Comparative Analysis of miRNA Expression Profiles under Salt Stress in Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Salt Treatment
2.2. Small RNA Library Construction and Sequencing
2.3. miRNA Identification
2.4. Detection of Differentially Expressed miRNAs
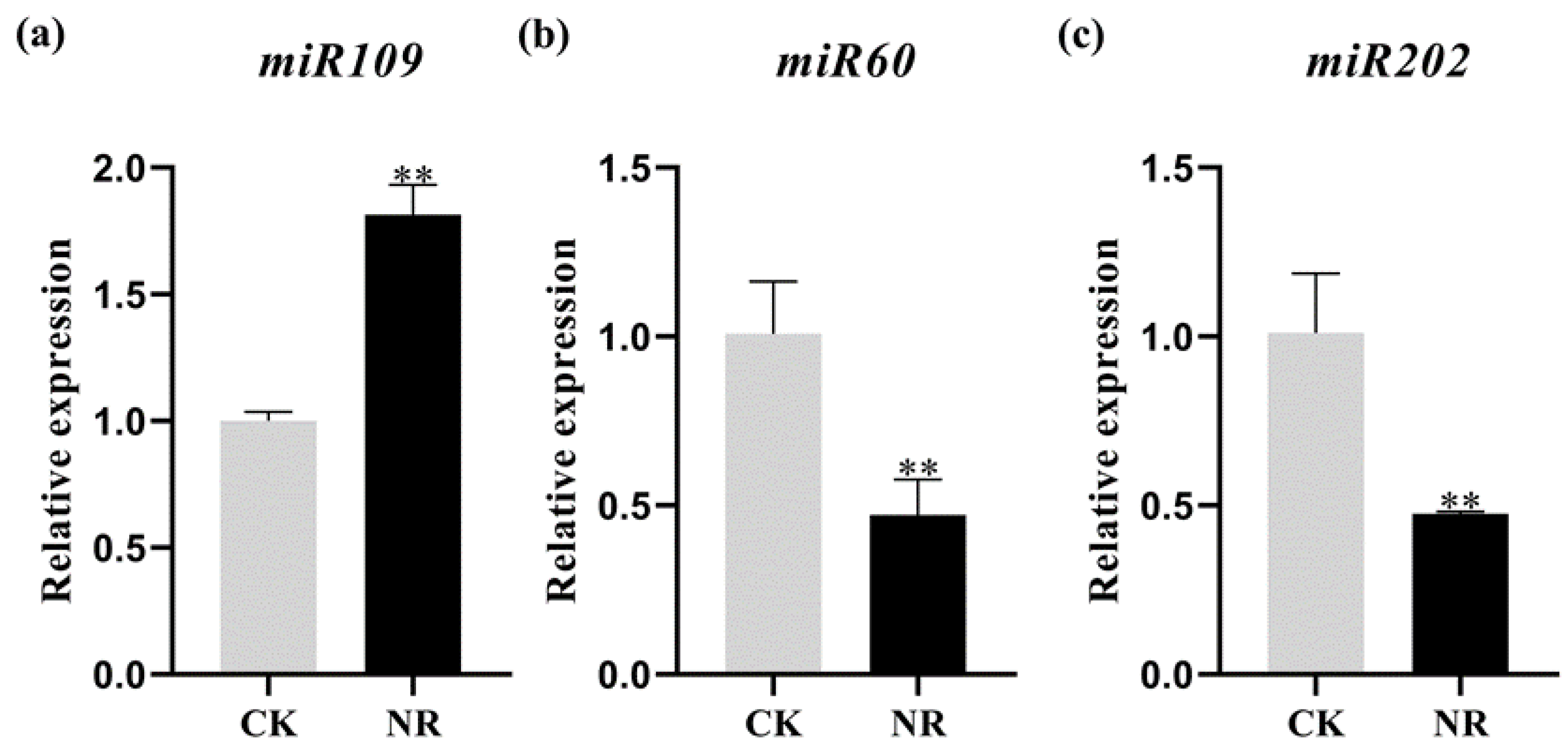
2.5. Validation of the Expression of miRNAs by qRT−PCR Analysis
2.6. Target Gene Prediction
2.7. Differential Expression of miRNA Phylogenetic Tree Analysis
3. Results
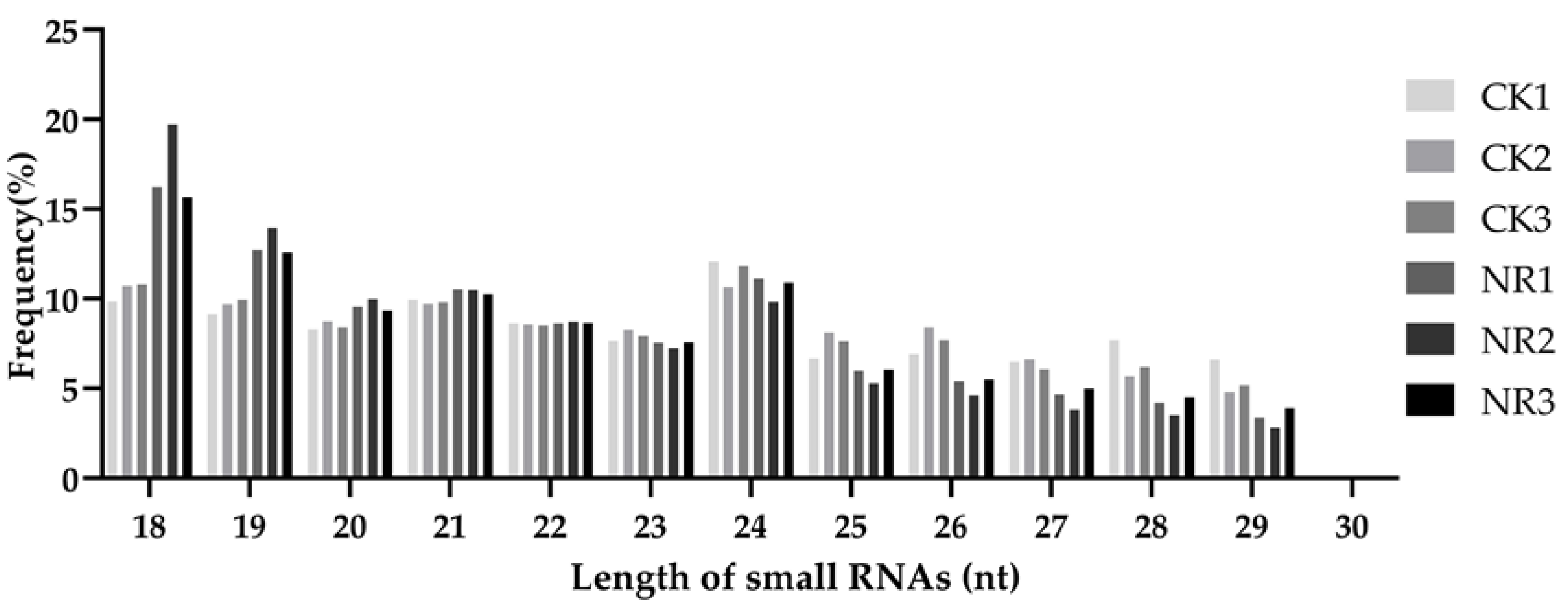
3.1. Small RNA Sequencing
3.2. Identification of Known miRNAs and of Novel miRNAs in Wheat Seedlings
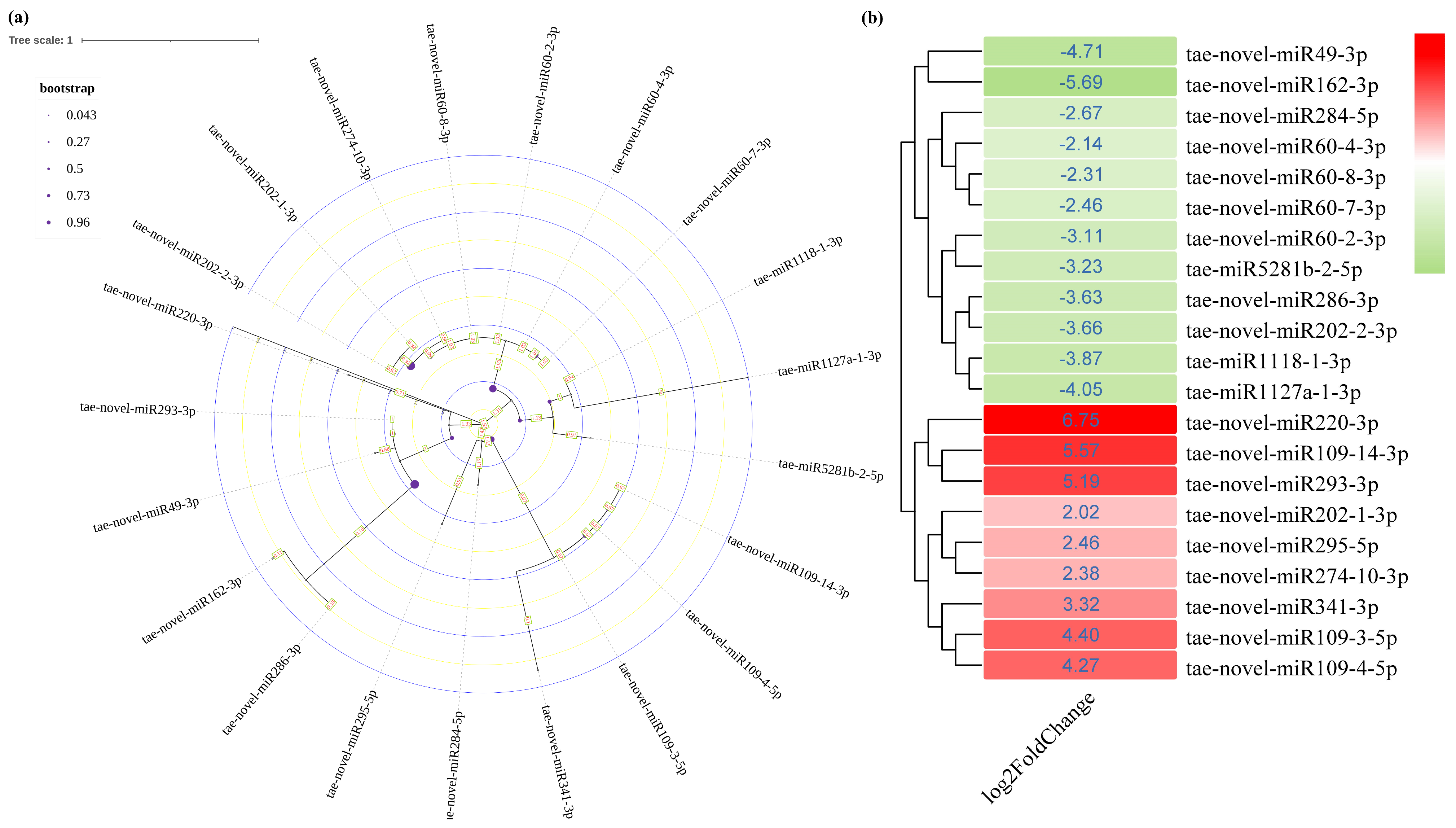
3.3. Identification and Characterization of Salinity-Responsive miRNAs
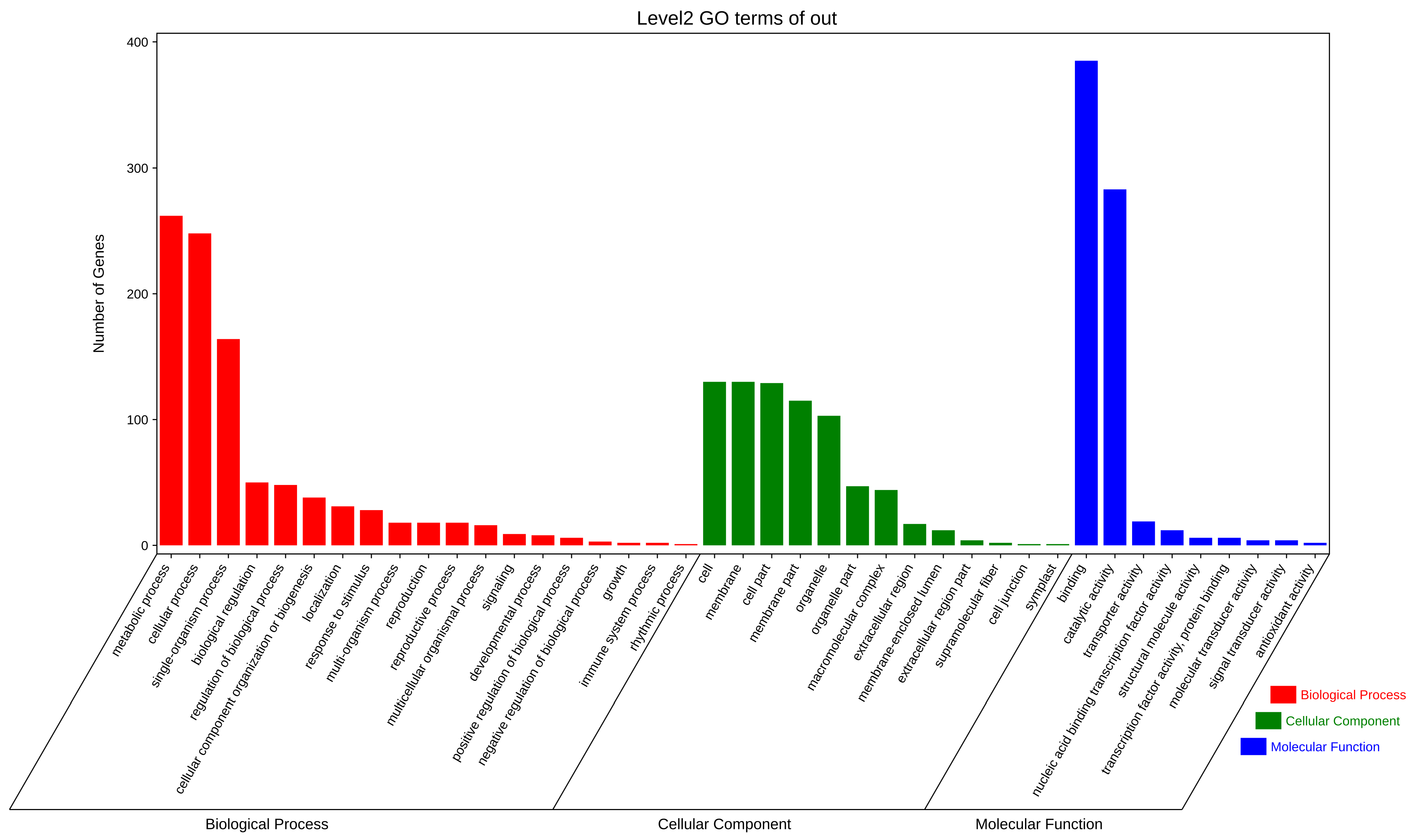
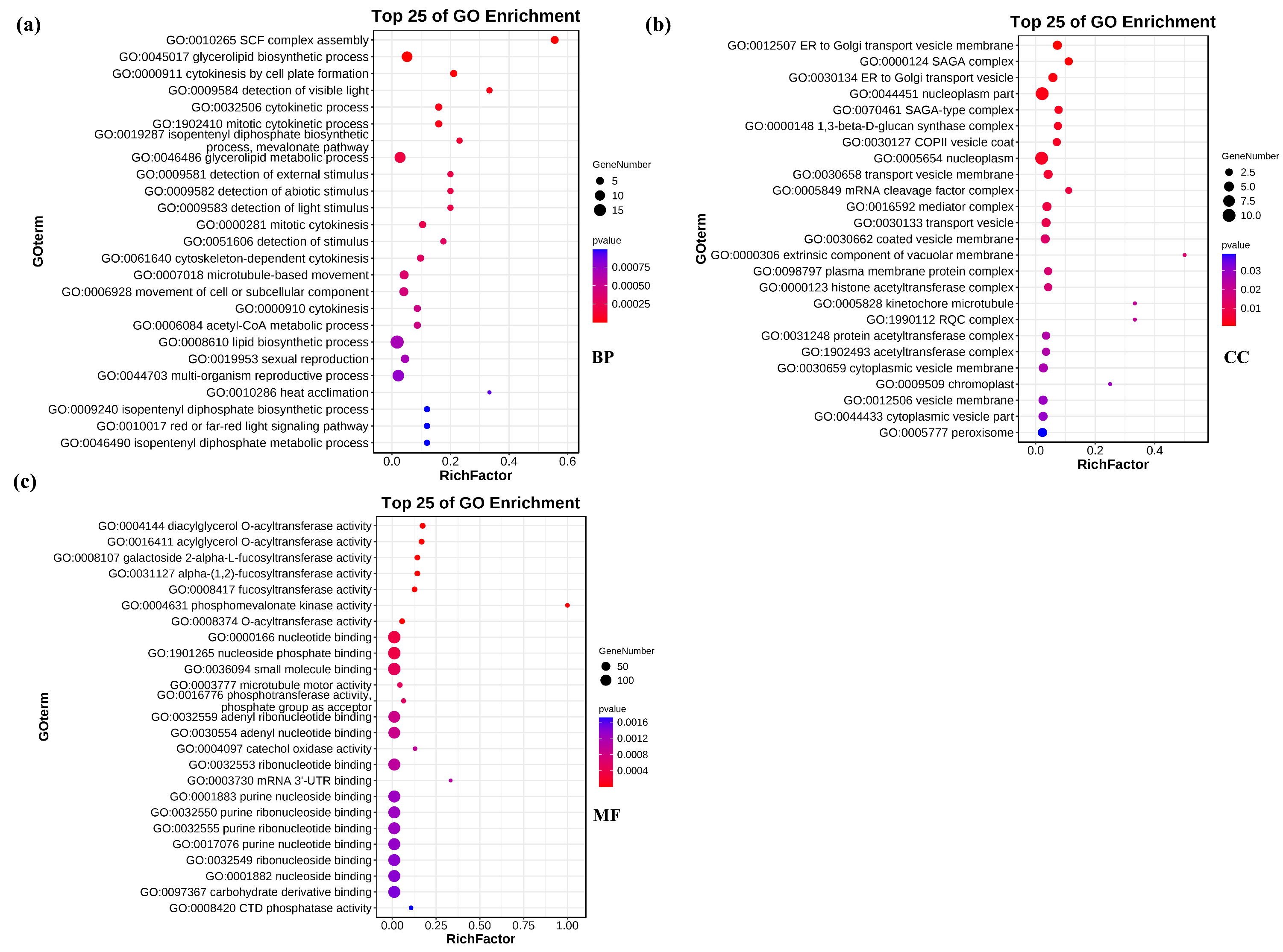
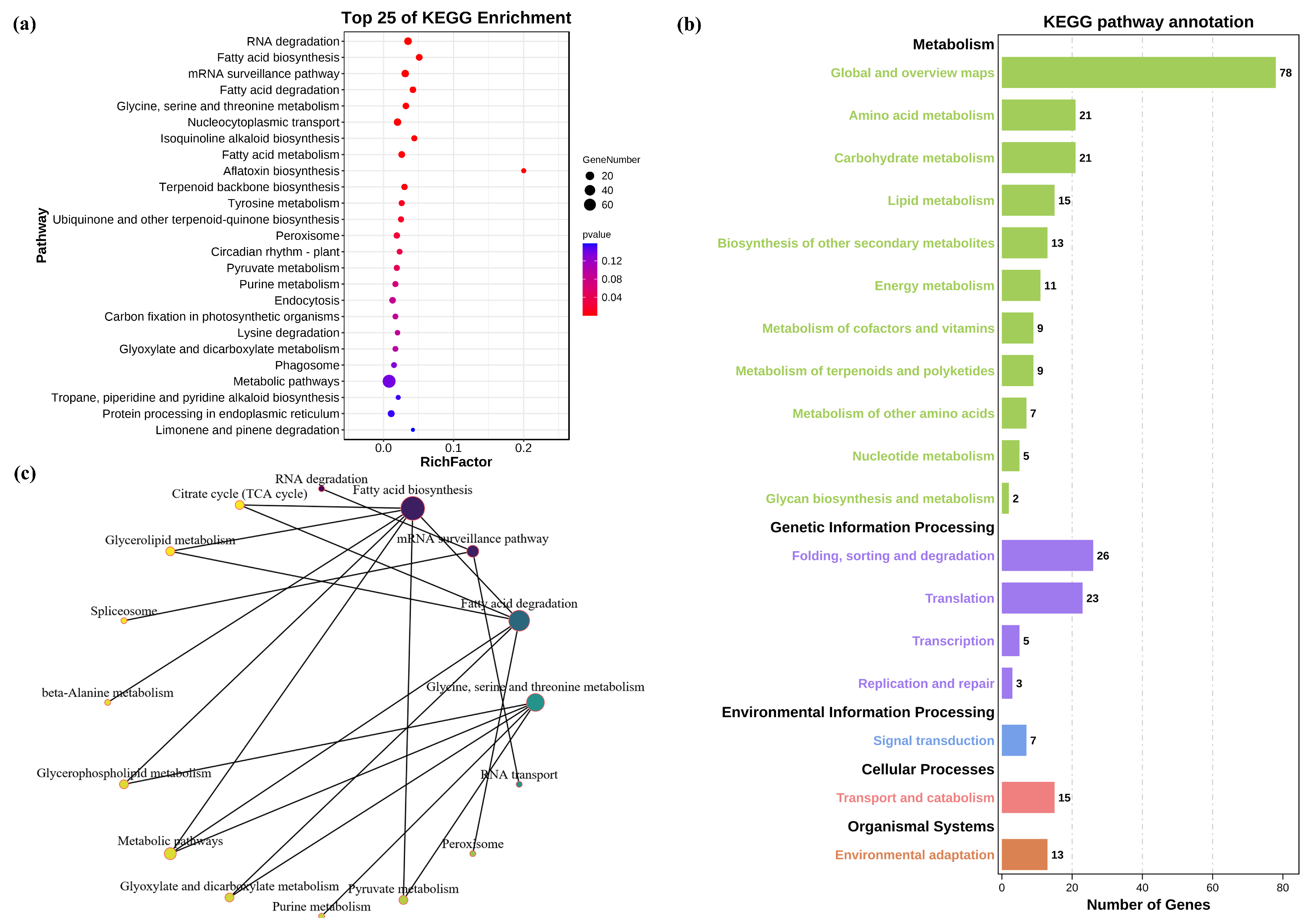
3.4. Candidate Target Genes for Differentially Expressed miRNA in Salt−Treated Wheat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, J.; Mao, X.; Li, C.; Li, L.; Yang, X.; Hao, C.; Chang, X.; Li, R.; Jing, R. Association Analysis Revealed That TaPYL4 Genes Are Linked to Plant Growth Related Traits in Multiple Environment. Front. Plant Sci. 2021, 12, 641087. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.; Oniszczuk, A.; Kasprzak, K.; Olech, M.; Mitrus, M.; Oniszczuk, T. Chemical composition and selected quality characteristics of new types of precooked wheat and spelt pasta products. Food Chem. 2020, 309, 125673. [Google Scholar] [CrossRef] [PubMed]
