Obesity Contributes to Transformation of Myometrial Stem-Cell Niche to Leiomyoma via Inducing Oxidative Stress, DNA Damage, Proliferation, and Extracellular Matrix Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Myometrial Stem Cells
2.2. Preparation of MSC-Adipocyte Co-Culture and Leptin and Adiponectin Treatments
2.3. Protein Extraction and Western Blot Analysis
2.4. MTT Analysis
2.5. Reactive Oxygen Species (ROS) Production Assay
2.6. Statistical Analysis
3. Results
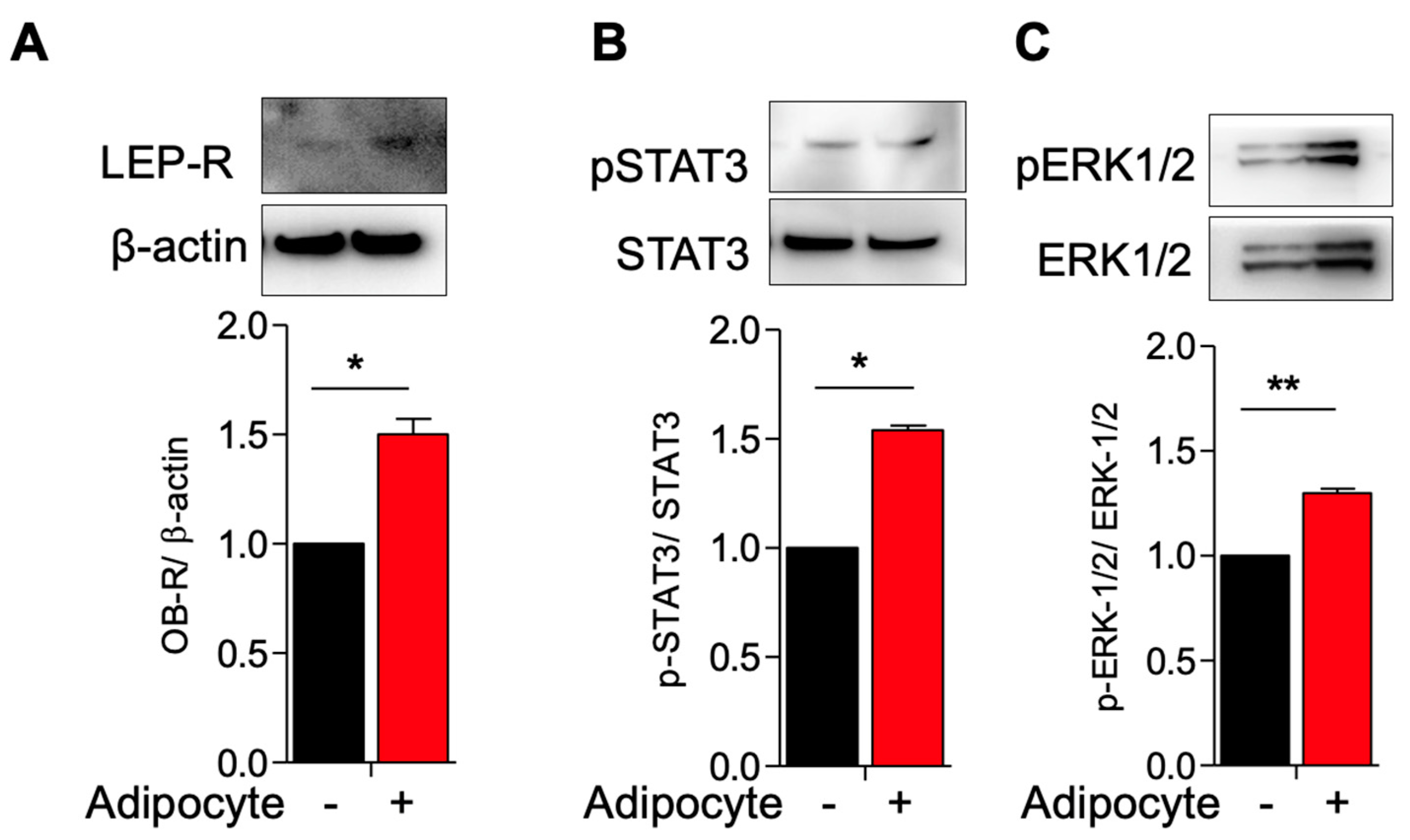
3.1. Activation of Leptin Receptor in Myometrial Stem Cells Co-Culture with Adipocyte
3.2. Activation of TGF-β3/SMAD2 and Wnt4/B-Catenin Pathway in Myometrial Stem Cells Co-Culture with Adipocyte
3.3. Adipocytes Co-Culture and Adipokine Treatment Increases Myometrial Stem Cells Proliferations and Type 1 Collagen Production
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmermann, A.; Bernuit, D.; Gerlinger, C.; Schaefers, M.; Geppert, K. Prevalence, symptoms and management of uterine fibroids: An international internet-based survey of 21,746 women. BMC Women’s Health 2012, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Shikora, S.A.; Niloff, J.M.; Bistrian, B.R.; Forse, R.A.; Blackburn, G.L. Relationship between obesity and uterine leiomyomata. Nutrition 1991, 7, 251–255. [Google Scholar]
- Qin, H.; Lin, Z.; Vasquez, E.; Luan, X.; Guo, F.; Xu, L. Association between obesity and the risk of uterine fibroids: A systematic review and meta-analysis. J. Epidemiol. Community Health 2021, 75, 197–204. [Google Scholar] [CrossRef]
- Brewster, L.M.; Haan, Y.; van Montfrans, G.A. Cardiometabolic Risk and Cardiovascular Disease in Young Women with Uterine Fibroids. Cureus 2022, 14, e30740. [Google Scholar] [CrossRef] [PubMed]
- Dandolu, V.; Singh, R.; Lidicker, J.; Harmanli, O. BMI and uterine size: Is there any relationship? Int. J. Gynecol. Pathol. 2010, 29, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; El Sabah, M.; Manzoor, A.; Miyashita-Ishiwata, M.; Reschke, L.; Borahay, M.A. Adipocyte coculture induces a pro-inflammatory, fibrotic, angiogenic, and proliferative microenvironment in uterine leiomyoma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166564. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Guo, L.; Peng, Y.; Shi, X.; Zhao, Y.; Liu, K.; Zhou, R.; Fu, J.; Peng, C. Correlation between inflammatory marker and lipid metabolism in patients with uterine leiomyomas. Front. Med. 2023, 10, 1124697. [Google Scholar] [CrossRef] [PubMed]
- Miyashita-Ishiwata, M.; El Sabeh, M.; Reschke, L.D.; Afrin, S.; Borahay, M.A. Hypoxia induces proliferation via NOX4-Mediated oxidative stress and TGF-beta3 signaling in uterine leiomyoma cells. Free Radic. Res. 2022, 56, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Asif, H.; Feng, Y.; Kohrn, B.F.; Kennedy, S.R.; Kim, J.J.; Wei, J.-J. Myometrial oxidative stress drives MED12 mutations in leiomyoma. Cell Biosci. 2022, 12, 111. [Google Scholar] [CrossRef]
