on Gut Microbiome and Mental Health via the Gut–Brain Axis. Microorganisms 2022, 10, 1457. [Google Scholar] [CrossRef]
- Hu, F.; Liang, W.; Zhang, L.; Wang, H.; Li, Z.; Zhou, Y. Hyperactivity of basolateral amygdala mediates behavioral deficits in mice following exposure to bisphenol A and its analogue alternative. Chemosphere 2022, 287, 132044. [Google Scholar] [CrossRef]
- Senyildiz, M.; Karaman, E.F.; Bas, S.S.; Pirincci, P.A.; Ozden, S. Effects of BPA on global DNA methylation and global histone 3 lysine modifications in SH-SY5Y cells: An epigenetic mechanism linking the regulation of chromatin modifiying genes. Toxicol. Vitr. 2017, 44, 313–321. [Google Scholar] [CrossRef]
- Desai, M.; Ferrini, M.G.; Han, G.; Jellyman, J.K.; Ross, M.G. In vivo maternal and in vitro BPA exposure effects on hypothalamic neurogenesis and appetite regulators. Environ. Res. 2018, 164, 45–52. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, L.; Yang, S.; Ni, L.; Ma, L.; Zhao, Y.; Zheng, A.; Jin, Y.; Fu, Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere 2021, 282, 130952. [Google Scholar] [CrossRef]
- AlOlaby, R.R.; Zafarullah, M.; Barboza, M.; Peng, G.; Varian, B.J.; Erdman, S.E.; Lebrilla, C.; Tassone, F. Differential Methylation Profile in Fragile X Syndrome-Prone Offspring Mice after in Utero Exposure to Lactobacillus Reuteri. Genes 2022, 13, 1300. [Google Scholar] [CrossRef]
- Chang, X.; Li, P.; Yan, K.; Lu, Y.; Tang, T.; Fan, X.; Fan, C.; Zhan, D.; Qi, K. Maternal dietary calcium status during pregnancy and lactation affects brain DHA accretion through modifying DNA methylation of fatty acid desaturases in the mouse offspring. Nutr. Res. 2019, 65, 29–42. [Google Scholar] [CrossRef]
- Jašarević, E.; Howerton, C.L.; Howard, C.D.; Bale, T.L. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 2015, 156, 3265–3276. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Kowal-Wiśniewska, E.; Jarmuż-Szymczak, M.; Filip, M. Alteration of the early development environment by maternal diet and the occurrence of autistic-like phenotypes in rat offspring. Int. J. Mol. Sci. 2021, 22, 9662. [Google Scholar] [CrossRef]
- McEwan, A.; Erickson, J.C.; Davidson, C.; Heijkoop, J.; Turnbull, Y.; Delibegovic, M.; Murgatroyd, C.; MacKenzie, A. The anxiety and ethanol intake controlling GAL5. 1 enhancer is epigenetically modulated by, and controls preference for, high-fat diet. Cell. Mol. Life Sci. 2021, 78, 3045–3055. [Google Scholar] [CrossRef]
- Di Gesù, C.M.; Matz, L.M.; Bolding, I.J.; Fultz, R.; Hoffman, K.L.; Gammazza, A.M.; Petrosino, J.F.; Buffington, S.A. Maternal gut microbiota mediate intergenerational effects of high-fat diet on descendant social behavior. Cell Rep. 2022, 41, 111461. [Google Scholar] [CrossRef] [PubMed]
- Arguelles-Lopez, A.; de la Barca, A.M.C. Can methyl donors in breastmilk prevent rapid growth in breastfed infants? Med. Hypotheses 2023, 174, 111065. [Google Scholar] [CrossRef]
- Mahajan, A.; Sapehia, D.; Thakur, S.; Mohanraj, P.S.; Bagga, R.; Kaur, J. Effect of imbalance in folate and vitamin B12 in maternal/parental diet on global methylation and regulatory miRNAs. Sci. Rep. 2019, 9, 17602. [Google Scholar] [CrossRef] [PubMed]
- Korsmo, H.W.; Dave, B.; Trasino, S.; Saxena, A.; Liu, J.; Caviglia, J.M.; Edwards, K.; Dembitzer, M.; Sheeraz, S.; Khaldi, S. Maternal Choline Supplementation and High-Fat Feeding Interact to Influence DNA Methylation in Offspring in a Time-Specific Manner. Front. Nutr. 2022, 9, 42. [Google Scholar] [CrossRef]
