Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications
Abstract
1. Introduction
1.1. Stem Cell Therapy for Tissue Regeneration
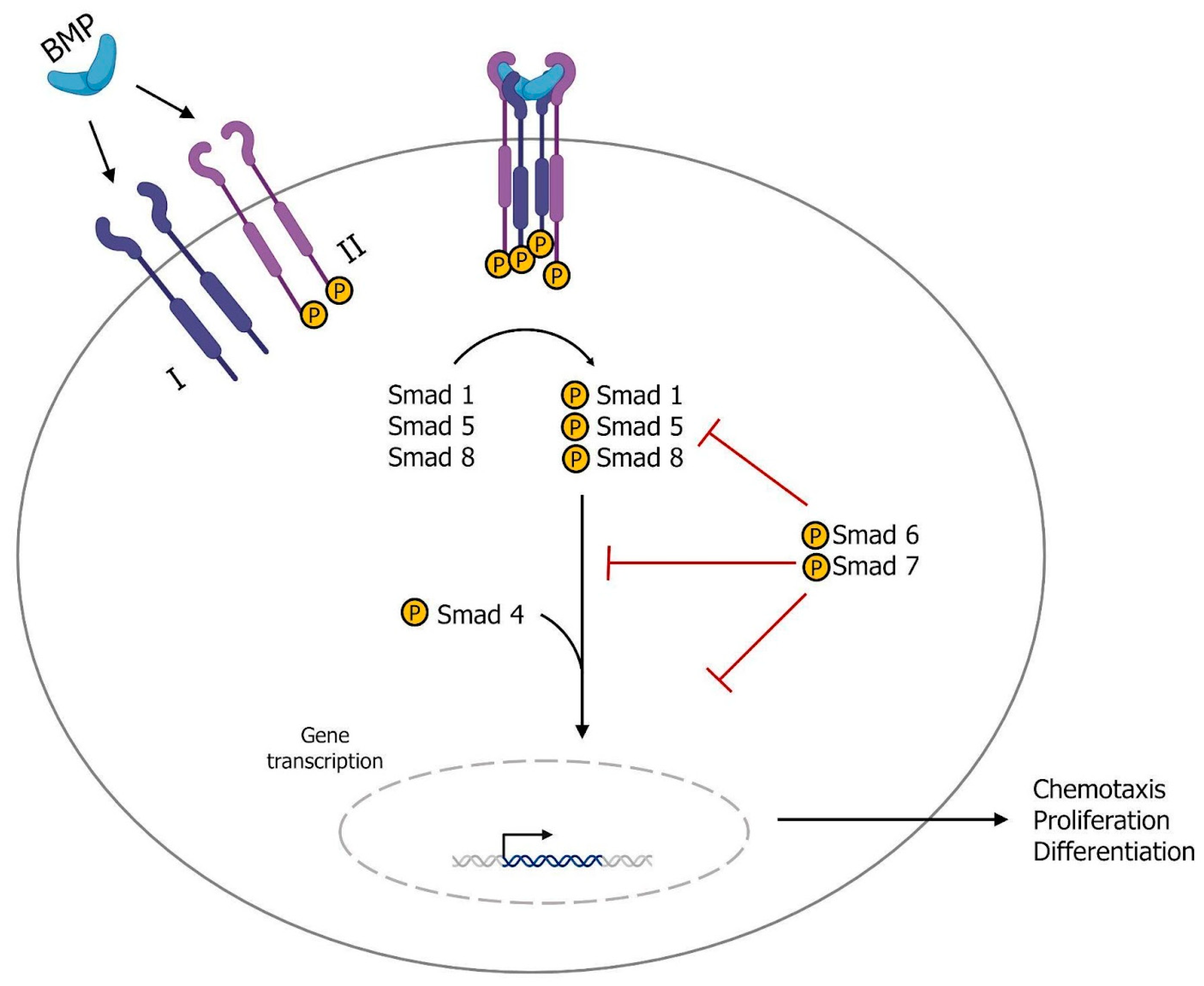
1.2. Growth Factors and Tissue Regeneration
2. Autologous Platelet Concentrates
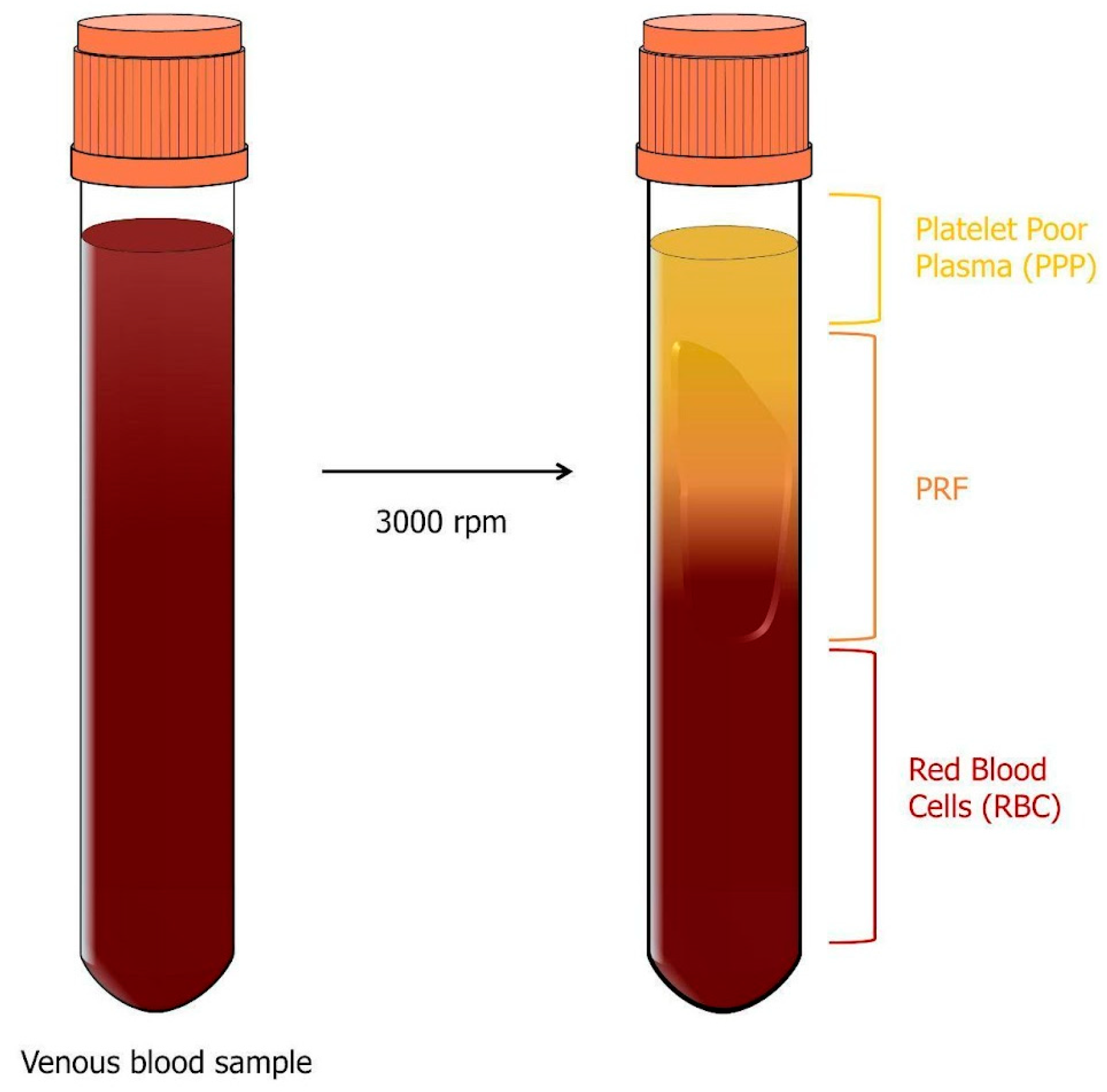
2.1. Platelet-Rich Plasma (PRP)
- 1.
- The first layer contains platelet-poor plasma (PPP) and represents 40% of the total volume;
- 2.
- The middle layer is called the buffy coat (BC) and contains platelets and leukocytes, making up only 5% of the total volume;
- 3.
- The bottom layer is made up of red blood cells (RBCs) and represents 55% of the total volume.
2.2. Platelet-Rich Fibrin (PRF)
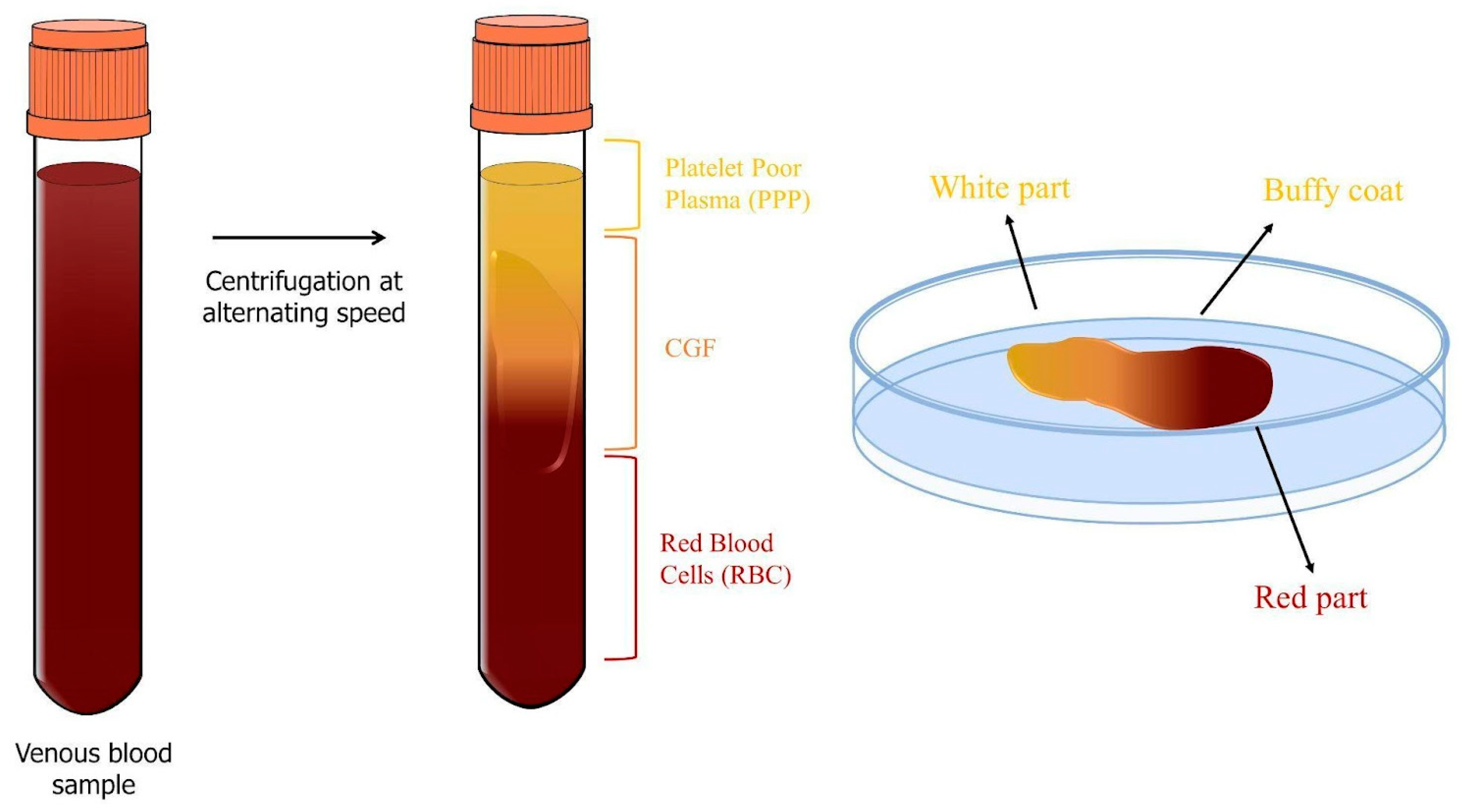
2.3. Concentrated Growth Factors (CGFs)
2.4. Comparison among the Three Generations of APCs
2.4.1. Commonly Used Devices
- −
- Centrifuges:
- −
- PRP Kits:
- −
- Double-Syringe Systems and Single-Syringe Systems:
- −
- PRF Boxes and Tubes:
- −
- i-PRF System:
- −
- Customizable Centrifuges:
2.4.2. Growth Factor Release
2.4.3. Cellular Content and Release
2.4.4. Applications in Regenerative Medicine
- −
- Topical use (skin or mucous surfaces): topical application involves directly applying APCs to the skin or mucous membranes. This method is commonly used in dermatology, wound care, and oral surgery.
- −
- Infiltrative use (intra-tissue or intra-articular infiltration): infiltration involves injecting APCs directly into tissues or joints. This method aims to enhance tissue healing and reduce inflammation. It is often used in orthopedics, sports medicine, and pain management.
- −
- Surgical use (local application in surgical fields): APCs can be used in surgical settings to promote tissue healing and regeneration. This method is commonly employed in plastic and reconstructive surgery, oral and maxillofacial surgery, and orthopedic surgery.
| Material | Effect of APCs | References |
|---|---|---|
| Facial rejuvenation | ||
| PRF | Improves facial wrinkles, skin texture, and elasticity through collagen synthesis and fibroblast proliferation. | [81,82,83] |
| CGF | Effects treating wrinkles by promoting fibroblast and endothelial cell proliferation and metabolic activity, leading to increased collagen synthesis. | [84] |
| Reconstruction of ears and noses | ||
| PRP on engineered cartilage grafts | This combination stimulates chondrocyte proliferation and inhibits fibrocartilage formation. | [87] |
| CGF | It stimulates chondrocyte proliferation, migration, and ECM synthesis. | [88] |
| Regeneration and repair of bone defects | ||
| CGF | It can induce osteogenic differentiation in human BMSCs. | [89] |
| It has a greater regenerative potential than PRF in the initial phase of femoral defect repair. | [54] | |
| CGF and A-PRF | CGFs and A-PRF are better than PRP in stimulating human periosteal cell proliferation. | [46] |
| CGF + scaffold | This combination has shown better regenerative capabilities in the later stages of bone repair. | [48,91] |
| Dental implantology | ||
| PRF | It can increase the expression of osteogenic differentiation markers in gingival stromal progenitor cells, periodontal ligament stem cells, alveolar bone osteoblasts, and periodontal ligament fibroblasts. | [99,100] |