- Hammond−Kosack, M.C.U.; King, R.; Kanyuka, K.; Hammond−Kosack, K.E. Exploring the diversity of promoter and 5′UTR sequences in ancestral, historic and modern wheat. Plant Biotechnol. J. 2021, 19, 2469–2487. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.; Chen, B.; Jian, C.; Mei, F.; Zhang, Y.; Li, F.; Chen, N.; Li, T.; Du, L.; et al. Variation in cis−regulation of a NAC transcription factor contributes to drought tolerance in wheat. Mol. Plant 2022, 15, 276–292. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt−tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, L.; Zhang, H.; Yang, Z.; Wang, H.; Wen, S.; Zhang, C.; Rustgi, S.; von Wettstein, D.; Liu, B. Evolution of physiological responses to salt stress in hexaploid wheat. Proc. Natl. Acad. Sci. USA 2014, 111, 11882–11887. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, Y.; Shi, Y.; Ma, L.; Wang, Y.; Song, C.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R. The calcium transporter ANNEXIN1 mediates cold−induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, J.; Qin, L.; Shi, W.; Xia, G.; Liu, S. TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnol. J. 2020, 18, 791–804. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Ji, D.; Zhang, W.; Wang, Y.; Yu, Y.; Zhao, S.; Lyu, M.; You, J.; Zhang, Y.; et al. A GmSIN1/GmNCED3s/GmRbohBs Feed−Forward Loop Acts as a Signal Amplifier That Regulates Root Growth in Soybean Exposed to Salt Stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Chun, H.J.; Kang, S.; Shin, G.; Park, S.J.; Hong, H.; Kim, C.; Kim, D.H.; Lee, S.Y.; Kim, M.C.; et al. A Role for Arabidopsis miR399f in Salt, Drought, and ABA Signaling. Mol. Cells 2016, 39, 111–118. [Google Scholar]
- Cheng, X.; He, Q.; Tang, S.; Wang, H.; Zhang, X.; Lv, M.; Liu, H.; Gao, Q.; Zhou, Y.; Wang, Q.; et al. The miR172/IDS1 signaling module confers salt tolerance through maintaining ROS homeostasis in cereal crops. New Phytol. 2021, 230, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef]
- Long, R.; Li, M.; Li, X.; Gao, Y.; Zhang, T.; Sun, Y.; Kang, J.; Wang, T.; Cong, L.; Yang, Q. A Novel miRNA Sponge Form. Efficiently Inhibits the Activity of miR393 and Enhances the Salt Tolerance and ABA Insensitivity in Arabidopsis thaliana. Plant Mol. Biol. Report. 2017, 35, 409–415. [Google Scholar] [CrossRef]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Pan, X.; Li, Y.; Cai, H.; Ji, W.; Chen, Q.; Zhu, Y. osa−MIR393, a salinity− and alkaline stress−related microRNA gene. Mol. Biol. Rep. 2011, 38, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Wang, X.; Chen, X.; Shi, G.; Liu, Z.; Guo, C.; Xiao, K. Wheat miRNA TaemiR408 Acts as an Essential Mediator in Plant Tolerance to Pi Deprivation and Salt Stress via Modulating Stress−Associated Physiological Processes. Front. Plant Sci. 