- Chiu, K.-C.; Sisca, F.; Ying, J.-H.; Tsai, W.-J.; Hsieh, W.-S.; Chen, P.-C.; Liu, C.-Y. Prenatal chlorpyrifos exposure in association with PPARγ H3K4me3 and DNA methylation levels and child development. Environ. Pollut. 2021, 274, 116511. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, C.; Morales-Navas, M.; Guardia-Escote, L.; Garrido-Cárdenas, J.A.; Colomina, M.T.; Giménez, E.; Sánchez-Santed, F. Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: A locomotor, pharmacological, brain gene expression and gut microbiome analysis. Food Chem. Toxicol. 2020, 135, 110865. [Google Scholar] [CrossRef]
- Hebert, J.C.; Radford-Smith, D.E.; Probert, F.; Ilott, N.; Chan, K.W.; Anthony, D.C.; Burnet, P.W. Mom’s diet matters: Maternal prebiotic intake in mice reduces anxiety and alters brain gene expression and the fecal microbiome in offspring. Brain Behav. Immun. 2021, 91, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhong, X.; He, Y.; Shi, Y. Butyrate, but not propionate, reverses maternal diet-induced neurocognitive deficits in offspring. Pharmacol. Res. 2020, 160, 105082. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zeng, Z.; Yan, S.; Li, L.; Yuan, S.; Zhao, S.; Dai, X. Gut Microbiota Mediates High-Fiber Diet Alleviation of Maternal Obesity-Induced Cognitive and Social Deficits in Offspring. Search Life-Sci. Lit. 2020; preprint. [Google Scholar]
- Cristiano, C.; Hoxha, E.; Lippiello, P.; Balbo, I.; Russo, R.; Tempia, F.; Miniaci, M.C. Maternal treatment with sodium butyrate reduces the development of autism-like traits in mice offspring. Biomed. Pharmacother. 2022, 156, 113870. [Google Scholar] [CrossRef]
- Ng, J.W. DNA Methylation Patterns Derived from Fetal Vulnerability to Maternal Smoking Relate to Future Child Outcomes. Ph.D. Thesis, Department of Physiology, University of Alberta, Edmonton, AB, Canada, 2021. [Google Scholar]
- Wang, S.; Zeng, Y.; He, X.; Liu, F.; Pei, P.; Zhang, T. Folate-deficiency induced acyl-CoA synthetase short-chain family member 2 increases lysine crotonylome involved in neural tube defects. Front. Mol. Neurosci. 2022, 15, 1064509. [Google Scholar] [CrossRef] [PubMed]
- Kleeman, E.A.; Gubert, C.; Hannan, A.J. Transgenerational epigenetic impacts of parental infection on offspring health and disease susceptibility. Trends Genet. 2022, 38, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chang, L.; Liu, G.; Wang, X.; Yang, Y.; Hashimoto, K. Long-lasting beneficial effects of maternal intake of sulforaphane glucosinolate on gut microbiota in adult offspring. J. Nutr. Biochem. 2022, 109, 109098. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, N.; Chen, Y.; Zhu, L.; Zhong, Q.; Liu, J.; Chen, T. Sodium butyrate modulates a methamphetamine-induced conditioned place preference. J. Neurosci. Res. 2017, 95, 1044–1052. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef]
- Doenyas, C. Potential role of epigenetics and redox signaling in the gut–brain communication and the case of autism spectrum disorder. Cell. Mol. Neurobiol. 2022, 42, 483–487. [Google Scholar] [CrossRef]
- Obrenovich, M.E. Leaky gut, leaky brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.-C.; Berk, M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, J.; Lee, H.-J. Flavonoid-rich orange juice intake and altered gut microbiome in young adults with depressive symptom: A randomized controlled study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.A.; Holscher, H.D. Microbiome-mediated effects of the Mediterranean diet on inflammation. Adv. Nutr. 2018, 9, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Gallè, F.; Valeriani, F.; Cattaruzza, M.S.; Gianfranceschi, G.; Liguori, R.; Antinozzi, M.; Mederer, B.; Liguori, G.; Romano Spica, V. Mediterranean diet, physical activity and gut microbiome composition: A cross-sectional study among healthy young Italian adults. Nutrients 2020, 12, 2164. [Google Scholar] [CrossRef]