| In vivo, PRF has been successfully used in maxillary sinus lifting, intraosseous defects, and dental extractions. | [40] | |
| PRF increases dental implant stability and dental bone density. | [101] | |
| CGF | CGFs, used in maxillary sinus floor augmentation, induce rapid new bone formation. | [102] |
| The treatment with CGFs has resulted in a better quality of bone formed around implants, indicating implant osseointegration. | [103] | |
| CGF permeation improves the success of titanium dental implants by reducing post-surgical complications. | [91] | |
| CGFs accelerate osteointegration and have a positive effect on implant stability values. | [103] | |
| Neural domain | ||
| CGF | CGFs can induce neuronal differentiation in human neuroblastoma cells (SHSY-5Y), like commonly used differentiation inducers. | [106] |
3. Allogeneic Platelet Gel Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenwood, H.L.; Singer, P.A.; Downey, G.P.; Martin, D.K.; Thorsteinsdóttir, H.; Daar, A.S. Regenerative medicine and the developing world. PLoS Med. 2006, 3, e381. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue engineering and regenerative medicine: Past, present, and future. Int. Rev. Neurobiol. 2013, 108, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.; Greco, R.; Snowden, J.A. Hematopoietic Stem Cell Transplantation for Autoimmune Disease. Annu. Rev. Med. 2021, 72, 215–228. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Galli, C.; Passeri, G.; Macaluso, G.M. Osteocytes and WNT: The Mechanical Control of Bone Formation. J. Dent. Res. 2010, 89, 331–343. [Google Scholar] [CrossRef]
- Talaat, W.; Ghoneim, M.; Salah, O.; Adly, O. Autologous Bone Marrow Concentrates and Concentrated Growth Factors Accelerate Bone Regeneration After Enucleation of Mandibular Pathologic Lesions. J. Craniofac. Surg. 2018, 29, 992–997. [Google Scholar] [CrossRef]
- Strauer, B.E.; Kornowski, R. Stem Cell Therapy in Perspective. Circulation 2003, 107, 929–934. [Google Scholar] [CrossRef]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–105. [Google Scholar] [CrossRef]
- Ando, Y.; Matsubara, K.; Ishikawa, J.; Fujio, M.; Shohara, R.; Hibi, H.; Ueda, M.; Yamamoto, A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014, 61, 82–90. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res. 2018, 22, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.P.; Wang, Y.; Lozano, G. Mesenchymal stem cells and the origin of Ewing’s sarcoma. Sarcoma 2011, 2011, 276463. [Google Scholar] [CrossRef] [PubMed]
- Krafts, K.P. Tissue repair. The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef]
- Moshiri, A.; Oryan, A. Role of platelet rich plasma in soft and hard connective tissue healing: An evidence based review from basic to clinical application. Hard Tissue 2013, 2, 6. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Attisano, L.; Wrana, J.L. Signal transduction by the TGFbeta superfamily. Science 2002, 296, 1646–1647. [Google Scholar] [CrossRef]
- Kang, J.S.; Liu, C.; Derynck, R. New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 2009, 19, 385–394. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Burnouf, T.; Goubran, H.A.; Chen, T.M.; Ou, K.L.; El-Ekiaby, M.; Radosevic, M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013, 27, 77–89. [Google Scholar] [CrossRef]
