Unraveling the Epigenetic Tapestry: Decoding the Impact of Epigenetic Modifications in Hidradenitis Suppurativa Pathogenesis
Abstract
:1. Introduction
2. Search Strategy
Criteria for Paper Selection
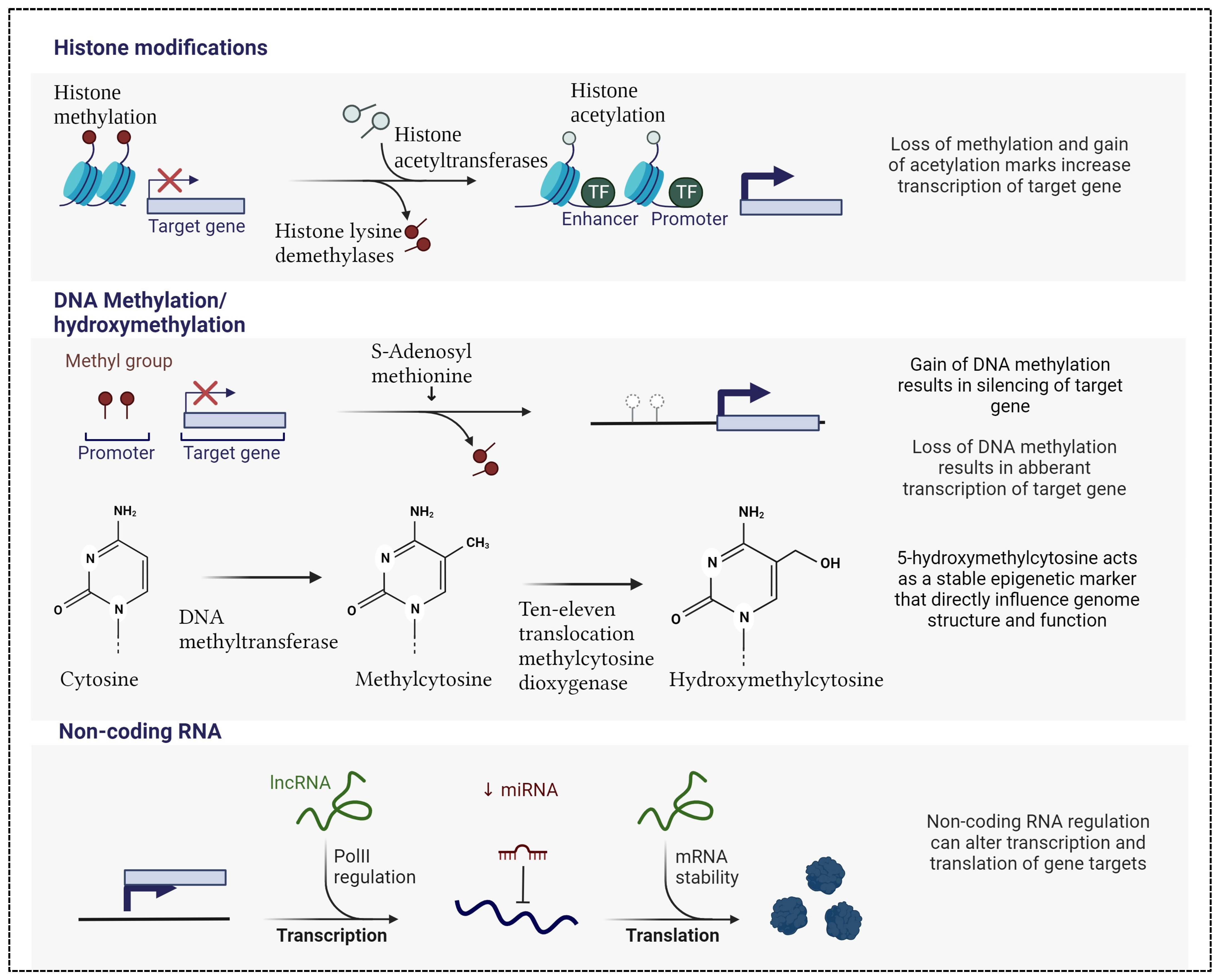
3. Harmony of Change: Exploring Epigenetic Variations and Their Influence on Gene Expression Modulation
4. Unraveling the Impact of Epigenetic Landscape in Hidradenitis Suppurativa
4.1. The Role of DNA Methylation
4.2. Investigating the Role of Histone Acetylation
4.3. The Role of microRNAs and Long Non-Coding RNA
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, Ł.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis Suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Jfri, A.; Nassim, D.; O’Brien, E.; Gulliver, W.; Nikolakis, G.; Zouboulis, C.C. Prevalence of Hidradenitis Suppurativa. JAMA Dermatol. 2021, 157, 924. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.; Willems, A.; Sinclair, R.D. Hidradenitis Suppurativa: An up-to-Date Review of Clinical Features, Pathogenesis and Therapeutic Approaches. Wound Pract. Res. 2022, 30, 40–49. [Google Scholar] [CrossRef]
- Garg, A.; Neuren, E.; Cha, D.; Kirby, J.S.; Ingram, J.R.; Jemec, G.B.E.; Esmann, S.; Thorlacius, L.; Villumsen, B.; del Marmol, V.; et al. Evaluating Patients’ Unmet Needs in Hidradenitis Suppurativa: Results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J. Am. Acad. Dermatol. 2020, 82, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Hallock, K.K.; Mizerak, M.R.; Dempsey, A.; MacZuga, S.; Kirby, J.S. Differences Between Children and Adults With Hidradenitis Suppurativa. JAMA Dermatol. 2021, 157, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Liy-Wong, C.; Kim, M.; Kirkorian, A.Y.; Eichenfield, L.F.; Diaz, L.Z.; Horev, A.; Tollefson, M.; Oranges, T.; Philips, R.; Chiu, Y.E.; et al. Hidradenitis Suppurativa in the Pediatric Population: An International, Multicenter, Retrospective, Cross-Sectional Study of 481 Pediatric Patients. JAMA Dermatol. 2021, 157, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Moltrasio, C.; Tricarico, P.M.; Romagnuolo, M.; Marzano, A.V.; Crovella, S. Hidradenitis Suppurativa: A Perspective on Genetic Factors Involved in the Disease. Biomedicines 2022, 10, 2039. [Google Scholar] [CrossRef] [PubMed]
- Moltrasio, C.; Romagnuolo, M.; Marzano, A.V. Epigenetic Mechanisms of Epidermal Differentiation. Int. J. Mol. Sci. 2022, 23, 4874. [Google Scholar] [CrossRef]
- Vișan, M.A.; Căruntu, C.; Costache, R.S.; Tiplica, S.G.; Costache, D.O. Hidradenitis Suppurativa: Detangling Phenotypes and Identifying Common Denominators. J. Eur. Acad. Dermatol. Venereol. 2023, 38, 62–76. [Google Scholar] [CrossRef]
- Gibson, F.; Hanly, A.; Grbic, N.; Grunberg, N.; Wu, M.; Collard, M.; Alani, R.M. Epigenetic Dysregulation in Autoimmune and Inflammatory Skin Diseases. Clin. Rev. Allergy Immunol. 2022, 63, 447–471. [Google Scholar] [CrossRef]
- Radhakrishna, U.; Ratnamala, U.; Jhala, D.D.; Uppala, L.V.; Vedangi, A.; Patel, M.; Vadsaria, N.; Shah, S.; Saiyed, N.; Rawal, R.M.; et al. Hidradenitis Suppurativa Presents a Methylome Dysregulation Capable to Explain the Pro-Inflammatory Microenvironment: Are These DNA Methylations Potential Therapeutic Targets? J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Dopytalska, K.; Czaplicka, A.; Szymańska, E.; Walecka, I. The Essential Role of MicroRNAs in Inflammatory and Autoimmune Skin Diseases—A Review. Int. J. Mol. Sci. 2023, 24, 9130. [Google Scholar] [CrossRef] [PubMed]