- Rejeski, J.J.; Wilson, F.M.; Nagpal, R.; Yadav, H.; Weinberg, R.B. The impact of a mediterranean diet on the gut microbiome in healthy human subjects: A pilot study. Digestion 2022, 103, 133–140. [Google Scholar] [CrossRef]
- Solch, R.; Engler-Chiurazzi, E.; Harper, C.; Wasson, S.; Ogbonna, S.; Ouvrier, B.; Wang, H.; McDonald, K.; Biose, I.; Gregory, B. A Mediterranean Diet Enhances Cognitive Function and Modulates the Gut Microbiota. Curr. Dev. Nutr. 2022, 6, 1029. [Google Scholar] [CrossRef]
- Lorite Mingot, D.; Gesteiro, E.; Bastida, S.; Sánchez-Muniz, F.J. Epigenetic effects of the pregnancy Mediterranean diet adherence on the offspring metabolic syndrome markers. J. Physiol. Biochem. 2017, 73, 495–510. [Google Scholar] [CrossRef]
- House, J.S.; Mendez, M.; Maguire, R.L.; Gonzalez-Nahm, S.; Huang, Z.; Daniels, J.; Murphy, S.K.; Fuemmeler, B.F.; Wright, F.A.; Hoyo, C. Periconceptional maternal mediterranean diet is associated with favorable offspring behaviors and altered CpG methylation of imprinted genes. Front. Cell Dev. Biol. 2018, 6, 107. [Google Scholar] [CrossRef]
- Remely, M.; Ferk, F.; Sterneder, S.; Setayesh, T.; Roth, S.; Kepcija, T.; Noorizadeh, R.; Rebhan, I.; Greunz, M.; Beckmann, J. EGCG prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Oxidative Med. Cell. Longev. 2017, 2017, 3079148. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-Y.; Jang, H.-M.; Kim, D.-H. High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci. Lett. 2019, 698, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of sesamin alleviates stress-induced behavioral and psychological disorders via reshaping the gut microbiota structure. J. Agric. Food Chem. 2019, 67, 12441–12451. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci. Rep. 2017, 7, 10776. [Google Scholar] [CrossRef]
- Matsumoto, A.K.; Maes, M.; Maes, A.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Moreira, E.; Kanchanatawan, B.; Barbosa, D.S. In schizophrenia, PON1 Q192R genotypes and/or lowered Paraoxonase 1 (PON1) enzymatic activity are significantly associated with the deficit syndrome, negative symptoms, formal thought disorders, psychomotor retardation, excitation and increased IgA levels to gram-negative microbiota. Med. Pharmacol. 2019; preprint. [Google Scholar]
- Wei, H.; Yuan, Y.; Zhu, C.; Ma, M.; Yang, F.; Lu, Z.; Wang, C.; Deng, H.; Zhao, J.; Tian, R. DNA hyper-methylation associated with schizophrenia may lead to increased levels of autoantibodies. Schizophr. Bull. Open 2022, sgac047. [Google Scholar] [CrossRef]
- Luo, C.; Pi, X.; Hu, N.; Wang, X.; Xiao, Y.; Li, S.; Sweeney, J.A.; Bishop, J.R.; Gong, Q.; Xie, D. Subtypes of schizophrenia identified by multi-omic measures associated with dysregulated immune function. Mol. Psychiatry 2021, 26, 6926–6936. [Google Scholar] [CrossRef]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 3546. [Google Scholar] [CrossRef]
- Wang, J.; Hodes, G.E.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 2018, 9, 477. [Google Scholar] [CrossRef]
- Dalile, B.; La Torre, D.; Kalc, P.; Zoppas, F.; Roye, C.; Loret, C.; Lamothe, L.; Bergonzelli, G.; Courtin, C.M.; Vervliet, B. Extruded wheat bran consumption increases serum short-chain fatty acids but does not modulate psychobiological functions in healthy men: A randomized, placebo-controlled trial. Front. Nutr. 2022, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, M.; D’Urso, F.; Piccininni, C.; Montagna, M.L.; Sardone, R.; Resta, E.; Dibello, V.; Daniele, A.; Giannelli, G.; Bellomo, A. The relationship between epigenetics and microbiota in neuropsychiatric diseases. Epigenomics 2020, 12, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Carrer, A.; Parris, J.L.; Trefely, S.; Henry, R.A.; Montgomery, D.C.; Torres, A.; Viola, J.M.; Kuo, Y.-M.; Blair, I.A.; Meier, J.L. Impact of a high-fat diet on tissue Acyl-CoA and histone acetylation levels. J. Biol. Chem. 2017, 292, 3312–3322. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Soliman, M.L.; Rosenberger, T.A. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol. Cell. Biochem. 2011, 352, 173–180. [Google Scholar] [CrossRef]