- Lowery, J.W.; Rosen, V. The BMP Pathway and Its Inhibitors in the Skeleton. Physiol. Rev. 2018, 98, 2431–2452. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. MRT 2003, 60, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Gaucher, J.-F.; Vidal, M.; Broussy, S. A Structural Overview of Vascular Endothelial Growth Factors Pharmacological Ligands: From Macromolecules to Designed Peptidomimetics. Molecules 2021, 26, 6759. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Heymach, J.V. Vascular Endothelial Growth Factor (VEGF) Pathway. J. Thorac. Oncol. 2006, 1, 768–770. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Li, L.; Asteriou, T.; Bernert, B.; Heldin, C.H.; Heldin, P. Growth factor regulation of hyaluronan synthesis and degradation in human dermal fibroblasts: Importance of hyaluronan for the mitogenic response of PDGF-BB. Biochem. J. 2007, 404, 327–336. [Google Scholar] [CrossRef]
- Fiorentino, S.; Roffi, A.; Filardo, G.; Marcacci, M.; Kon, E. European Definitions, Current Use, and EMA Stance of Platelet-Rich Plasma in Sports Medicine. J. Knee Surg. 2015, 28, 51–54. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef]
- Masoudi, E.; Ribas, J.; Kaushik, G.; Leijten, J.; Khademhosseini, A. Platelet-Rich Blood Derivatives for Stem Cell-Based Tissue Engineering and Regeneration. Curr. Stem Cell Rep. 2016, 2, 33–42. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Ouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Intini, G. The use of platelet-rich plasma in bone reconstruction therapy. Biomaterials 2009, 30, 4956–4966. [Google Scholar] [CrossRef] [PubMed]
- Mijiritsky, E.; Assaf, H.D.; Peleg, O.; Shacham, M.; Cerroni, L.; Mangani, L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation-A Narrative Review. Biology 2021, 10, 317. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Desai, R.; Kaye, A.D. Is Platelet-Rich Plasma a Future Therapy in Pain Management? Med. Clin. N. Am. 2016, 100, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF-Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef]
- Xu, Z.; Yin, W.; Zhang, Y.; Qi, X.; Chen, Y.; Xie, X.; Zhang, C. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci. Rep. 2017, 7, 43301. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Anitua, E.; Nurden, P.; Prado, R.; Nurden, A.T.; Padilla, S. Autologous fibrin scaffolds: When platelet- and plasma-derived biomolecules meet fibrin. Biomaterials 2019, 192, 440–460. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Yu, J.; Wang, J.; Zhai, P.; Chen, S.; Liu, M.; Zhou, Y. Platelet-Rich Fibrin as a Bone Graft Material in Oral and Maxillofacial Bone Regeneration: Classification and Summary for Better Application. BioMed Res. Int. 2019, 2019, 3295756. [Google Scholar] [CrossRef]
