Transcriptome Profiling Identifies Differentially Expressed Genes in Skeletal Muscle Development in Native Chinese Ducks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Ethics Statement
2.2. RNA Extraction, Library Construction and Sequencing
2.3. Quality Evaluation and Alignment Analysis
2.4. Interaction Network of DEGs
2.5. Transcript Assembly, Differentially Expressed Gene and Enrichment Analysis
2.6. Verification of Results by qRT-PCR
3. Results
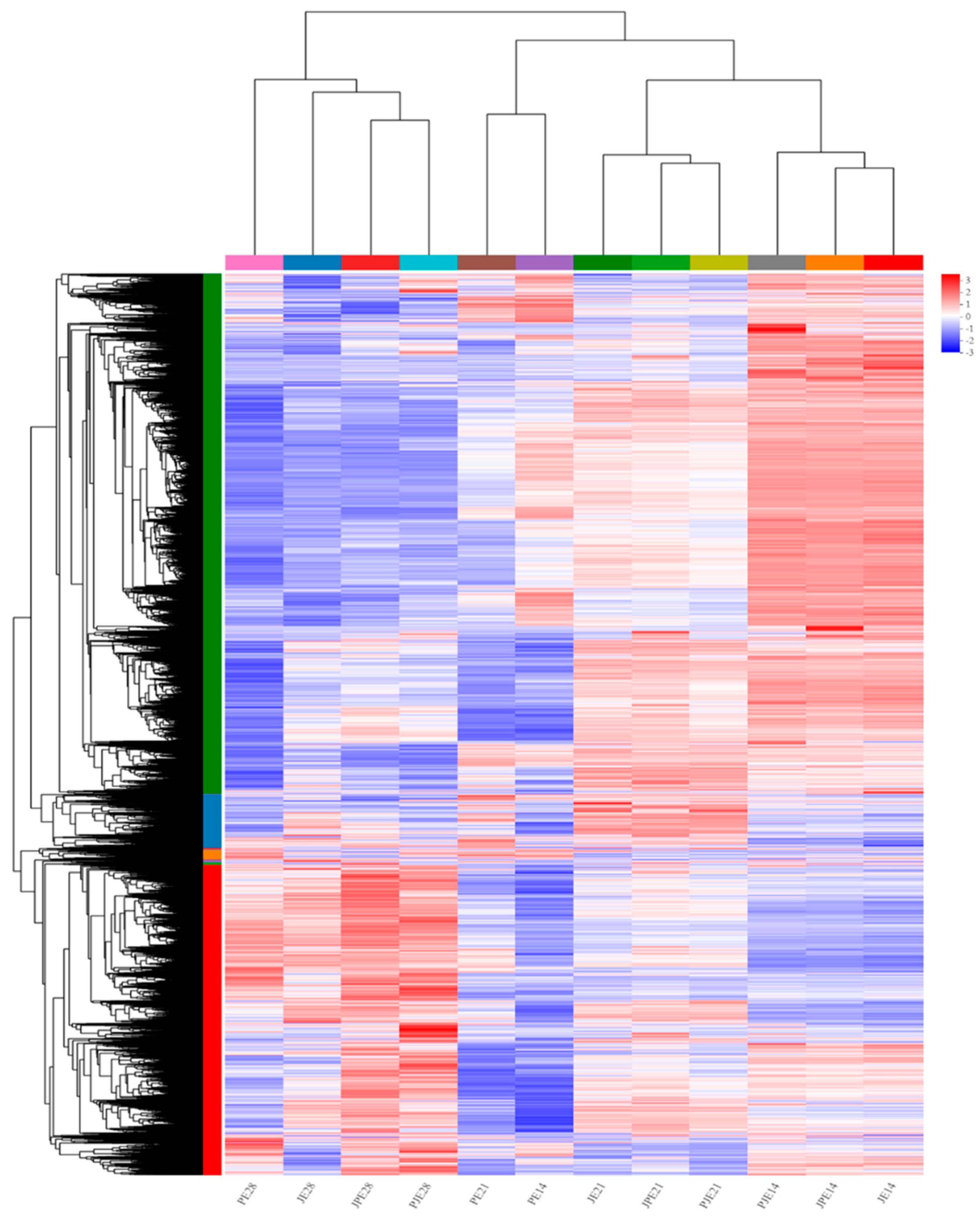
3.1. Transcriptomic Screening and Analysis of Duck Breast Muscle Tissue
