Involvement of Nucleotide Excision Repair and Rec-Dependent Pathway Genes for UV Radiation Resistance in Deinococcus irradiatisoli 17bor-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culturing and Radiation Resistance Analysis
2.2. Genome Sequence Project
2.3. Library Construction and Assembly
2.4. Genome Annotation
3. Results
3.1. Morphology and Radiation Resistance Analysis
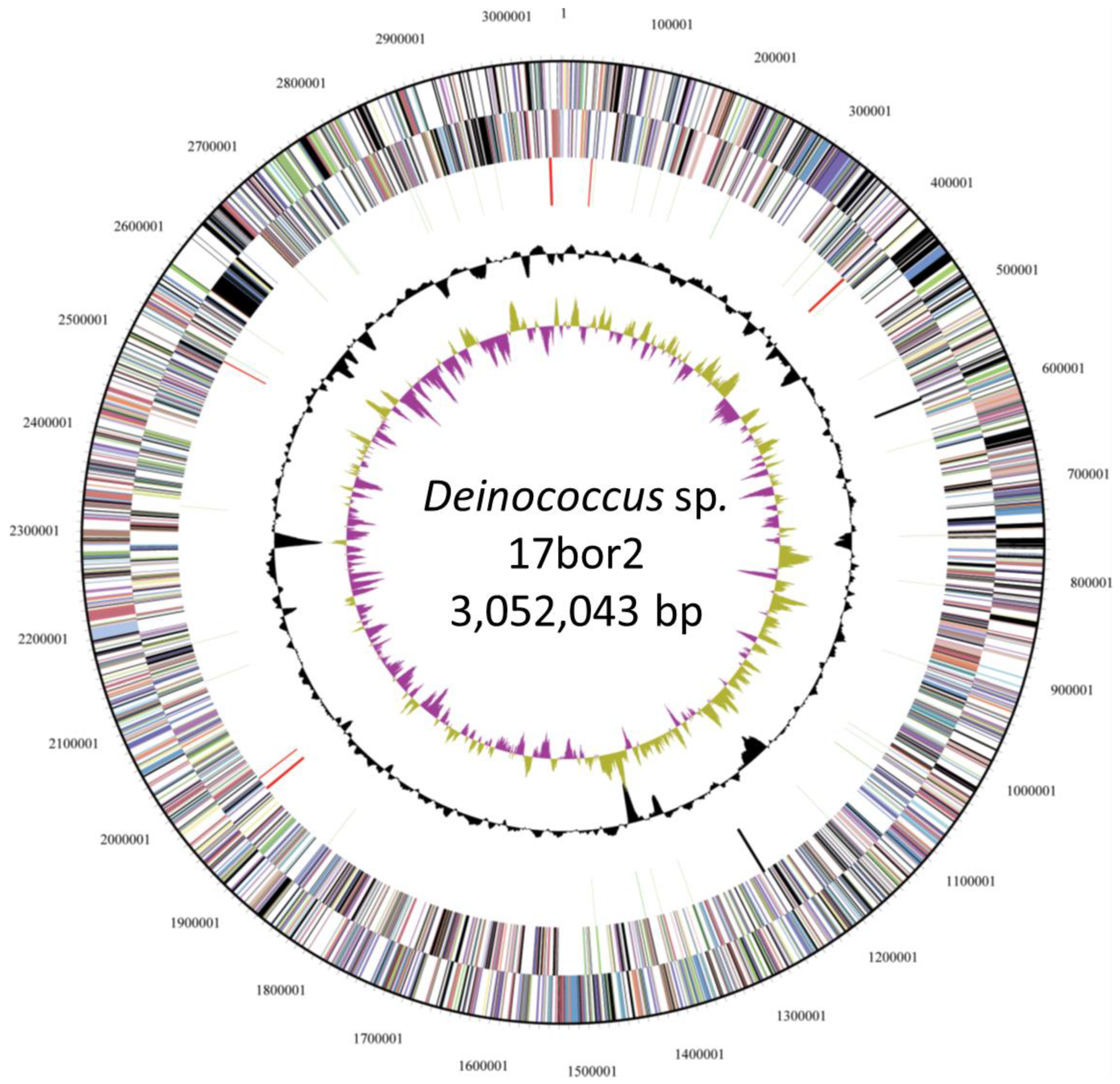
3.2. Genomic Properties
3.3. Perceptions from the Genome Sequence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinak, M. Chapter 10—Enzymatic recognition of radiation-produced oxidative DNA lesion. Molecular dynamics approach. In Modern Methods for Theoretical Physical Chemistry of Biopolymers; Starikov, E.B., Lewis, J.P., Tanaka, S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 191–210. [Google Scholar]
- Ortiz de Orue Lucana, D.; Wedderhoff, I.; Groves, M.R. ROS-Mediated Signalling in Bacteria: Zinc-Containing Cys-X-X-Cys Redox Centres and Iron-Based Oxidative Stress. J. Signal Transduct. 2012, 2012, 605905. [Google Scholar] [CrossRef]
- Waldeck, W.; Heidenreich, E.; Mueller, G.; Wiessler, M.; Toth, K.; Braun, K. ROS-mediated killing efficiency with visible light of bacteria carrying different red fluorochrome proteins. J. Photochem. Photobiol. B Biol. 2012, 109, 28–33. [Google Scholar] [CrossRef]
- Yu, S.-L.; Lee, S.-K. Ultraviolet radiation: DNA damage, repair, and human disorders. Mol. Cell. Toxicol. 2017, 13, 21–28. [Google Scholar] [CrossRef]
- Zimmerman, J.M.; Battista, J.R. A ring-like nucleoid is not necessary for radioresistance in the Deinococcaceae. BMC Microbiol. 2005, 5, 17. [Google Scholar] [CrossRef]
- Daly, M.J. Death by protein damage in irradiated cells. DNA Repair 2012, 11, 12–21. [Google Scholar] [CrossRef]
- Zahradka, K.; Slade, D.; Bailone, A.; Sommer, S.; Averbeck, D.; Petranovic, M.; Lindner, A.B.; Radman, M. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature 2006, 443, 569–573. [Google Scholar] [CrossRef]
- Byrne, R.T.; Klingele, A.J.; Cabot, E.L.; Schackwitz, W.S.; Martin, J.A.; Martin, J.; Wang, Z.; Wood, E.A.; Pennacchio, C.; Pennacchio, L.A.; et al. Evolution of extreme resistance to ionizing radiation via genetic adaptation of DNA repair. eLife 2014, 3, e01322. [Google Scholar] [CrossRef]
- Holcomb, N.; Goswami, M.; Han, S.G.; Scott, T.; D’Orazio, J.; Orren, D.K.; Gairola, C.G.; Mellon, I. Inorganic arsenic inhibits the nucleotide excision repair pathway and reduces the expression of XPC. DNA Repair 2017, 52, 70–80. [Google Scholar] [CrossRef]
- Nieto Moreno, N.; Olthof, A.M.; Svejstrup, J.Q. Transcription-Coupled Nucleotide Excision Repair and the Transcriptional Response to UV-Induced DNA Damage. Annu. Rev. Biochem. 2023, 92, 81–113. [Google Scholar] [CrossRef]