- Varela, H.A.; Souza, J.C.M.; Nascimento, R.M.; Araújo, R.F., Jr.; Vasconcelos, R.C.; Cavalcante, R.S.; Guedes, P.M.; Araújo, A.A. Injectable platelet rich fibrin: Cell content, morphological, and protein characterization. Clin. Oral. Investig. 2019, 23, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; He, X.; Li, S.; Pan, H.; Yin, L. Injectable platelet-rich fibrin positively regulates osteogenic differentiation of stem cells from implant hole via the ERK1/2 pathway. Platelets 2023, 34, 2159020. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.R.; Levy, F.M. Autologous platelet concentrates for facial rejuvenation. J. Appl. Oral Sci. 2022, 30, e20220020. [Google Scholar] [CrossRef] [PubMed]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- Stanca, E.; Calabriso, N.; Giannotti, L.; Nitti, P.; Damiano, F.; Stanca, B.D.C.; Carluccio, M.A.; De Benedetto, G.E.; Demitri, C.; Palermo, A.; et al. Analysis of CGF Biomolecules, Structure and Cell Population: Characterization of the Stemness Features of CGF Cells and Osteogenic Potential. Int. J. Mol. Sci. 2021, 22, 8867. [Google Scholar] [CrossRef]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Aghamohammadi, Z.; Tayebi, L. In vitro and in vivo effects of concentrated growth factor on cells and tissues. J. Biomed Mater. Res. A 2020, 108, 1338–1350. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, H. A Comprehensive Review of Concentrated Growth Factors and Their Novel Applications in Facial Reconstructive and Regenerative Medicine. Aesthetic Plast. Surg. 2020, 44, 1047–1057. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Collins, T.; Alexander, D.; Barkatali, B. Platelet-rich plasma: A narrative review. EFORT Open Rev. 2021, 6, 225–235. [Google Scholar] [CrossRef]
- Dashore, S.; Chouhan, K.; Nanda, S.; Sharma, A. Platelet-Rich Fibrin, Preparation and Use in Dermatology. Indian Dermatol. Online J. 2021, 12, S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; An, N.; Ouyang, X. Quantification of growth factors in different platelet concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Kim, S.G.; Oh, J.S.; You, J.S.; Kim, J.S.; Lim, S.C.; Jeong, M.A.; Kim, J.S.; Jung, C.; Kwon, Y.S.; et al. Early bone formation at a femur defect using CGF and PRF grafts in adult dogs. Implant Dent. 2016, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral. Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Bonazza, V.; Hajistilly, C.; Patel, D.; Patel, J.; Woo, R.; Cocchi, M.A.; Buffoli, B.; Lancini, D.; Gheno, E.; Rezzani, R.; et al. Growth Factors Release From Concentrated Growth Factors: Effect of β-Tricalcium Phosphate Addition. J. Craniofac. Surg. 2018, 29, 2291–2295. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jime’nez, P.; Corso, M.D.; Kang, B.S.; Nally, M.; Lanata, N.; Wang, H.L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Huang, L.; Zou, R.; He, J.; Ouyang, K.; Piao, Z. Comparing osteogenic effects between concentrated growth factors and the acellular dermal matrix. Braz. Oral. Res. 2018, 32, e29. [Google Scholar] [CrossRef]
- Borsani, E.; Bonazza, V.; Buffoli, B.; Cocchi, M.A.; Castrezzati, S.; Scarì, G.; Baldi, F.; Pandini, S.; Licenziati, S.; Parolini, S.; et al. Biological Characterization and In Vitro Effects of Human Concentrated Growth Factor Preparation: An Innovative Approach to Tissue Regeneration. Biol. Med. 2015, 7, 256. [Google Scholar] [CrossRef]