- Lukac, D.; Pagani, K.; Collender, P.A.; Fadadu, R.P.; Cardenas, A.; McGee, J.S. Increased Epigenetic Age Acceleration in the Hidradenitis Suppurativa Skin. Arch. Dermatol. Res. 2023, 315, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.D. DNA Methylation and Human Disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Gambichler, T.; Skrygan, M.; Sand, M.; Rüddel, I.; Scholl, L.; Bechara, F.G. Reduced Ten-Eleven Translocation and Isocitrate Dehydrogenase Expression in Inflammatory Hidradenitis Suppurativa Lesions. Eur. J. Dermatol. 2018, 28, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, K.; Shen, Q.; Han, Y.; Gu, Y.; Li, X.; Zhao, D.; Liu, Y.; Wang, C.; Zhang, X.; et al. Tet2 Is Required to Resolve Inflammation by Recruiting Hdac2 to Specifically Repress IL-6. Nature 2015, 525, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Shi, Y.G. Tet Family Proteins and 5-Hydroxymethylcytosine in Development and Disease. Development 2012, 139, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. The Histone Modification Code in the Pathogenesis of Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 2608605. [Google Scholar] [CrossRef]
- Green, D.; Dalmay, T.; Chapman, T. Microguards and Micromessengers of the Genome. Heredity 2016, 116, 125–134. [Google Scholar] [CrossRef]
- Saliminejad, K.; Reza Khorram Khorshid, H.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell. Physiol. 2018, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Ialongo, A.; Yeh, S.-Y.; Rhee, H.S. Crosstalk between Chromatin, Chromosomes, and Epigenetics. Biochem. Cell Biol. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Reddy, P.H. Are Circulating MicroRNAs Peripheral Biomarkers for Alzheimer’s Disease? Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Baylin, S.B. The Fundamental Role of Epigenetic Events in Cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic Regulation in Cardiovascular Disease: Mechanisms and Advances in Clinical Trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Feng, X.; He, T.; Wu, Y.; He, T.; Yue, Z.; Zhou, W. Discussion on Structure Classification and Regulation Function of Histone Deacetylase and Their Inhibitor. Chem. Biol. Drug Des. 2023, 103, e14366. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A. Epigenetic Regulation of Epidermal Development and Keratinocyte Differentiation. J. Investig. Dermatol. Symp. Proc. 2015, 17, 18–19. [Google Scholar] [CrossRef]
- Hessam, S.; Sand, M.; Lang, K.; Käfferlein, H.U.; Scholl, L.; Gambichler, T.; Skrygan, M.; Brüning, T.; Stockfleth, E.; Bechara, F.G. Altered Global 5-Hydroxymethylation Status in Hidradenitis Suppurativa: Support for an Epigenetic Background. Dermatology 2017, 233, 129–135. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, S.; Liu, J.; Zhang, L.; Guo, X.; Lu, J.; Li, L.; Yang, C.; Fu, Q.; Zeng, B. Epigenetic Regulation of Programmed Cell Death in Hypoxia-Induced Pulmonary Arterial Hypertension. Front. Immunol. 