2018, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, L.; Zeng, H.Q.; Zhou, Z.S.; Yang, Z.M.; Li, H.; Sun, D.; Xie, F.; Zhang, B. A cotton miRNA is involved in regulation of plant response to salt stress. Sci. Rep. 2016, 6, 19736. [Google Scholar] [CrossRef]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Li, Y.; Cai, H.; Ji, W.; Guo, D.; Zhu, Y. Over−expression of osa−MIR396c decreases salt and alkali stress tolerance. Planta 2010, 231, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Song, J.B.; Gao, S.; Sun, D.; Li, H.; Shu, X.X.; Yang, Z.M. miR394 and LCR are involved in Arabidopsis salt and drought stress responses in an abscisic acid−dependent manner. BMC Plant Biol. 2013, 13, 210. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Y.; Yu, J.; Liu, Y.; Li, C.; Han, X.; Shen, F. Difference in miRNA expression profiles between two cotton cultivars with distinct salt sensitivity. Mol. Biol. Rep. 2012, 39, 4961–4970. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhang, L.; Wang, H.; Liu, Z.; Zhang, Z.; Zheng, Y. Differential expression of miRNAs in response to salt stress in maize roots. Ann. Bot. 2009, 103, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, W.; Yang, W.; Qi, L.; Han, S. Identification of microRNAs in Caragana intermedia by highthroughput sequencing and expression analysis of 12 microRNAs and their targets under salt stress. Plant Cell Rep. 2013, 32, 1339–1349. [Google Scholar] [CrossRef]