- Kim, J.S.; Kurie, J.M.; Ahn, Y.H. BMP4 depletion by miR-200 inhibits tumorigenesis and metastasis of lung adenocarcinoma cells. Mol. Cancer 2015, 14, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschen, G.W.; Hessami, K.; AlAshqar, A.; Afrin, S.; Lulseged, B.; Borahay, M. Uterine Transcriptome: Understanding Physiology and Disease Processes. Biology 2023, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Chae, B.; Kim, M.R. The Potential of Transforming Growth Factor-beta Inhibitor and Vascular Endothelial Growth Factor Inhibitor as Therapeutic Agents for Uterine Leiomyoma. Int. J. Med. Sci. 2022, 19, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Sessions-Bresnahan, D.R.; Carnevale, E.M. The effect of equine metabolic syndrome on the ovarian follicular environment. J. Anim. Sci. 2014, 92, 1485–1494. [Google Scholar] [CrossRef] [Green Version]
- Spoto, B.; Di Betta, E.; Mattace-Raso, F.; Sijbrands, E.; Vilardi, A. Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, V.; Procaccini, C.; Cali, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Real, J.M.; Castro, A.; Vazquez, G.A.B.R.I.E.L.; Casamitjana, R.; López-Bermejo, A.; Peñarroja, G.; Ricart, W. Adiponectin is associated with vascular function independent of insulin sensitivity. Diabetes Care 2004, 27, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.C.; Xu, A.; Chow, W.S.; Lam, M.C.W.; Ai VH, G.; Tam, S.C.F.; Lam, K.S.L. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J. Clin. Endocrinol. Metab. 2004, 89, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Afrin, S.; Ali, M.; El Sabeh, M.; Yang, Q.; Al-Hendy, A.; Borahay, M.A. Simvastatin inhibits stem cell proliferation in human leiomyoma via TGF-beta3 and Wnt/beta-Catenin pathways. J. Cell Mol. Med. 2022, 26, 1684–1698. [Google Scholar] [CrossRef]
- Afrin, S.; Ramaiyer, M.; Begum, U.A.M.; Borahay, M.A. Adipocyte and Adipokines Promote a Uterine Leiomyoma Friendly Microenvironment. Nutrients 2023, 15, 715. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Piro, S.; Anello, M.; Di Pietro, C.; Lizzio, M.N.; Patane, G.; Rabuazzo, A.M.; Vigneri, R.; Purrello, M.; Purrello, F. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: Possible role of oxidative stress. Metabolism 2002, 51, 1340–1347. [Google Scholar] [CrossRef]
- Martyn, K.D.; Frederick, L.M.; von Loehneysen, K.; Dinauer, M.C.; Knaus, U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006, 18, 69–82. [Google Scholar] [CrossRef]
- Munoz, M.; Lopez-Oliva, M.E.; Rodriguez, C.; Martinez, M.P.; Saenz-Medina, J.; Sanchez, A.; Climent, B.; Benedito, S.; García-Sacristán, A.; Rivera, L.; et al. Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol. 2020, 28, 101330. [Google Scholar] [CrossRef]
- Revet, I.; Feeney, L.; Bruguera, S.; Wilson, W.; Dong, T.K.; Oh, D.H.; Dankort, D.; Cleaver, J.E. Functional relevance of the histone gammaH2Ax in the response to DNA damaging agents. Proc. Natl. Acad. Sci. USA 2011, 108, 8663–8667. [Google Scholar] [CrossRef] [PubMed]
- Enriori, P.J.; Evans, A.E.; Sinnayah, P.; Cowley, M.A. Leptin resistance and obesity. Obesity 2006, 14 (Suppl. S5), 254S–258S. [Google Scholar] [CrossRef]