2023, 14, 1206452. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and Gene Expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Brunmeir, R.; Lagger, S.; Seiser, C. Histone Deacetylase 1 and 2-Controlled Embryonic Development and Cell Differentiation. Int. J. Dev. Biol. 2009, 53, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Jin, L.; Gallagher, S.; Mijatov, B.; Zhang, X.D.; Hersey, P. Histone Deacetylases (HDACs) as Mediators of Resistance to Apoptosis in Melanoma and as Targets for Combination Therapy with Selective BRAF Inhibitors. Adv. Pharmacol. 2012, 65, 27–43. [Google Scholar] [CrossRef] [PubMed]
- LeBoeuf, M.; Terrell, A.; Trivedi, S.; Sinha, S.; Epstein, J.A.; Olson, E.N.; Morrisey, E.E.; Millar, S.E. Hdac1 and Hdac2 Act Redundantly to Control P63 and P53 Functions in Epidermal Progenitor Cells. Dev. Cell 2010, 19, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ponandai-Srinivasan, S.; Nandakumar, K.S.; Fabre, S.; Xu Landén, N.; Mavon, A.; Khmaladze, I. Targeting MicroRNA for Improved Skin Health. Health Sci. Rep. 2021, 4, e374. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Li, C. ΔNp63α-Mediated Epigenetic Regulation in Keratinocyte Senescence. Epigenetics 2023, 18, 2173931. [Google Scholar] [CrossRef] [PubMed]
- Mervis, J.S.; McGee, J.S. DNA Methylation and Inflammatory Skin Diseases. Arch. Dermatol. Res. 2020, 312, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Horvath, C.M. The Jak-STAT Pathway Stimulated by Interferon Gamma. Sci. STKE 2004, 2004, tr8-tr8. [Google Scholar]
- Bendickova, K.; Fric, J. Roles of IL-2 in Bridging Adaptive and Innate Immunity, and as a Tool for Cellular Immunotherapy. J. Leukoc. Biol. 2020, 108, 427–437. [Google Scholar] [CrossRef]
- Balato, A.; Caiazzo, G.; Annunziata, M.C.; Marasca, C.; Scala, E.; Cacciapuoti, S.; Fabbrocini, G. Anti-TNF-α Therapy Modulates MTORC1 Signalling in Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e43–e45. [Google Scholar] [CrossRef]
- Nomura, T. Hidradenitis Suppurativa as a Potential Subtype of Autoinflammatory Keratinization Disease. Front. Immunol. 2020, 11, 847. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Ashrafzadeh, S.; Kim, Y.; Peters, G.A.; Lee, H.; Asgari, M.M. Risk of Keratinocyte Carcinoma among Patients with Hidradenitis Suppurativa. Br. J. Dermatol. 2020, 183, 962–964. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Zhai, W.; Zhang, X.; Li, L.; Yuan, C.; Li, Y.; Li, Y.; Yan, Y.; Wang, B.; et al. Reduced Expression of CXCL16/CXCR6 Is Involved in the Pathogenesis of Hidradenitis Suppurativa. Exp. Dermatol. 2023, 32, 359–367. [Google Scholar] [CrossRef]
- Tohyama, M.; Sayama, K.; Komatsuzawa, H.; Hanakawa, Y.; Shirakata, Y.; Dai, X.; Yang, L.; Tokumaru, S.; Nagai, H.; Hirakawa, S.; et al. CXCL16 Is a Novel Mediator of the Innate Immunity of Epidermal Keratinocytes. Int. Immunol. 2007, 19, 1095–1102. [Google Scholar] [CrossRef]