- Eren, H.; Pekmezci, M.Y.; Okay, S.; Turktas, M.; Inal, B.; Ilhan, E.; Atak, M.; Erayman, M.; Unver, T. Hexaploid wheat (Triticum aestivum) root miRNome analysis in response to salt stress. Ann. Appl. Biol. 2015, 167, 208–216. [Google Scholar] [CrossRef]
- Feng, K.; Nie, X.; Cui, L.; Deng, P.; Wang, M.; Song, W. Genome−Wide Identification and Characterization of Salinity Stress−Responsive miRNAs in Wild Emmer Wheat (Triticum turgidum ssp. dicoccoides). Genes 2017, 8, 156. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y. −F.; Song, N.; Wei, J.−P.; Wang, X.−J.; Feng, H.; Yin, Z.−Y.; Kang, Z.−S. MicroRNAs involving in cold, wounding and salt stresses in Triticum aestivum L. Plant Physiol. Biochem. 2014, 80, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.P.; Meena, N.L.; Sharma, I.; Sharma, P. Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat. Mol. Biol. Rep. 2014, 41, 4623–4629. [Google Scholar] [CrossRef]
- Duan, H.; Lu, X.; Lian, C.; An, Y.; Xia, X.; Yin, W. Genome−Wide Analysis of MicroRNA Responses to the Phytohormone Abscisic Acid in Populus euphratica. Front. Plant Sci. 2016, 7, 1184. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Xue, F.; Zhang, H.; Ji, W. Wheat NAC transcription factor TaNAC29 is involved in response to salt stress. Plant Physiol. Biochem. 2015, 96, 356–363. [Google Scholar] [CrossRef]