- Honda, H.; Tamai, N.; Naka, N.; Yoshikawa, H.; Myoui, A. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J. Artif. Organs 2013, 16, 305–315. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P. The Role of Vascular Endothelial Growth Factor in Angiogenesis. Acta Haematol. 2001, 106, 148–156. [Google Scholar] [CrossRef]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Flavell, R.A. TGF-β: A Master of All T Cell Trades. Cell 2008, 134, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Xi, Q. TGF-β control of stem cell differentiation genes. FEBS Lett. 2012, 586, 1953–1958. [Google Scholar] [CrossRef]
- Xue, T.; Wei, L.; Qiao, L.; Qiu, J.; Zha, D. Does bone morphogenetic proteins play an important role in chronic rhinosinusitis? Med. Hypotheses 2009, 72, 228. [Google Scholar] [CrossRef] [PubMed]
- Kalén, A.; Wahlström, O.; Linder, C.H.; Magnusson, P. The content of bone morphogenetic proteins in platelets varies greatly between different platelet donors. Biochem. Biophys. Res. Commun. 2008, 375, 261–264. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, Q.; Li, Y.; Xu, C.; Tang, X.; Ci, J.; Sun, S.; Xu, B.; Li, Y. Acidic pH environment induces autophagy in osteoblasts. Sci. Rep. 2017, 7, 46161. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Isobe, K.; Watanebe, T.; Kawabata, H.; Kitamura, Y.; Kawase, T. Mechanical and degradation properties of advanced platelet-rich fibrin (APRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int. J. Implant. Dent. 2017, 3, 17. [Google Scholar] [CrossRef]
- Yu, M.; Wang, X.; Liu, Y.; Qiao, J. Cytokine release kinetics of concentrated growth factors in different scaffolds. Clin. Oral. Investig. 2018, 23, 1663–1671. [Google Scholar] [CrossRef]
- Lei, L.; Yu, Y.; Han, J.; Shi, D.; Sun, W.; Zhang, D.; Chen, L. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J. Periodontol. 2020, 91, 462–472. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Ní Ríordáin, R. Autologous platelet concentrates in oral surgery: Protocols, properties, and clinical applications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Di Liddo, R.; Bertalot, T.; Borean, A.; Pirola, I.; Argentoni, A.; Schrenk, S.; Cenzi, C.; Capelli, S.; Conconi, M.T.; Parnigotto, P.P. Leucocyte and Platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J. Cell Mol. Med. 2018, 22, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, M.; Okazaki, Y.; Kodama, H.; Izumi, K.; Yasuoka, H.; Ogawa, Y.; Kawakami, Y.; Ikeda, Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J. Leukoc. Biol. 2003, 74, 833–845. [Google Scholar] [CrossRef]
- Cesselli, D.; Beltrami, A.P.; Rigo, S.; Bergamin, N.; D’Aurizio, F.; Verardo, R.; Piazza, S.; Klaric, E.; Fanin, R.; Toffoletto, B.; et al. Multipotent progenitor cells are present in human peripheral blood. Circ. Res. 2009, 104, 1225–1234. [Google Scholar] [CrossRef]
- Graf, T.; Stadtfeld, M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell 2008, 3, 480–483. [Google Scholar] [CrossRef]
- Giannotti, L.; Di Chiara Stanca, B.; Nitti, P.; Spedicato, F.; Damiano, F.; Demitri, C.; Calabriso, N.; Carluccio, M.A.; Palermo, A.; Ferrante, F.; et al. Hydroxyapatite-Silicon Scaffold Promotes Osteogenic Differentiation of CGF Primary Cells. Biology 2023, 12, 528. [Google Scholar] [CrossRef]
- Calabriso, N.; Stanca, E.; Rochira, A.; Damiano, F.; Giannotti, L.; Di Chiara Stanca, B.; Massaro, M.; Scoditti, E.; Demitri, C.; Nitti, P.; et al. Angiogenic Properties of Concentrated Growth Factors (CGFs): The Role of Soluble Factors and Cellular Components. Pharmaceutics 2021, 13, 635. [Google Scholar] [CrossRef]