- Gualillo, O.; Eiras, S.; Lago, F.; Dieguez, C.; Casanueva, F.F. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000, 67, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Takeda, T.; Sakata, M.; Isobe, A.; Miyake, A.; Nishimoto, F.; Ota, Y.; Kamiura, S.; Kimura, T. Relationship between metabolic syndrome and uterine leiomyomas: A case-control study. Gynecol. Obstet. Investig. 2008, 66, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Spiegelman, D.; Harlow, B.L.; Stewart, E.A.; Adams-Campbell, L.L.; Rosenberg, L. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology 2005, 16, 346–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, F.; Nishi, M.; Kudo, R.; Miyake, H. Body fat distribution and uterine leiomyomas. J. Epidemiol. 1998, 8, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlAshqar, A.; Reschke, L.; Kirschen, G.W.; Borahay, M.A. Role of inflammation in benign gynecologic disorders: From pathogenesis to novel therapies. Biol. Reprod. 2021, 105, 7–31. [Google Scholar] [CrossRef]
- Kurachi, O.; Matsuo, H.; Samoto, T.; Maruo, T. Tumor necrosis factor-alpha expression in human uterine leiomyoma and its down-regulation by progesterone. J. Clin. Endocrinol. Metab. 2001, 86, 2275–2280. [Google Scholar]
- Protic, O.; Islam, M.S.; Greco, S.; Giannubilo, S.R.; Lamanna, P.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; Hinz, B.; Ciarmela, P. Activin A in Inflammation, Tissue Repair, and Fibrosis: Possible Role as Inflammatory and Fibrotic Mediator of Uterine Fibroid Development and Growth. Semin. Reprod. Med. 2017, 35, 499–509. [Google Scholar] [CrossRef]
- Ciebiera, M.; Wlodarczyk, M.; Zgliczynska, M.; Lukaszuk, K.; Meczekalski, B.; Kobierzycki, C.; Łoziński, T.; Jakiel, G. The Role of Tumor Necrosis Factor alpha in the Biology of Uterine Fibroids and the Related Symptoms. Int. J. Mol. Sci. 2018, 19, 3869. [Google Scholar] [CrossRef] [Green Version]
- Moridi, I.; Mamillapalli, R.; Kodaman, P.H.; Habata, S.; Dang, T.; Taylor, H.S. CXCL12 Attracts Bone Marrow-Derived Cells to Uterine Leiomyomas. Reprod. Sci. 2020, 27, 1724–1730. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. miR-200c regulates IL8 expression by targeting IKBKB: A potential mediator of inflammation in leiomyoma pathogenesis. PLoS ONE 2014, 9, e95370. [Google Scholar] [CrossRef]
- Sitar-Taut, A.V.; Cozma, A.; Fodor, A.; Coste, S.C.; Orasan, O.H.; Negrean, V.; Pop, D.; Sitar-Tǎut, D.-A. New Insights on the Relationship between Leptin, Ghrelin, and Leptin/Ghrelin Ratio Enforced by Body Mass Index in Obesity and Diabetes. Biomedicines 2021, 9, 1657. [Google Scholar] [CrossRef]
- Edman, C.D.; MacDonald, P.C. Effect of obesity on conversion of plasma androstenedione to estrone in ovulatory and anovulator young women. Am. J. Obstet. Gynecol. 1978, 130, 456–461. [Google Scholar] [CrossRef] [PubMed]
- AlAshqar, A.; Patzkowsky, K.; Afrin, S.; Wild, R.; Taylor, H.S.; Borahay, M.A. Cardiometabolic Risk Factors and Benign Gynecologic Disorders. Obstet. Gynecol. Surv. 2019, 74, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Alashqar, A.; El Ouweini, H.; Gornet, M.; Yenokyan, G.; Borahay, M.A. Cardiometabolic profile of women with uterine leiomyoma: A cross-sectional study. Minerva Obstet. Gynecol. 2023, 75, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; AlAshqar, A.; El Sabeh, M.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A. Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies. Nutrients 2021, 13, 1747. [Google Scholar] [CrossRef] [PubMed]