- Ma, X.; Cui, W.; Liang, W.; Huang, Z. Wheat TaSP gene improves salt tolerance in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2015, 97, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant−Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths−Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, C.; Zhai, Z.; Peng, X.; Wang, Y.; Sun, Y.; Li, J.; Shen, X.; Xiao, Y.; Zhu, S.; et al. The Apple microR171i−SCARECROW−LIKE PROTEINS26.1 Module Enhances Drought Stress Tolerance by Integrating Ascorbic Acid Metabolism. Plant Physiol. 2020, 184, 194–211. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Islam, W.; Waheed, A.; Naveed, H.; Zeng, F. MicroRNAs Mediated Plant Responses to Salt Stress. Cells 2022, 11, 2806. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, H.X.; Li, S.; Gao, Y.; Dang, Y.; Chen, Z.; Wu, F.; Wang, X.; Xie, Q.; Sui, N. MicroRNAs balance growth and salt stress responses in sweet sorghum. Plant J. 2022, 113, 677–697. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dong, T.; Dai, X.; Wei, Y.; Fang, Y.; Zhang, L.; Zhu, M.; Nawaz, G.; Cao, Q.; Xu, T. Comparative Analysis of Salt Responsive MicroRNAs in Two Sweetpotato [Ipomoea batatas (L.) Lam.] Cultivars With Different Salt Stress Resistance. Front. Plant Sci. 2022, 13, 879819. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, Y.; Liu, H.; Liang, Z.; Zhang, M.; Zou, C.; Yuan, G.; Gao, S.; Pan, G.; Shen, Y.; et al. A Combination of a Genome−Wide Association Study and a Transcriptome Analysis Reveals circRNAs as New Regulators Involved in the Response to Salt Stress in Maize. Int. J. Mol. Sci. 2022, 23, 9755. [Google Scholar] [CrossRef] [PubMed]