- Mallet, J.D.; Dorr, M.M.; Drigeard Desgarnier, M.C.; Bastien, N.; Gendron, S.P.; Rochette, P.J. Faster DNA Repair of Ultraviolet-Induced Cyclobutane Pyrimidine Dimers and Lower Sensitivity to Apoptosis in Human Corneal Epithelial Cells than in Epidermal Keratinocytes. PLoS ONE 2016, 11, e0162212. [Google Scholar] [CrossRef]
- You, Y.H.; Lee, D.H.; Yoon, J.H.; Nakajima, S.; Yasui, A.; Pfeifer, G.P. Cyclobutane pyrimidine dimers are responsible for the vast majority of mutations induced by UVB irradiation in mammalian cells. J. Biol. Chem. 2001, 276, 44688–44694. [Google Scholar] [CrossRef]
- Bohm, K.A.; Wyrick, J.J. Damage mapping techniques and the light they have shed on canonical and atypical UV photoproducts. Front. Genet. 2023, 13, 1102593. [Google Scholar] [CrossRef]
- Kciuk, M.; Marciniak, B.; Mojzych, M.; Kontek, R. Molecular Sciences Review Focus on UV-Induced DNA Damage and Repair-Disease Relevance and Protective Strategies. Int. J. Mol. Sci. 2020, 21, 7264. [Google Scholar] [CrossRef]
- Jones, D.L.; Baxter, B.K. DNA Repair and Photoprotection: Mechanisms of Overcoming Environmental Ultraviolet Radiation Exposure in Halophilic Archaea. Front. Microbiol. 2017, 8, 1882. [Google Scholar] [CrossRef]
- Barve, A.; Galande, A.A.; Ghaskadbi, S.S.; Ghaskadbi, S. DNA Repair Repertoire of the Enigmatic Hydra. Front. Genet. 2021, 12, 670695. [Google Scholar] [CrossRef]
- Krasikova, Y.; Rechkunova, N.; Lavrik, O. Nucleotide Excision Repair: From Molecular Defects to Neurological Abnormalities. Int. J. Mol. Sci. 2021, 22, 6220. [Google Scholar] [CrossRef]
- Kraithong, T.; Hartley, S.; Jeruzalmi, D.; Pakotiprapha, D. A Peek Inside the Machines of Bacterial Nucleotide Excision Repair. Int. J. Mol. Sci. 2021, 22, 952. [Google Scholar] [CrossRef]
- Borsos, B.N.; Majoros, H.; Pankotai, T. Emerging Roles of Post-Translational Modifications in Nucleotide Excision Repair. Cells 2020, 9, 1466. [Google Scholar] [CrossRef]
- Kurzbauer, M.T.; Uanschou, C.; Chen, D.; Schlogelhofer, P. The recombinases DMC1 and RAD51 are functionally and spatially separated during meiosis in Arabidopsis. Plant Cell 2012, 24, 2058–2070. [Google Scholar] [CrossRef]
- Shinohara, A.; Shinohara, M. Roles of RecA homologues Rad51 and Dmc1 during meiotic recombination. Cytogenet. Genome Res. 2004, 107, 201–207. [Google Scholar] [CrossRef]
- Earl, A.M.; Rankin, S.K.; Kim, K.P.; Lamendola, O.N.; Battista, J.R. Genetic evidence that the uvsE gene product of Deinococcus radiodurans R1 is a UV damage endonuclease. J. Bacteriol. 2002, 184, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Lee, J.J.; Lim, S.Y.; Joe, M.H.; Im, S.H.; Kim, M.K. Deinococcus radioresistens sp. nov., a UV and gamma radiation-resistant bacterium isolated from mountain soil. Antonie Leeuwenhoek 2015, 107, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Srinivasan, S.; Seo, T.; Kim, M.K. Deinococcus soli sp. nov., a gamma-radiation-resistant bacterium isolated from rice field soil. Curr. Microbiol. 2014, 68, 777–783. [Google Scholar] [CrossRef]