- Marchetti, E.; Mancini, L.; Bernardi, S.; Bianchi, S.; Cristiano, L.; Torge, D.; Marzo, G.; Macchiarelli, G. Evaluation of Different Autologous Platelet Concentrate Biomaterials: Morphological and Biological Comparisons and Considerations. Materials 2020, 13, 2282. [Google Scholar] [CrossRef]
- Cabrera-Ramírez, J.O.; Puebla-Mora, A.G.; González-Ojeda, A.; García-Martínez, D.; Cortés-Lares, J.A.; Márquez-Valdés, A.R.; Contreras-Hernández, G.I.; Bracamontes-Blanco, J.; Saucedo Ortiz, J.A.; Fuentes-Orozco, C. Platelet-rich plasma for the treatment of photodamage of the skin of the hands. Actas Dermosifiliogr. 2017, 108, 746–751. [Google Scholar] [CrossRef]
- Kamakura, T.; Kataoka, J.; Maeda, K.; Teramachi, H.; Mihara, H.; Miyata, K.; Ooi, K.; Sasaki, N.; Kobayashi, M.; Ito, K. Platelet-rich plasma with basic fibroblast growth factor for Treatment of wrinkles and depressed areas of the skin. Plast. Reconstr. Surg. 2015, 136, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Elnehrawy, N.Y.; Ibrahim, Z.A.; Eltoukhy, A.M.; Nagy, H.M. Assessment of the efficacy and safety of single platelet-rich plasma injection on different types and grades of facial wrinkles. J. Cosmet. Dermatol. 2017, 16, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Zhang, Y.; Miron, R.J. Fluid platelet-rich fibrin stimulates greater dermal skin fibroblast cell migration, proliferation, and collagen synthesis when compared to platelet- rich plasma. J. Cosmet. Dermatol. 2019, 18, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Bonazza, V.; Borsani, E.; Buffoli, B.; Parolini, S.; Inchingolo, F.; Rezzani, R.; Rodella, L.F. In vitro treatment with concentrated growth factors (CGF) and sodium orthosilicate positively affects cell renewal in three different human cell lines. Cell Biol. Int. 2018, 42, 353–364. [Google Scholar] [CrossRef]
- Topkara, A.; Özkan, A.; Özcan, R.H.; Öksüz, M.; Akbulut, M. Effect of concentrated growth factor on survival of diced cartilage graft. Aesthet. Surg. J. 2016, 36, 1176–1187. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Chen, Y.; Hou, Y.; Song, P.; Zhou, M.; Nie, M.; Liu, X. Modulation of proliferation and differentiation of gingiva-derived mesenchymal stem cells by concentrated growth factors: Potential implications in tissue engineering for dental regeneration and repair. Int. J. Mol. Med. 2019, 44, 37–46. [Google Scholar] [CrossRef]
- Fang, D.; Jin, P.; Huang, Q.; Yang, Y.; Zhao, J.; Zheng, L. Platelet-rich plasma promotes the regeneration of cartilage engineered by mesenchymal stem cells and collagen hydrogel via the TGF-b/SMAD signaling pathway. J. Cell Physiol. 2019, 234, 15627–15637. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Zhang, Q.; Xu, Z.; Xu, F.; Li, D.; Li, Y. Microtia patients: Auricular chondrocyte ECM is promoted by CGF through IGF-1 activation of the IGF-1R/PI3K/AKT path-way. J. Cell Physiol. 2019, 234, 21817–21824. [Google Scholar] [CrossRef]
- Rochira, A.; Siculella, L.; Damiano, F.; Palermo, A.; Ferrante, F.; Carluccio, M.A.; Calabriso, N.; Giannotti, L.; Stanca, E. Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells. Biology 2020, 9, 370. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.H.; Sándor, G.K.; Kim, Y.D. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth, factor (CGF) in rabbit-skull defect healing. Arch. Oral Biol. 2014, 59, 550–558. [Google Scholar] [CrossRef]