- Rehman, O.U.; Uzair, M.; Chao, H.; Fiaz, S.; Khan, M.R.; Chen, M. Role of the type−B authentic response regulator gene family in fragrant rice under alkaline salt stress. Physiol. Plant 2022, 174, e13696. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pan, W.; Cao, R.; Guo, Q.; Cheng, Y.; Zhao, Q.; Cui, L.; Nie, X. Multi−omics reveals the key and specific miRNA−mRNA modules underlying salt tolerance in wild emmer wheat (Triticum dicoccoides L.). BMC Genom. 2022, 23, 724. [Google Scholar] [CrossRef]
- He, X.; Han, Z.; Yin, H.; Chen, F.; Dong, Y.; Zhang, L.; Lu, X.; Zeng, J.; Ma, W.; Mu, P. High−Throughput Sequencing−Based Identification of miRNAs and Their Target mRNAs in Wheat Variety Qing Mai 6 Under Salt Stress Condition. Front. Genet. 2021, 12, 724527. [Google Scholar] [CrossRef]
- Shamloo−Dashtpagerdi, R.; Sisakht, J.N.; Tahmasebi, A. MicroRNA miR1118 contributes to wheat (Triticum aestivum L.) salinity tolerance by regulating the Plasma Membrane Intrinsic Proteins1;5 (PIP1;5) gene. J. Plant Physiol. 2022, 278, 153827. [Google Scholar] [CrossRef]
- Muhammad, D.; Smith, K.A.; Bartel, B. Plant peroxisome proteostasis−establishing, renovating, and dismantling the peroxisomal proteome. Essays Biochem. 2022, 66, 229–242. [Google Scholar]