- Osman, A.; Mervosh, N.L.; Strat, A.N.; Meckel, K.R.; Euston, T.J.; Zipursky, G.D.; Drapeau, E.; Buxbaum, J.D.; Breen, M.S.; Kiraly, D.D. Effects of gene by microbiome interactions on behavioral and neurobiological phenotypes in a mouse model for autism spectrum disorder. bioRxiv, 2020; preprint. [Google Scholar]
- Wolugbom Jr, J.A.; Areloegbe, S.E.; Olaniyi, K.S. Acetate meliorates depressive-like behaviour in a rat model of PCOS through suppression of HDAC2 expression and DNA methylation. Res. Sq. 2022; preprint. [Google Scholar]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef]
- Rode, J.; Yang, L.; König, J.; Hutchinson, A.N.; Wall, R.; Venizelos, N.; Brummer, R.-J.; Rangel, I.; Vumma, R. Butyrate rescues oxidative stress-induced transport deficits of tryptophan: Potential implication in affective or gut-brain axis disorders. Neuropsychobiology 2021, 80, 253–263. [Google Scholar] [CrossRef]
- Wei, H.; Yu, C.; Zhang, C.; Ren, Y.; Guo, L.; Wang, T.; Chen, F.; Li, Y.; Zhang, X.; Wang, H. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed. Pharmacother. 2023, 160, 114308. [Google Scholar] [CrossRef]
- Intlekofer, K.A.; Berchtold, N.C.; Malvaez, M.; Carlos, A.J.; McQuown, S.C.; Cunningham, M.J.; Wood, M.A.; Cotman, C.W. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology 2013, 38, 2027–2034. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Simões, L.R.; Faller, C.J.; Ceretta, R.A.; Petronilho, F.; Lopes-Borges, J.; Valvassori, S.S.; Quevedo, J. Sodium butyrate prevents memory impairment by re-establishing BDNF and GDNF expression in experimental pneumococcal meningitis. Mol. Neurobiol. 2015, 52, 734–740. [Google Scholar] [CrossRef]
- Kratsman, N.; Getselter, D.; Elliott, E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology 2016, 102, 136–145. [Google Scholar] [CrossRef]
- Chen, L.; Miao, Z.; Xu, X. β-hydroxybutyrate alleviates depressive behaviors in mice possibly by increasing the histone3-lysine9-β-hydroxybutyrylation. Biochem. Biophys. Res. Commun. 2017, 490, 117–122. [Google Scholar] [CrossRef]
- Serger, E. Intermittent Fasting Promotes Axonal Regeneration through the Gut Microbiome Derived Metabolite: Indole-3-Propionic Acid. Ph.D. Thesis, Department of Brain Sciences, Imperial College London, London, UK, 2020. [Google Scholar]
- Xiao, H.-W.; Cui, M.; Li, Y.; Dong, J.-L.; Zhang, S.-Q.; Zhu, C.-C.; Jiang, M.; Zhu, T.; Wang, B.; Wang, H.-C. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 2020, 8, 69. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, X.; Cheng, Z.; Dong, Q.; Ruan, G. The anti-inflammatory effect of preventive intervention with ketogenic diet mediated by the histone acetylation of mGluR5 promotor region in rat Parkinson’s disease model: A dual-tracer PET study. Park. Dis. 2022, 2022, 3506213. [Google Scholar] [CrossRef]
- Thau-Zuchman, O.; Svendsen, L.; Dyall, S.C.; Paredes-Esquivel, U.; Rhodes, M.; Priestley, J.V.; Feichtinger, R.G.; Kofler, B.; Lotstra, S.; Verkuyl, J.M. A new ketogenic formulation improves functional outcome and reduces tissue loss following traumatic brain injury in adult mice. Theranostics 2021, 11, 346. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Izquierdo, A.G.; Primo, D.; Milagro, F.I.; Sajoux, I.; Jácome, A.; Fernandez-Quintela, A.; Portillo, M.P.; Martínez, J.A.; Martinez-Olmos, M.A. Epigenetic landscape in blood leukocytes following ketosis and weight loss induced by a very low calorie ketogenic diet (VLCKD) in patients with obesity. Clin. Nutr. 2021, 40, 3959–3972. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef]