- Palermo, A.; Giannotti, L.; Di Chiara Stanca, B.; Ferrante, F.; Gnoni, A.; Nitti, P.; Calabriso, N.; Demitri, C.; Damiano, F.; Batani, T.; et al. Use of CGF in Oral and Implant Surgery: From Laboratory Evidence to Clinical Evaluation. Int. J. Mol. Sci. 2022, 23, 15164. [Google Scholar] [CrossRef] [PubMed]
- Karaman, O.; Kumar, A.; Moeinzadeh, S.; He, X.; Cui, T.; Jabbari, E. Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J. Tissue Eng. Regen. Med. 2013, 10, E132–E146. [Google Scholar] [CrossRef] [PubMed]
- Onak, G.; Şen, M.; Horzum, N.; Ercan, U.K.; Yaralı, Z.B.; Garipcan, B.; Karaman, O. Aspartic and Glutamic Acid Templated Peptides Conjugation on Plasma Modified Nanofibers for Osteogenic Differentiation of Human Mesenchymal Stem Cells: A Comparative Study. Sci. Rep. 2018, 8, 17620. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, M.; Kitase, Y.; Kimble, M.; O’Connell, T.M.; Bonewald, L.F. Taurine, an osteocyte metabolite, protects against oxidative stress-induced cell death and decreases inhibitors of the Wnt/β-catenin signaling pathway. Bone 2020, 137, 115374. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Xu, L.; Wu, T.; Cui, L.; Xu, D. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids 2014, 46, 1673–1680. [Google Scholar] [CrossRef]
- Anghelina, M.; Krishnan, P.; Moldovan, L.; Moldovan, N.I. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair. Am. J. Pathol. 2006, 168, 529–541. [Google Scholar] [CrossRef]
- Manole, E.; Niculite, C.; Lambrescu, I.M.; Gaina, G.; Ioghen, O.; Ceafalan, L.C.; Hinescu, M.E. Macrophages and Stem Cells-Two to Tango for Tissue Repair? Biomolecules 2021, 11, 697. [Google Scholar] [CrossRef]
- Verma, U.P.; Yadav, R.K.; Dixit, M.; Gupta, A. Platelet-rich fibrin: A paradigm in periodontal therapy—A systematic review. J. Int. Soc. Prev. Community Dent. 2017, 7, 227–233. [Google Scholar] [CrossRef]
- Li, Q.; Pan, S.; Dangaria, S.J.; Gopinathan, G.; Kolokythas, A.; Chu, S.; Geng, Y.; Zhou, Y.; Luan, X. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. Biomed Res. Int. 2013, 2013, 638043. [Google Scholar] [CrossRef]
- Duan, X.; Lin, Z.; Lin, X.; Wang, Z.; Wu, Y.; Ji, M.; Lu, W.; Wang, X.; Zhang, D. Study of platelet-rich fibrin combined with rat periodontal ligament stem cells in periodontal tissue regeneration. J. Cell Mol. Med. 2018, 22, 1047–1055. [Google Scholar]
- Mijiritsky, E.; Assaf, H.D.; Kolerman, R.; Mangani, L.; Ivanova, V.; Zlatev, S. Autologous Platelet Concentrates (APCs) for Hard Tissue Regeneration in Oral Implantology, Sinus Floor Elevation, Peri-Implantitis, Socket Preservation, and Medication-Related Osteonecrosis of the Jaw (MRONJ): A Literature Review. Biology 2022, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.S.; Heo, J.U.; Kwak, D.H.; Kim, D.E.; Kim, J.M.; Moon, J.W.; Lee, J.H.; Park, I.S. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant. Dent. 2011, 20, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Lokwani, B.V.; Gupta, D.; Agrawal, R.S.; Mehta, S.; Nirmal, N.J. The use of concentrated growth factor in dental implantology: A systematic review. J. Indian Prosthodont. Soc. 2020, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shetye, A.G.; Rathee, M.; Jain, P.; Agarkar, V.; Kaushik, S.; Alam, M. Effect of advanced platelet-rich fibrin and concentrated growth factor on tissues around implants in maxillary anterior region. J. Indian Prosthodont. Soc. 2022, 22, 169–178. [Google Scholar] [CrossRef]