- Frick, E.M.; Strader, L.C. Kinase MPK17 and the Peroxisome Division Factor PMD1 Influence Salt−induced Peroxisome Proliferation. Plant Physiol. 2018, 176, 340–351. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Khan, A.A.; Lin, Q.; Han, Y.; Mu, P.; Liu, Y.; Zhang, H.; Li, L.; Meng, X.; et al. Expression partitioning of homeologs and tandem duplications contribute to salt tolerance in wheat (Triticum aestivum L.). Sci. Rep. 2016, 6, 21476. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, H.; Jiao, B.; Wang, J.; Yang, Y.; Yang, F.; Geng, Z.; Zhao, G.; Liu, Y.; Dong, F.; Wang, Y.; et al. Comparative Analysis of miRNA Expression Profiles under Salt Stress in Wheat. Genes 2023, 14, 1586. https://doi.org/10.3390/genes14081586
Qiao H, Jiao B, Wang J, Yang Y, Yang F, Geng Z, Zhao G, Liu Y, Dong F, Wang Y, et al. Comparative Analysis of miRNA Expression Profiles under Salt Stress in Wheat. Genes. 2023; 14(8):1586. https://doi.org/10.3390/genes14081586
Chicago/Turabian StyleQiao, Hualiang, Bo Jiao, Jiao Wang, Yang Yang, Fan Yang, Zhao Geng, Guiyuan Zhao, Yongwei Liu, Fushuang Dong, Yongqiang Wang, and et al. 2023. "Comparative Analysis of miRNA Expression Profiles under Salt Stress in Wheat" Genes 14, no. 8: 1586. https://doi.org/10.3390/genes14081586
APA StyleQiao, H., Jiao, B., Wang, J., Yang, Y., Yang, F., Geng, Z., Zhao, G., Liu, Y., Dong, F., Wang, Y., & Zhou, S. (2023). Comparative Analysis of miRNA Expression Profiles under Salt Stress in Wheat. Genes, 14(8), 1586. https://doi.org/10.3390/genes14081586