- Lee, R.W.; Corley, M.J.; Pang, A.; Arakaki, G.; Abbott, L.; Nishimoto, M.; Miyamoto, R.; Lee, E.; Yamamoto, S.; Maunakea, A.K. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol. Behav. 2018, 188, 205–211. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Qin, L.; Ma, K.; Yan, Z. Rescue of histone hypoacetylation and social deficits by ketogenic diet in a Shank3 mouse model of autism. Neuropsychopharmacology 2022, 47, 1271–1279. [Google Scholar] [CrossRef]
- Olivito, I.; Avolio, E.; Minervini, D.; Soda, T.; Rocca, C.; Angelone, T.; Iaquinta, F.S.; Bellizzi, D.; De Rango, F.; Bruno, R. Ketogenic diet ameliorates autism spectrum disorders-like behaviors via reduced inflammatory factors and microbiota remodeling in BTBR mice. Exp. Neurol. 2023, 366, 114432. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.L.; Cowan, C.S.; Richardson, R. Treating generational stress: Effect of paternal stress on development of memory and extinction in offspring is reversed by probiotic treatment. Psychol. Sci. 2016, 27, 1171–1180. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H. PROVIT: Supplementary probiotic treatment and vitamin B7 in depression—A randomized controlled trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Di Minno, A.; Esposito, C.; El-Seedi, H.R.; Khalifa, S.A.; Baldi, A.; Greco, A.; Santonastaso, S.; Cioffi, V.; Sperandeo, R. Efficacy of a food supplement based on S-adenosyl methionine and probiotic strains in subjects with subthreshold depression and mild-to-moderate depression: A monocentric, randomized, cross-over, double-blind, placebo-controlled clinical trial. Biomed. Pharmacother. 2022, 156, 113930. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Bengesser, S.A.; Hauschild, A.-C.; Birkl-Töglhofer, A.-M.; Fellendorf, F.T.; Platzer, M.; Färber, T.; Seidl, M.; Mendel, L.-M.; Unterweger, R. Interleukin-6 gene expression changes after a 4-week intake of a multispecies probiotic in major depressive disorder—Preliminary Results of the PROVIT Study. Nutrients 2020, 12, 2575. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, B.; Tian, P.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Daily intake of Lactobacillus alleviates autistic-like behaviors by ameliorating the 5-hydroxytryptamine metabolic disorder in VPA-treated rats during weaning and sexual maturation. Food Funct. 2021, 12, 2591–2604. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, T.; Xu, Y.; Gu, X.; Li, D.; Niu, K.; Wang, T.; Zhao, J.; Zhou, R.; Wang, H.-L. Long-term probiotic intervention mitigates memory dysfunction through a novel H3K27me3-based mechanism in lead-exposed rats. Transl. Psychiatry 2020, 10, 25. [Google Scholar] [CrossRef]
- Nobile, V.; Giardina, S.; Puoci, F. The effect of a probiotic complex on the gut-brain axis: A translational study. Neuropsychobiology 2022, 81, 116–126. [Google Scholar] [CrossRef]
- Song, L.; Sun, Q.; Zheng, H.; Zhang, Y.; Wang, Y.; Liu, S.; Duan, L. Roseburia hominis Alleviates Neuroinflammation via Short-Chain Fatty Acids through Histone Deacetylase Inhibition. Mol. Nutr. Food Res. 2022, 66, 2200164. [Google Scholar] [CrossRef]
- Bakker, G.J.; Nieuwdorp, M. Fecal microbiota transplantation: Therapeutic potential for a multitude of diseases beyond Clostridium difficile. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Wortelboer, K.; Nieuwdorp, M.; Herrema, H. Fecal microbiota transplantation beyond Clostridioides difficile infections. eBioMedicine 2019, 44, 716–729. [Google Scholar] [CrossRef]