- Srinivasan, S.; Kim, M.K.; Lim, S.; Joe, M.; Lee, M. Deinococcus daejeonensis sp. nov., isolated from sludge in a sewage disposal plant. Int. J. Syst. Evol. Microbiol. 2012, 62, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Garrity, G.; Gray, T.; Morrison, N.; Selengut, J.; Sterk, P.; Tatusova, T.; Thomson, N.; Allen, M.J.; Angiuoli, S.V.; et al. The minimum information about a genome sequence (MIGS) specification. Nat. Biotechnol. 2008, 26, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, V.M.; Mavromatis, K.; Ivanova, N.N.; Chen, I.M.A.; Chu, K.; Kyrpides, N.C. IMG ER: A system for microbial genome annotation expert review and curation. Bioinformatics 2009, 25, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rodland, E.A.; Staerfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Nawrocki, E.P.; Kolbe, D.L.; Eddy, S.R. Infernal 1.0: Inference of RNA alignments. Bioinformatics 2009, 25, 1335–1337. [Google Scholar] [CrossRef]
- de Groot, A.; Dulermo, R.; Ortet, P.; Blanchard, L.; Guérin, P.; Fernandez, B.; Vacherie, B.; Dossat, C.; Jolivet, E.; Siguier, P.; et al. Alliance of Proteomics and Genomics to Unravel the Specificities of Sahara Bacterium Deinococcus deserti. PLoS Genet. 2009, 5, e1000434. [Google Scholar] [CrossRef]
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Tatusov, R.L.; Minton, K.W.; Koonin, E.V.; Daly, M.J. Genome of the Extremely Radiation-Resistant Bacterium Deinococcus radiodurans Viewed from the Perspective of Comparative Genomics. Microbiol. Mol. Biol. Rev. 2001, 65, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Sancar, A. Nucleotide excision repair: From E. coli to man. Biochimie 1999, 81, 15–25. [Google Scholar] [CrossRef]
- Seck, A.; De Bonis, S.; Saint-Pierre, C.; Gasparutto, D.; Ravanat, J.-L.; Timmins, J. In vitro reconstitution of an efficient nucleotide excision repair system using mesophilic enzymes from Deinococcus radiodurans. Commun. Biol. 2022, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Truglio, J.J.; Croteau, D.L.; Van Houten, B.; Kisker, C. Prokaryotic nucleotide excision repair: The UvrABC system. Chem. Rev. 2006, 106, 233–252. [Google Scholar] [CrossRef]
- Dai, S.; Jin, Y.; Li, T.; Weng, Y.; Xu, X.; Zhang, G.; Li, J.; Pang, R.; Tian, B.; Hua, Y. DR1440 is a potential iron efflux protein involved in maintenance of iron homeostasis and resistance of Deinococcus radiodurans to oxidative stress. PLoS ONE 2018, 13, e0202287. [Google Scholar] [CrossRef]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef]