- Radhakrishna, U.; Ratnamala, U.; Jhala, D.D.; Uppala, L.V.; Vedangi, A.; Saiyed, N.; Patel, M.; Vadsaria, N.; Shah, S.R.; Rawal, R.M.; et al. Hidradenitis suppurativa associated telomere-methylome dysregulations in blood. J. Eur. Acad. Dermatol. Venereol. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Radhakrishna, U.; Ratnamala, U.; Jhala, D.D.; Vadsaria, N.; Patel, M.; Uppala, L.V.; Vishweswaraiah, S.; Vedangi, A.; Saiyed, N.; Damiani, G.; et al. Methylated MiRNAs May Serve as Potential Biomarkers and Therapeutic Targets for Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2199–2213. [Google Scholar] [CrossRef]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA Approval Summary: Vorinostat for Treatment of Advanced Primary Cutaneous T-Cell Lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, S.; Lai, Y.; Wang, L. Topical Histone Deacetylase 1 Inhibitor Entinostat Ameliorates Psoriasiform Dermatitis through Suppression of IL-17A Response. J. Dermatol. Sci. 2023, 110, 89–98. [Google Scholar] [CrossRef]
- Choo, Q.Y.; Ho, P.C.; Tanaka, Y.; Lin, H.S. Histone Deacetylase Inhibitors MS-275 and SAHA Induced Growth Arrest and Suppressed Lipopolysaccharide-Stimulated NF-ΚB P65 Nuclear Accumulation in Human Rheumatoid Arthritis Synovial Fibroblastic E11 Cells. Rheumatology 2010, 49, 1447–1460. [Google Scholar] [CrossRef]
- Leoni, F.; Zaliani, A.; Bertolini, G.; Porro, G.; Pagani, P.; Pozzi, P.; Donà, G.; Fossati, G.; Sozzani, S.; Azam, T.; et al. The Antitumor Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid Exhibits Antiinflammatory Properties via Suppression of Cytokines. Proc. Natl. Acad. Sci. USA 2002, 99, 2995–3000. [Google Scholar] [CrossRef]
- Reilly, C.M.; Mishra, N.; Miller, J.M.; Joshi, D.; Ruiz, P.; Richon, V.M.; Marks, P.A.; Gilkeson, G.S. Modulation of Renal Disease in MRL/Lpr Mice by Suberoylanilide Hydroxamic Acid. J. Immunol. 2004, 173, 4171–4178. [Google Scholar] [CrossRef]
- Samuelov, L.; Bochner, R.; Magal, L.; Malovitski, K.; Sagiv, N.; Nousbeck, J.; Keren, A.; Fuchs-Telem, D.; Sarig, O.; Gilhar, A.; et al. Vorinostat, a Histone Deacetylase Inhibitor, as a Potential Novel Treatment for Psoriasis. Exp. Dermatol. 2022, 31, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Sand, M.; Skrygan, M.; Gambichler, T.; Bechara, F.G. Expression of MiRNA-155, MiRNA-223, MiRNA-31, MiRNA-21, MiRNA-125b, and MiRNA-146a in the Inflammatory Pathway of Hidradenitis Suppurativa. Inflammation 2017, 40, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Sand, M.; Skrygan, M.; Gambichler, T.; Bechara, F.G. Inflammation Induced Changes in the Expression Levels of Components of the MicroRNA Maturation Machinery Drosha, Dicer, Drosha Co-Factor DGRC8 and Exportin-5 in Inflammatory Lesions of Hidradenitis Suppurativa Patients. J. Dermatol. Sci. 2016, 82, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Sand, M.; Skrygan, M.; Bechara, F.G. The MicroRNA Effector RNA-Induced Silencing Complex in Hidradenitis Suppurativa: A Significant Dysregulation within Active Inflammatory Lesions. Arch. Dermatol. Res. 2017, 309, 557–565. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Montanino, C.; Mallardo, M.; Babino, G.; Mattera, E.; Ragozzino, G.; Argenziano, G.; Daniele, A.; Nigro, E. Circulating MicroRNAs in Hidradenitis Suppurativa. Genes 2022, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, C.; Xu, H.; Duan, Z.; Liu, Y.; Zeng, R.; Li, M.; Wang, B. AKT-Dependent Hyperproliferation of Keratinocytes in Familial Hidradenitis Suppurativa with a NCSTN Mutation: A Potential Role of Defective MiR-100-5p. Br. J. Dermatol. 