- Li, Z.; Sun, T.; He, Z.; Li, Z.; Zhang, W.; Wang, J.; Xiang, H. SCFAs Ameliorate Chronic Postsurgical Pain–Related Cognition Dysfunction via the ACSS2-HDAC2 Axis in Rats. Mol. Neurobiol. 2022, 59, 6211–6227. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Y.; Wu, P.; Zhou, H.; Lasanajak, Y.; Fang, Y.; Tang, L.; Ye, L.; Li, X.; Cai, Z. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 2019, 148, 104403. [Google Scholar] [CrossRef]
- Lai, J.; Zhang, P.; Jiang, J.; Mou, T.; Li, Y.; Xi, C.; Wu, L.; Gao, X.; Zhang, D.; Chen, Y. New evidence of gut microbiota involvement in the neuropathogenesis of bipolar depression by TRANK1 modulation: Joint clinical and animal data. Front. Immunol. 2021, 12, 789647. [Google Scholar] [CrossRef]
- Abuaish, S.; Al-Otaibi, N.M.; Abujamel, T.S.; Alzahrani, S.A.; Alotaibi, S.M.; AlShawakir, Y.A.; Aabed, K.; El-Ansary, A. Fecal transplant and Bifidobacterium treatments modulate gut Clostridium bacteria and rescue social impairment and hippocampal BDNF expression in a rodent model of autism. Brain Sci. 2021, 11, 1038. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Torres-Espin, A.; Raposo, P.J.; Madsen, K.L.; Kigerl, K.A.; Popovich, P.G.; Fenrich, K.K.; Fouad, K. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS ONE 2020, 15, e0226128. [Google Scholar] [CrossRef]
- Koppenol, E.; Terveer, E.M.; Vendrik, K.E.; van Lingen, E.; Verspaget, H.W.; Keller, J.J.; Kuijper, E.J.; Giltay, E.J.; Netherlands Donor Feces Bank Study Group. Fecal microbiota transplantation is associated with improved aspects of mental health of patients with recurrent Clostridioides difficile infections: Effect of FMT on affect in rCDI patients. J. Affect. Disord. Rep. 2022, 9, 100355. [Google Scholar] [CrossRef]
- van der Vossen, E.W.; Bastos, D.; Stols-Gonçalves, D.; de Goffau, M.C.; Davids, M.; Pereira, J.P.; Li Yim, A.Y.; Henneman, P.; Netea, M.G.; de Vos, W.M. Effects of fecal microbiota transplant on DNA methylation in subjects with metabolic syndrome. Gut Microbes 2021, 13, 1993513. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Olivito, I.; Rosina, E.; Romano, L.; Angelone, T.; De Bartolo, A.; Scimeca, M.; Bellizzi, D.; D’Aquila, P.; Passarino, G. Modifications of Behavior and Inflammation in Mice Following Transplant with Fecal Microbiota from Children with Autism. Neuroscience 2022, 498, 174–189. [Google Scholar] [CrossRef]

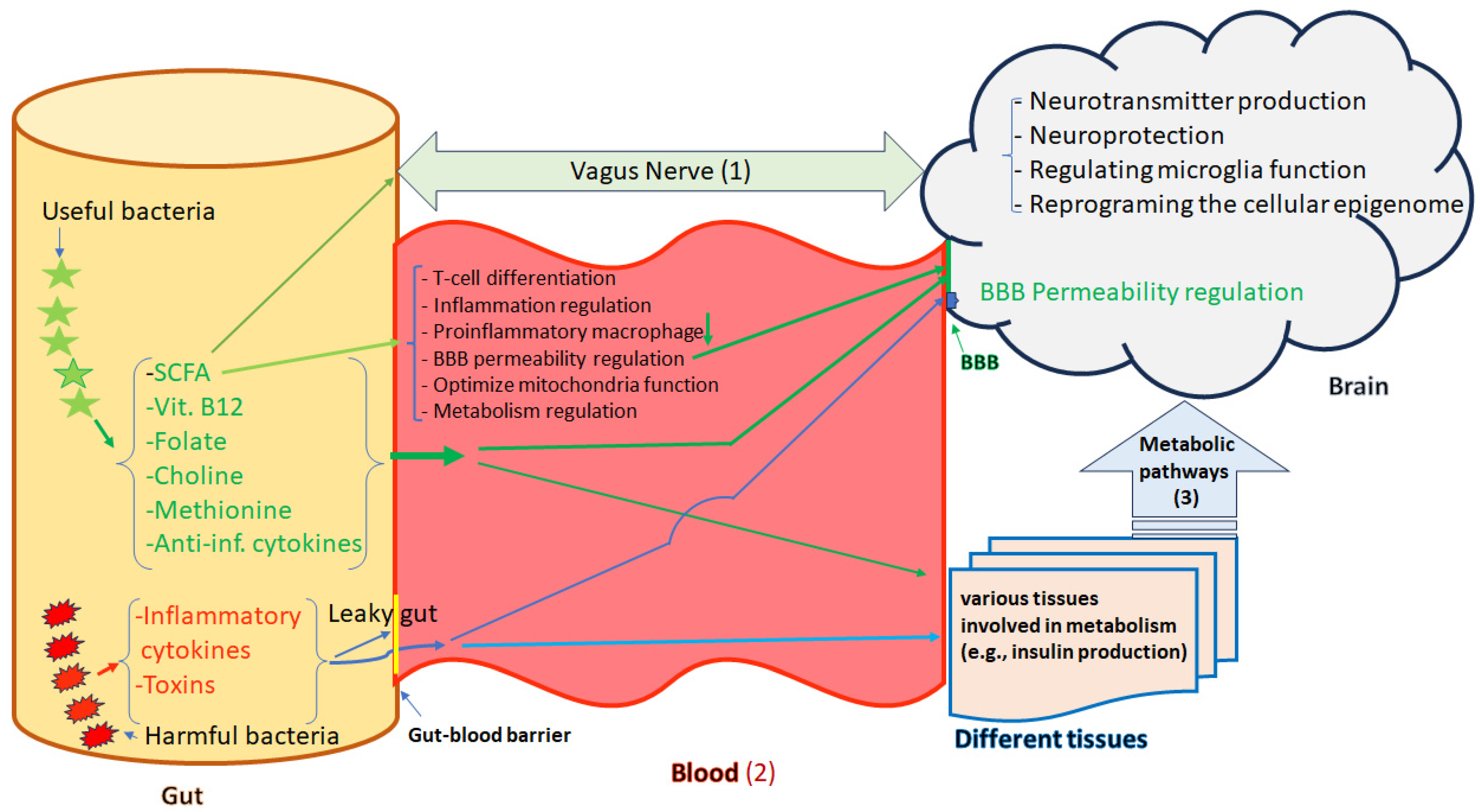


| Mental Disorders | Altered Gut Microbiota or Its Products | Effects or Outcomes | Epigenetic Alterations | Ref. |
|---|---|---|---|---|
| Schizophrenia | Increased microbial diversity in schizophrenia patients | A link between schizophrenia, immunity, and microbial products in blood | DNA methylation | [93] |
| Schizophrenia | Butyric acid as a microbiome-derived metabolite | Increasing serum levels of butyric acid as a microbiome-derived metabolite in schizophrenia patients after 24-week risperidone treatment | Histone acetylation | [94] |
| Schizophrenia | Metabolic alterations in the gut microbiome | Abnormal short-chain fatty acid-producing bacteria in patients | Histone acetylation | [95] |
| Depression | Changes in microbiome composition | Higher plasma concentrations of pro-inflammatory cytokines (IL-8 and TNF-α) and differential DNA methylation at immune-metabolic genes in monocytes | DNA methylation | [96] |
| MDD | Possible role of microbiome | ----------- | DNA methylation | [97] |
| MDD | Gut microbiota dysbiosis and pathogenesis of MDD | - Identifying 986 lysine acetylation sites in 543 proteins - Close association between lysine acetylation alterations and mitochondrial dysfunction in the brain | Lysine acetylation | [98] |