- Sancar, A. DNA EXCISION REPAIR. Annu. Rev. Biochem. 1996, 65, 43–81. [Google Scholar] [CrossRef]
- Friedberg, E.C. DNA damage and repair. Nature 2003, 421, 436–440. [Google Scholar] [CrossRef]
- Cox, M.M. Regulation of bacterial RecA protein function. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 41–63. [Google Scholar] [CrossRef]
| MIGS * ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | PacBio library |
| MIGS-29 | Sequencing platforms | Pacific Biosciences RS II |
| MIGS-31.2 | Sequencing coverage | 156× |
| MIGS-30 | Assemblers | PacBio SMRT Analysis 2.3.0 |
| NCBI accession | CP029494 | |
| GOLD ID | Ga0307136 | |
| NCBI bioproject ID | PRJNA471975 | |
| MIGS-13 | Source material identifier | 17bor-2 |
| Attribute | Value | % of Total a |
|---|---|---|
| Genome size (bp) | 3,052,043 | 100.0 |
| DNA-coding region (bp) | 2,492,501 | 88.4 |
| DNA G + C content (bp) | 2,112,710 | 67.0 |
| No. of contigs | 1 | 100.0 |
| Total genes | 2911 | 100.0 |
| rRNA genes | 9 | 0.1 |
| tRNA | 49 | 1.7 |
| Protein-coding genes | 2128 | 97.2 |
| Pseudo genes | 0 | 0.0 |
| Genes with function prediction | 2003 | 83.0 |
| Genes assigned to COGs | 1535 | 72.4 |
| Genes assigned Pfam domains | 1421 | 83.6 |
| Genes with signal peptides | 306 | 9.6 |
| Genes with transmembrane helices | 824 | 23.0 |
| Code | Value | % | COG Color * | Description |
|---|---|---|---|---|
| J | 141 | 6 |  | Translation |
| A | 0 | - |  | RNA processing and modification |
| K | 162 | 5.4 |  | Transcription |
| L | 124 | 4.6 |  | Replication, recombination, and repair |
| B | 5 | 0.1 |  | Chromatin structure and dynamics |
| D | 31 | 0.8 |  | Cell cycle control, mitosis, and meiosis |
| Y | 0 | - |  | Nuclear structure |
| V | 48 | 1.5 |  | Defense mechanisms |
| T | 43 | 3.4 |  | Signal transduction mechanisms |
| M | 97 | 4.5 |  | Cell wall/membrane biogenesis |
| N | 19 | 0.6 |  | Cell motility |
| Z | 3 | 0.1 |  | Cytoskeleton |
| W | 0 | - |  | Extracellular structures |
| U | 28 | 1.2 |  | Intracellular trafficking and secretion |
| O | 86 | 3.8 |  | Posttranslational modification, protein turnover, chaperones |
| C | 142 | 5.7 |  | Energy production and conversion |
| G | 163 | 7.1 |  | Carbohydrate transport and metabolism |
| E | 236 | 11.8 |  | Amino acid transport and metabolism |
| F | 76 | 3.4 |  | Nucleotide transport and metabolism |
| H | 92 | 3.7 |  | Coenzyme transport and metabolism |
| I | 84 | 3.7 |  | Lipid transport and metabolism |
| P | 126 | 4.8 |  | Inorganic ion transport and metabolism |
| Q | 56 | 23 |  | Secondary metabolite biosynthesis, transport, and catabolism |
| R | 318 | 13.6 |  | General function prediction only |
| S | 314 | 9.5 |  | Function unknown |
| - | 1181 | 35.1 |  | Not in COGs |