2020, 182, 500–502. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, H.; Li, C.; Zhang, X.; Zhou, P.; Xiao, X.; Zhang, W.; Wu, Y.; Zeng, R.; Wang, B. Nicastrin/miR-30a-3p/RAB31 Axis Regulates Keratinocyte Differentiation by Impairing EGFR Signaling in Familial Acne Inversa. J. Investig. Dermatol. 2019, 139, 124–134. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Moltrasio, C.; Tricarico, P.M.; Moura, R.R.; Brandão, L.; Crovella, S.; Marzano, A.V. Clinical and Molecular Characterization of Hidradenitis Suppurativa: A Practical Framework for Novel Therapeutic Targets. Dermatology 2023, 239, 836–839. [Google Scholar] [CrossRef]
- Brandão, L.A.C.; Tricarico, P.M.; Gratton, R.; Agrelli, A.; Zupin, L.; Abou-Saleh, H.; Moura, R.; Crovella, S. Multiomics Integration in Skin Diseases with Alterations in Notch Signaling Pathway: PlatOMICs Phase 1 Deployment. Int. J. Mol. Sci. 2021, 22, 1523. [Google Scholar] [CrossRef]
- Sun, Q.; Broadaway, K.A.; Edmiston, S.N.; Fajgenbaum, K.; Miller-Fleming, T.; Westerkam, L.L.; Melendez-Gonzalez, M.; Bui, H.; Blum, F.R.; Levitt, B.; et al. Genetic Variants Associated With Hidradenitis Suppurativa. JAMA Dermatol. 2023, 159, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Li, X.; Lee, H.S.; Kim, K.; Chaparala, V.; Murphy, W.; Zhou, W.; Cao, J.; Lowes, M.A.; et al. Single-Cell Transcriptomics Suggest Distinct Upstream Drivers of IL-17A/F in Hidradenitis versus Psoriasis. J. Allergy Clin. Immunol. 2023, 152, 656–666. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.S.L.E.; Bloise, G.; Moltrasio, C.; Coelho, A.; Agrelli, A.; Moura, R.; Tricarico, P.M.; Jamain, S.; Marzano, A.V.; Crovella, S.; et al. Transcriptome Meta-Analysis Confirms the Hidradenitis Suppurativa Pathogenic Triad: Upregulated Inflammation, Altered Epithelial Organization, and Dysregulated Metabolic Signaling. Biomolecules 2022, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Navrazhina, K.; Garcet, S.; Frew, J.W.; Zheng, X.; Coats, I.; Guttman-Yassky, E.; Krueger, J.G. The Inflammatory Proteome of Hidradenitis Suppurativa Skin Is More Expansive than That of Psoriasis Vulgaris. J. Am. Acad. Dermatol. 2022, 86, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Moltrasio, C.; Gradišek, A.; Marzano, A.V.; Flacher, V.; Boufenghour, W.; von Stebut, E.; Schmuth, M.; Jaschke, W.; Gams, M.; et al. Holistic Health Record for Hidradenitis Suppurativa Patients. Sci. Rep. 2022, 12, 8415. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Boniotto, M.; Genovese, G.; Zouboulis, C.C.; Marzano, A.V.; Crovella, S. An Integrated Approach to Unravel Hidradenitis Suppurativa Etiopathogenesis. Front. Immunol. 2019, 10, 457712. [Google Scholar] [CrossRef]
- Jin, L.; Chen, Y.; Muzaffar, S.; Li, C.; Mier-Aguilar, C.A.; Khan, J.; Kashyap, M.P.; Liu, S.; Srivastava, R.; Deshane, J.S.; et al. Epigenetic switch reshapes epithelial progenitor cell signatures and drives inflammatory pathogenesis in hidradenitis suppurativa. Proc. Natl. Acad. Sci. USA 2023, 120, e2315096120. [Google Scholar] [CrossRef]


| Peripheral Blood Leukocytes | ||
|---|---|---|
| miRNA | Effect | Reference |
miRNA-24-1-5p  | Inhibition of interferon gamma expression | [56] |