| Polycystic ovarian syndrome-associated depression | Significant differences in bacterial diversity and community, and stress responses in patients vs. healthy group | Lower FK506-binding protein 5 (FKBP5) DNA methylation in PCOS-associated depression | DNA methylation (reduced) | [99] |
| Depression | Gut microbiota dysbiosis-induced depression in Mice | Altered expression of 624 succinylation sites on 494 proteins and 315 acetylation sites on 223 proteins in gut microbiota dysbiosis | Acetylation and succinylation of proteins | [100] |
| ASD | Autism-gut microbiome associations | Microbiome differences in ASD may reflect dietary preferences | DNA methylation | [101] |
| ASD | Impairment in microbiome-related metabolites | Reducing protein and DNA methylation in autistic children, with concomitant lower concentrations of vitamins B6, B9 and B12 | DNA methylation (reduced) | [102] |
| ASD | Changes in microbiome-related metabolites | Increased abundance of valeric acid-associated bacteria (Acidobacteria) and decreased abundances of key butyrate-producing taxa (Ruminococcaceae, Eubacterium, Lachnospiraceae, and Erysipelotrichaceae) in autistics | Histone acetylation | [103] |
| ASD | Compositional changes in the gut microbiome and its secondary metabolites | Decreased abundances of Faecalibacterium and Agathobacter and reduced 3-hydroxybutyric acid and melatonin levels in ASD children with a sleep disorder | Histone acetylation | [104] |
| ASD | Association between microbiome composition and dysregulated immune profiles | Altered hematopoiesis during embryogenesis and reduced expression of AP-1 complex for microglia development via dysregulation of HDAC1-mediated epigenetic machinery | Histone acetylation | [105] |
| Bipolar disorder | Alterations in the gut microbiome diversity | Negative correlation between CpG methylation status of the clock gene ARNTL and gut microbiome diversity | DNA methylation | [106] |
| Factors | Effects on Gut Microbiome | Effects on Brain Functions | Epigenetic Changes | Ref. |
|---|---|---|---|---|
| Probiotic intake (feeding of a probiotic organism, Lactobacillus reuteri to pregnant animals) | Maternal gut microbiome alterations | Changing neurological functions (neurogenesis synaptogenesis, and synaptic modulation/transmission) | Differentially methylated genes in FXS-like mice descended from mothers treated and non-treated with L. reuteri | [125] |
| Dietary insufficient or excessive calcium intake during pregnancy | Long-lasting adverse effects on the gut microbiome (unpublished) | Detrimental effects on brain development and function | Hypomethylation of Fads2 promoter in the brain of 21-day-old offspring in the group with reproductive diet, with low calcium concentration (LC 0.25%) | [126] |
| Maternal Stress | Alterations in the vaginal microbiome | Loss of maternal vaginal Lactobacillus leads to reduced transmission of this bacterium to offspring, altering reprogramming of the developing brain | Changes in genomic DNA | [127] |
| Maternal high-fat diet (HFD) | Alterations in the offspring gut microbiome | Predisposition to an ASD-like phenotype in male adolescent offspring | Enhanced cortical global DNA methylation and the expression of miR-423 and miR-494 | [128] |
| Maternal HFD (>60% calories from fat) | Alterations in the offspring gut microbiome | Close association between maternal HFD with transgenerational susceptibility to chronic anxiety and alcohol abuse | DNA methylation changes (5 mC/5 hmC) in the genome regulatory regions | [129] |
| Maternal HFD | Alterations in the offspring gut microbiome | Social dysfunction and deficits in synaptic plasticity deficits in male offspring | Changes in histone acetylation | [130] |