| Subsystem | Gene Name | Deinococcus irradiatisoli 17bor-2 | Deinococcus radiodurans R1T | Deinococcus geothermalis DSM 11300T |
|---|---|---|---|---|
| UvrABC system | Excinuclease ABC subunit A | + | + | + |
| Excinuclease ABC subunit B | + | + | + | |
| Excinuclease ABC subunit C | + | + | + | |
| Bacterial | A/G-specific adenine glycosylase | + | + | + |
| DNA repair protein RadA | + | + | + | |
| DNA repair protein RecN | + | + | + | |
| Exodeoxyribonuclease III | + | + | + | |
| Exodeoxyribonuclease VII large subunit | + | + | + | |
| Exodeoxyribonuclease VII small subunit | + | + | + | |
| Exonuclease SbcC | + | + | + | |
| RecA protein | + | + | + | |
| Single-stranded DNA-binding protein | + | + | + | |
| Exonuclease SbcD | + | – | – | |
| Bacterial MutL-MutS system | DNA mismatch repair protein MutL | + | + | + |
| DNA mismatch repair protein MutS | + | + | + | |
| Recombination inhibitory protein MutS2 | + | + | + | |
| Bacterial RecBCD pathway | RecD-like DNA helicase YrrC | + | + | + |
| ATP-dependent DNA helicase RecQ | + | + | + | |
| DNA recombination and repair protein RecF | + | + | + | |
| DNA recombination and repair protein RecO | + | + | + | |
| RecA protein | + | + | + | |
| Recombination protein RecR | + | + | + | |
| Single-stranded DNA-binding protein | + | + | + | |
| Single-stranded DNA-specific exonuclease RecJ | + | + | + | |
| UvrD and related helicases | ATP-dependent DNA helicase UvrD/PcrA | + | + | – |
| RecA and MutS | DNA mismatch repair protein MutS | + | + | + |
| RecA protein | + | + | + | |
| Uracil-DNA glycosylase | G: T/U mismatch-specific uracil/thymine DNA-glycosylase | + | + | – |
| Uracil-DNA glycosylase, family 1 | + | + | – | |
| Uracil-DNA glycosylase, family 4 | + | + | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramani, G.; Srinivasan, S. Involvement of Nucleotide Excision Repair and Rec-Dependent Pathway Genes for UV Radiation Resistance in Deinococcus irradiatisoli 17bor-2. Genes 2023, 14, 1803. https://doi.org/10.3390/genes14091803
Subramani G, Srinivasan S. Involvement of Nucleotide Excision Repair and Rec-Dependent Pathway Genes for UV Radiation Resistance in Deinococcus irradiatisoli 17bor-2. Genes. 2023; 14(9):1803. https://doi.org/10.3390/genes14091803
Chicago/Turabian StyleSubramani, Gayathri, and Sathiyaraj Srinivasan. 2023. "Involvement of Nucleotide Excision Repair and Rec-Dependent Pathway Genes for UV Radiation Resistance in Deinococcus irradiatisoli 17bor-2" Genes 14, no. 9: 1803. https://doi.org/10.3390/genes14091803
APA StyleSubramani, G., & Srinivasan, S. (2023). Involvement of Nucleotide Excision Repair and Rec-Dependent Pathway Genes for UV Radiation Resistance in Deinococcus irradiatisoli 17bor-2. Genes, 14(9), 1803. https://doi.org/10.3390/genes14091803