miRNA-26a-5p  | Effects on cell proliferation, apoptosis, migration and interferon gamma pathway | [56] |
miRNA-206  | Effects on inflammation mediated by chemokine and cytokine signaling pathway; insulin/IGF pathway-mitogen activated protein kinase kinase/MAP kinase cascade; interferon gamma pathway | [56] |
miRNA-338-3p  | Dysfunction of regulatory T cells | [56] |
miRNA-146a-5p  | Effects on inflammation mediated by chemokine and cytokine signaling pathway; interferon gamma pathway | [56] |
| HS lesional skin | ||
miRNA-125b-5p  | Inhibition of TNF-alpha, regulating the differentiation and proliferation of keratinocytes through its inhibitory effect on the FGFR2 | [53] |
miRNA-100-5b  | Effects on cellular self-renewal and wound healing | [57] |
miRNA-30a-3p  | In the presence of NCSTN mutations and RAB31, aberrant keratinocytes differentiation | [58] |
miRNA-146a-5p  | Effects on TRAF6/IRAK-1 and inhibition of TNF-alpha production | [53] |
miRNA-21-5p  | Effects on T cell-derived skin inflammation | [53] |
miRNA-31-5p  | Overexpression of proinflammatory mediators in keratinocytes | [53] |
miRNA-155-5p  | Effects on Th17 cells differentiation and function; keratinocyte proliferation and differentiation; apoptosis; overexpression of TNF-alpha | [53] |
miRNA-223-5p  | Effects on proliferation of progenitors and differentiation and activation of granulocytes | [53] |
miRNA-338-5p  | Stimulation of cell proliferation, invasion, and the production of cytokines IL-1a, IL-6, and COX2 | [56] |
| Whole blood | ||
| miRNA-200 family— altered methylation | Effects on wound healing and regeneration | [46] |
| miRNA-132 family— altered methylation | Upregulation during the inflammation phase of wound repair | [46] |
| miRNA-548 family— altered methylation | Effects on cellular viability, proliferation and migration | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardacchione, E.M.; Tricarico, P.M.; Moura, R.; d’Adamo, A.P.; Thasneem, A.; Suleman, M.; Marzano, A.V.; Crovella, S.; Moltrasio, C. Unraveling the Epigenetic Tapestry: Decoding the Impact of Epigenetic Modifications in Hidradenitis Suppurativa Pathogenesis. Genes 2024, 15, 38. https://doi.org/10.3390/genes15010038
Nardacchione EM, Tricarico PM, Moura R, d’Adamo AP, Thasneem A, Suleman M, Marzano AV, Crovella S, Moltrasio C. Unraveling the Epigenetic Tapestry: Decoding the Impact of Epigenetic Modifications in Hidradenitis Suppurativa Pathogenesis. Genes. 2024; 15(1):38. https://doi.org/10.3390/genes15010038
Chicago/Turabian StyleNardacchione, Elena Maria, Paola Maura Tricarico, Ronald Moura, Adamo Pio d’Adamo, Ayshath Thasneem, Muhammad Suleman, Angelo Valerio Marzano, Sergio Crovella, and Chiara Moltrasio. 2024. "Unraveling the Epigenetic Tapestry: Decoding the Impact of Epigenetic Modifications in Hidradenitis Suppurativa Pathogenesis" Genes 15, no. 1: 38. https://doi.org/10.3390/genes15010038
APA StyleNardacchione, E. M., Tricarico, P. M., Moura, R., d’Adamo, A. P., Thasneem, A., Suleman, M., Marzano, A. V., Crovella, S., & Moltrasio, C. (2024). Unraveling the Epigenetic Tapestry: Decoding the Impact of Epigenetic Modifications in Hidradenitis Suppurativa Pathogenesis. Genes, 15(1), 38. https://doi.org/10.3390/genes15010038