| Methyl donor-containing foods in the maternal diet such as betaine compound | Alterations in the offspring gut microbiome | Direct association between a prolonged period of postnatal maturation of the prefrontal cortex and increased DNA methylation over time | DNA methylation | [131] |
| Imbalance in folate and vitamin B12 in maternal/parental diet | Alterations in gut microbiome composition | Folate and vitamin B12 are master regulators of brain DNA methylation | Changing global DNA methylation in the brain | [132] |
| Maternal choline supplementation and high-fat feeding | Alterations of gut microbiome composition | Potent modifier of brain DNA methylation | Brain DNA methylation | [133] |
| Prenatal chlorpyrifos exposure | Alterations in gut microbiome composition | Changing the cognitive and language domains | Increasing PPARγ DNA methylation | [134,135] |
| Maternal prebiotic supplementation (maternal galacto-oligosaccharide intake) | Increasing fecal microbiome-derived metabolites (butyrate and propionate) | - Altering brain and behavior in naïve and endotoxin-challenged offspring, increasing social preference and reducing anxiety | Increasing histone acetylation | [136] |
| Maternal low-fiber diet (MLFD) | Altering microbiome-derived metabolites, mostly butyrate | Impairment of cognitive function and synaptic plasticity in offspring | Reducing histone acetylation | [137] |
| High-dietary fiber intake | Reshaping gut microbiome | Reducing maternal obesity-induced cognitive and social dysfunctions | Changing histone acetylation | [138] |
| Maternal sodium butyrate intake | Altering the gut microbial metabolite butyrate | - Preventing long-term synaptic plasticity deficits, cerebellar cortex hypertrophy, and Purkinje cells firing - Improving ASD-like symptoms in offspring | Changing histone acetylation | [139] |
| Maternal smoking | Alterations of gut microbiome composition | Developing mental health diseases in offspring | Changing DNA methylation patterns | [140] |
| Maternal folate deficiency | Alterations of gut microbiome composition | Increasing the risk of neural tube defects (NTDs) | -Decreasing nuclear acetyl CoA levels and consequently reducing histone acetylation -Increasing lysine crotonylome as an epigenetic mark | [141] |
| Parental infection | Alterations in the offspring gut microbiome | Epigenetic alterations in the developing brain | Changes in germline epigenetics (expression of DNA methyltransferases and histone deacetylases) | [142] |
| Maternal intake of sulforaphane glucosinolate | Alterations in gut microbiome α and β-diversity in 3-week-old offspring | Reducing stress-related psychiatric diseases in offspring | Possible role of epigenetic modifications by sulforaphane glucosinolate | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nohesara, S.; Abdolmaleky, H.M.; Thiagalingam, S. Epigenetic Aberrations in Major Psychiatric Diseases Related to Diet and Gut Microbiome Alterations. Genes 2023, 14, 1506. https://doi.org/10.3390/genes14071506
Nohesara S, Abdolmaleky HM, Thiagalingam S. Epigenetic Aberrations in Major Psychiatric Diseases Related to Diet and Gut Microbiome Alterations. Genes. 2023; 14(7):1506. https://doi.org/10.3390/genes14071506
Chicago/Turabian StyleNohesara, Shabnam, Hamid Mostafavi Abdolmaleky, and Sam Thiagalingam. 2023. "Epigenetic Aberrations in Major Psychiatric Diseases Related to Diet and Gut Microbiome Alterations" Genes 14, no. 7: 1506. https://doi.org/10.3390/genes14071506
APA StyleNohesara, S., Abdolmaleky, H. M., & Thiagalingam, S. (2023). Epigenetic Aberrations in Major Psychiatric Diseases Related to Diet and Gut Microbiome Alterations. Genes, 14(7), 1506. https://doi.org/10.3390/genes14071506