- Özveri Koyuncu, B.; İçpınar Çelik, K.; Özden Yüce, M.; Günbay, T.; Çömlekoğlu, M.E. The role of concentrated growth factor on implant stability: A preliminary study. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 363–367. [Google Scholar] [CrossRef]
- Borsani, E.; Buffoli, B.; Bonazza, V.; Brunelli, G.; Monini, L.; Inchingolo, F.; Ballini, A.; Rezzani, R.; Rodella, L.F. In vitro effects of concentrated growth factors (CGF) on human SH-SY5Y neuronal cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 304–314. [Google Scholar] [CrossRef]
- Perseghin, P.; Sciorelli, G.; Belotti, D.; Speranza, T.; Pogliani, E.M.; Ferro, O.; Gianoli, M.; Porta, F.; Paolini, G. Frozen-and-thawed allogeneic platelet gels for treating postoperative chronic wounds. Transfusion 2005, 45, 1544.e6. [Google Scholar] [CrossRef]
- Smrke, D.; Gubina, B.; Domanoviç, D.; Rozman, P. Allogeneic platelet gel with autologous cancellous bone graft for the treatment of a large bone defect. Eur. Surg. Res. 2007, 39, 170.e4. [Google Scholar] [CrossRef]
- Greppi, N.; Mazzucco, L.; Galetti, G.; Bona, F.; Petrillo, E.; Smacchia, C.; Raspollini, E.; Cossovich, P.; Caprioli, R.; Borzini, P.; et al. Treatment of recalcitrant ulcers with allogeneic platelet gel from pooled platelets in aged hypomobile patients. Biologicals 2011, 39, 73–80. [Google Scholar] [CrossRef]
- Wang, S.; Ding, W.; Du, Y.; Qi, Q.; Luo, K.; Luan, J.; Shen, Y.; Chen, B. Allogeneic platelet gel therapy for refractory abdominal wound healing: A preliminary study. Adv. Clin. Exp. Med. 2023. online ahead of print. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannotti, L.; Di Chiara Stanca, B.; Spedicato, F.; Nitti, P.; Damiano, F.; Demitri, C.; Calabriso, N.; Carluccio, M.A.; Palermo, A.; Siculella, L.; et al. Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications. Genes 2023, 14, 1669. https://doi.org/10.3390/genes14091669
Giannotti L, Di Chiara Stanca B, Spedicato F, Nitti P, Damiano F, Demitri C, Calabriso N, Carluccio MA, Palermo A, Siculella L, et al. Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications. Genes. 2023; 14(9):1669. https://doi.org/10.3390/genes14091669
Chicago/Turabian StyleGiannotti, Laura, Benedetta Di Chiara Stanca, Francesco Spedicato, Paola Nitti, Fabrizio Damiano, Christian Demitri, Nadia Calabriso, Maria Annunziata Carluccio, Andrea Palermo, Luisa Siculella, and et al. 2023. "Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications" Genes 14, no. 9: 1669. https://doi.org/10.3390/genes14091669
APA StyleGiannotti, L., Di Chiara Stanca, B., Spedicato, F., Nitti, P., Damiano, F., Demitri, C., Calabriso, N., Carluccio, M. A., Palermo, A., Siculella, L., & Stanca, E. (2023). Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications. Genes, 14(9), 1669. https://doi.org/10.3390/genes14091669