- Reschke, L.; Afrin, S.; El Sabah, M.; Charewycz, N.; Miyashita-Ishiwata, M.; Borahay, M.A. Leptin induces leiomyoma cell proliferation and extracellular matrix deposition via JAK2/STAT3 and MAPK/ERK pathways. F&S Sci. 2022, 3, 383–391. [Google Scholar]
- Kirschen, G.W.; Yanek, L.; Borahay, M. Relationship Among Surgical Fibroid Removal, Blood Pressure, and Biomarkers of Renin-Angiotensin-Aldosterone System Activation. Reprod. Sci. 2023, 30, 1–7. [Google Scholar] [CrossRef]
- Kirschen, G.W.; AlAshqar, A.; Miyashita-Ishiwata, M.; Reschke, L.; El Sabeh, M.; Borahay, M.A. Vascular biology of uterine fibroids: Connecting fibroids and vascular disorders. Reproduction 2021, 162, R1–R18. [Google Scholar] [CrossRef]
- Fischer, N.M.; Nieuwenhuis, T.O.; Hazimeh, D.; Voegtline, K.; Singh, B.; Segars, J.H. Beta blockers reduce uterine fibroid incidence in hypertensive women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 287, 119–125. [Google Scholar] [CrossRef]
- Fischer, N.M.; Nieuwenhuis, T.O.; Singh, B.; Yenokyan, G.; Segars, J.H. Angiotensin-Converting Enzyme Inhibitors Reduce Uterine Fibroid Incidence in Hypertensive Women. J. Clin. Endocrinol. Metab. 2021, 106, e650–e659. [Google Scholar] [CrossRef]
- Forney, J.P.; Milewich, L.; Chen, G.T.; Garlock, J.L.; Schwarz, B.E.; Edman, C.D.; Macdonald, P.C. Aromatization of androstenedione to estrone by human adipose tissue in vitro. Correlation with adipose tissue mass, age, and endometrial neoplasia. J. Clin. Endocrinol. Metab. 1981, 53, 192–199. [Google Scholar] [CrossRef]
- Song, H.; Lu, D.; Navaratnam, K.; Shi, G. Aromatase inhibitors for uterine fibroids. Cochrane Database Syst. Rev. 2013, 10, CD009505. [Google Scholar] [CrossRef] [Green Version]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen Receptors and Signaling in Fibroids: Role in Pathobiology and Therapeutic Implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Moroni, R.M.; Martins, W.P.; Dias, S.V.; Vieira, C.S.; Ferriani, R.A.; Nastri, C.O.; Brito, L.G. Combined oral contraceptive for treatment of women with uterine fibroids and abnormal uterine bleeding: A systematic review. Gynecol. Obstet. Investig. 2015, 79, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wu, T.; Chen, X.Y.; Xie, L.; Yang, J. Selective estrogen receptor modulators (SERMs) for uterine leiomyomas. Cochrane Database Syst. Rev. 2012, 10, CD005287. [Google Scholar] [CrossRef]
- Lethaby, A.; Vollenhoven, B.; Sowter, M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst. Rev. 2000, 2, CD000547. [Google Scholar] [CrossRef]
- Enazy, S.A.; Kirschen, G.W.; Vincent, K.; Yang, J.; Saada, J.; Shah, M.; Oberhauser, A.F.; Bujalowski, P.J.; Motamedi, M.; Salama, S.A.; et al. PEGylated Polymeric Nanoparticles Loaded with 2-Methoxyestradiol for the Treatment of Uterine Leiomyoma in a Patient-Derived Xenograft Mouse Model. J. Pharm. Sci. 2023, in press. [Google Scholar] [CrossRef]
- Billiar, R.B.; Richardson, D.; Anderson, E.; Mahajan, D.; Little, B. The effect of chronic and acyclic elevation of circulating androstenedione or estrone concentrations on ovarian function in the rhesus monkey. Endocrinology 1985, 116, 2209–2220. [Google Scholar] [CrossRef]
- Chun, S. Relationship between early follicular serum estrone level and other hormonal or ultrasonographic parameters in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2020, 36, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kuang, H.; Sun, F.; Diamond, M.P.; Legro, R.S.; Coutifaris, C.; Alvero, R.; Robinson, R.D.; Casson, P.R.; Christman, G.M.; et al. Lower prevalence of non-cavity-distorting uterine fibroids in patients with polycystic ovary syndrome than in those with unexplained infertility. Fertil. Steril. 2019, 111, 1011–1019.e1. [Google Scholar] [CrossRef]
- Wise, L.A.; Palmer, J.R.; Stewart, E.A.; Rosenberg, L. Polycystic ovary syndrome and risk of uterine leiomyomata. Fertil. Steril. 2007, 87, 1108–1115. [Google Scholar] [CrossRef] [Green Version]
- Furuhata, R.; Kabe, Y.; Kanai, A.; Sugiura, Y.; Tsugawa, H.; Sugiyama, E.; Hirai, M.; Yamamoto, T.; Koike, I.; Yoshikawa, N.; et al. Progesterone receptor membrane associated component 1 enhances obesity progression in mice by facilitating lipid accumulation in adipocytes. Commun. Biol. 2020, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Baranov, V.S.; Osinovskaya, N.S.; Yarmolinskaya, M.I. Pathogenomics of Uterine Fibroids Development. Int. J. Mol. Sci. 2019, 20, 6151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, M.; De Quattro, C.; Rossato, M.; Lazzarini, R.; Delli Carpini, G.; Ciavattini, A.; Orciani, M. A Possible Cause for the Differential Expression of a Subset of miRNAs in Mesenchymal Stem Cells Derived from Myometrium and Leiomyoma. Genes 2022, 13, 1106. [Google Scholar] [CrossRef]
- Sprenkle, N.T.; Winn, N.C.; Bunn, K.E.; Zhao, Y.; Park, D.J.; Giese, B.G.; Karijolich, J.J.; Ansel, K.M.; Serezani, C.H.; Hasty, A.H.; et al. The miR-23-27-24 clusters drive lipid-associated macrophage proliferation in obese adipose tissue. Cell Rep. 2023, 42, 112928. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrin, S.; Kirschen, G.W.; Borahay, M.A. Obesity Contributes to Transformation of Myometrial Stem-Cell Niche to Leiomyoma via Inducing Oxidative Stress, DNA Damage, Proliferation, and Extracellular Matrix Deposition. Genes 2023, 14, 1625. https://doi.org/10.3390/genes14081625
Afrin S, Kirschen GW, Borahay MA. Obesity Contributes to Transformation of Myometrial Stem-Cell Niche to Leiomyoma via Inducing Oxidative Stress, DNA Damage, Proliferation, and Extracellular Matrix Deposition. Genes. 2023; 14(8):1625. https://doi.org/10.3390/genes14081625
Chicago/Turabian StyleAfrin, Sadia, Gregory W. Kirschen, and Mostafa A. Borahay. 2023. "Obesity Contributes to Transformation of Myometrial Stem-Cell Niche to Leiomyoma via Inducing Oxidative Stress, DNA Damage, Proliferation, and Extracellular Matrix Deposition" Genes 14, no. 8: 1625. https://doi.org/10.3390/genes14081625