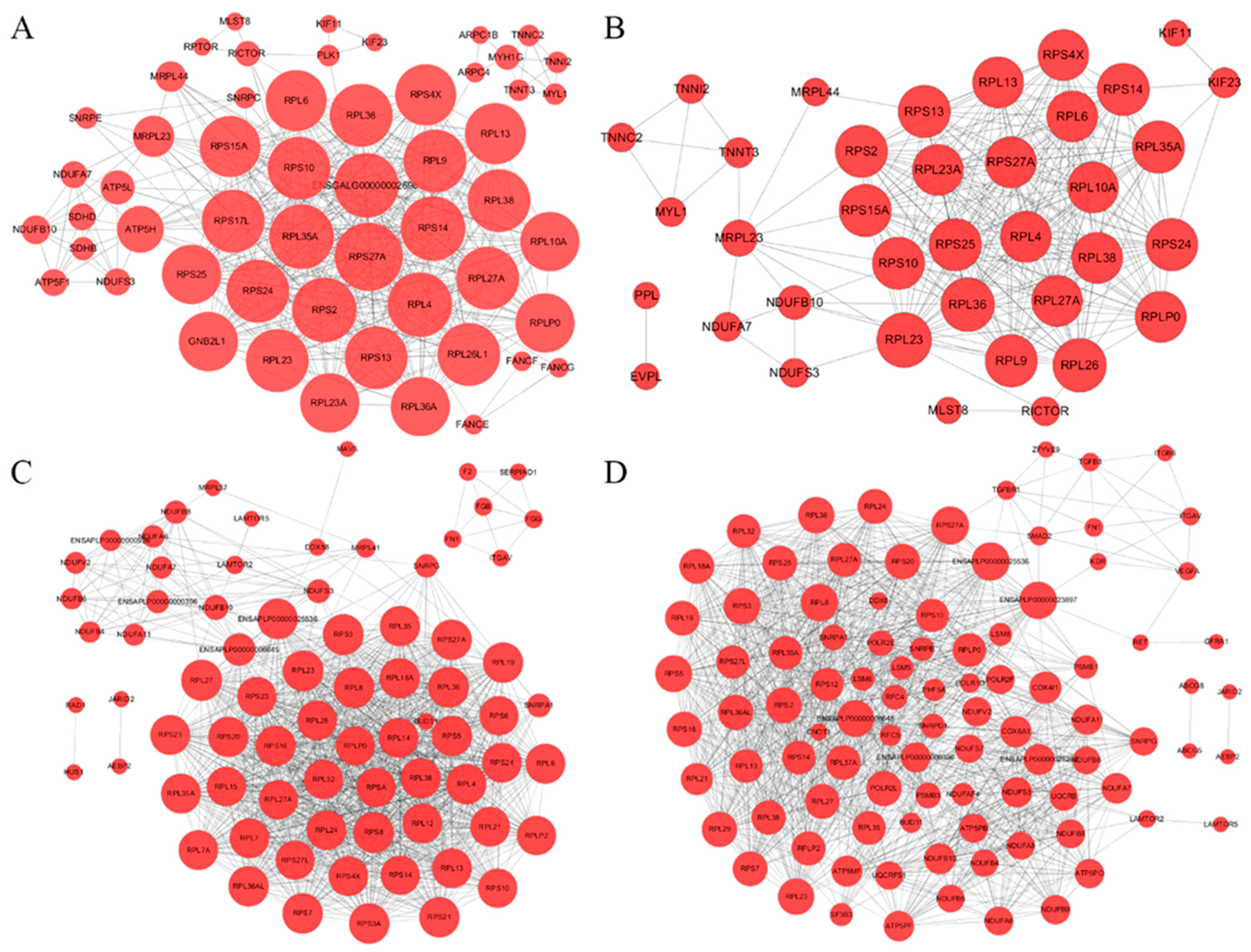
3.2. Protein-Protein Interaction Network Predictive Analysis
3.3. DEG Screening for the Early Development of Ducks
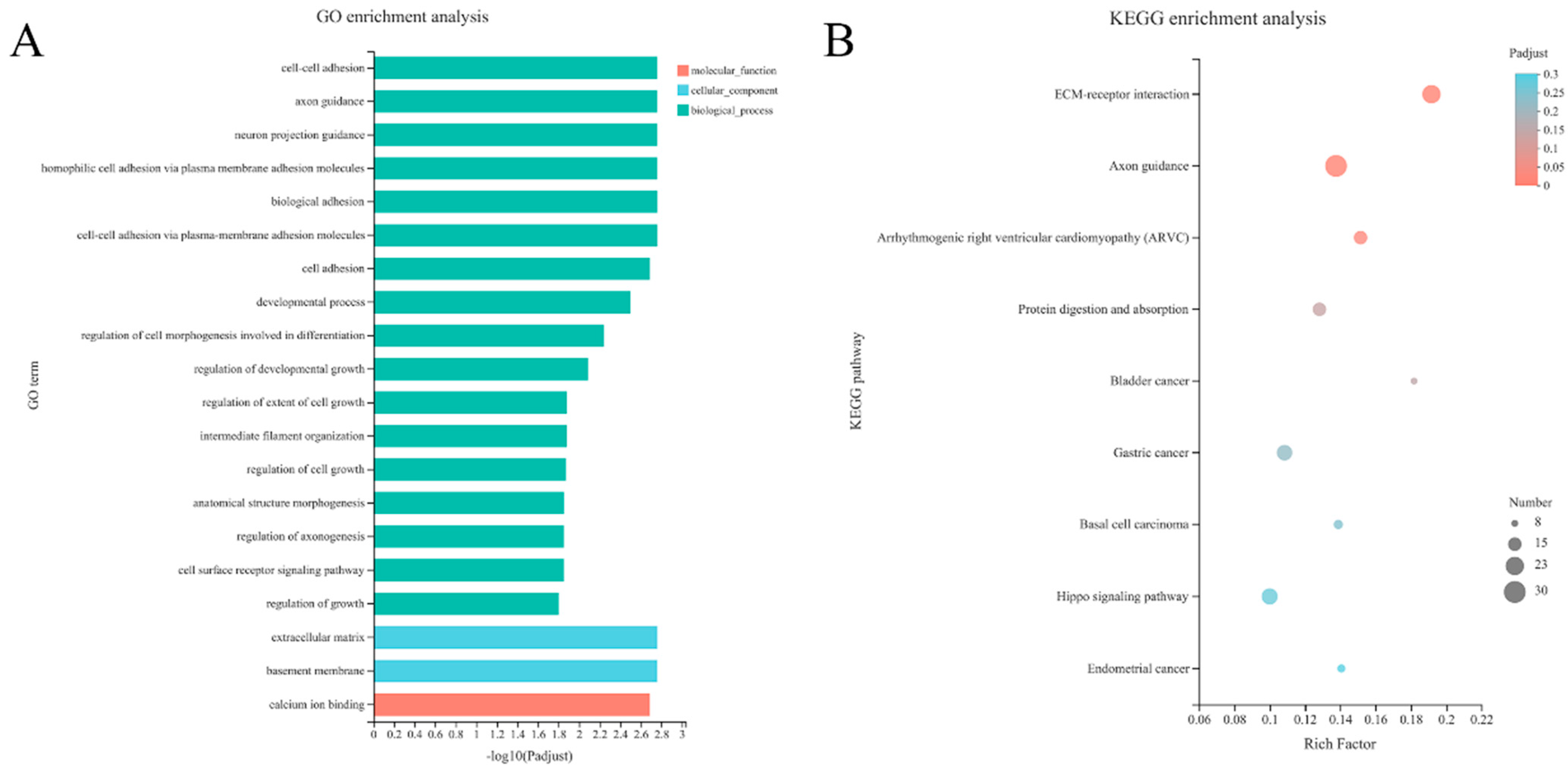
3.4. GO Enrichment and KEGG Function Analysis
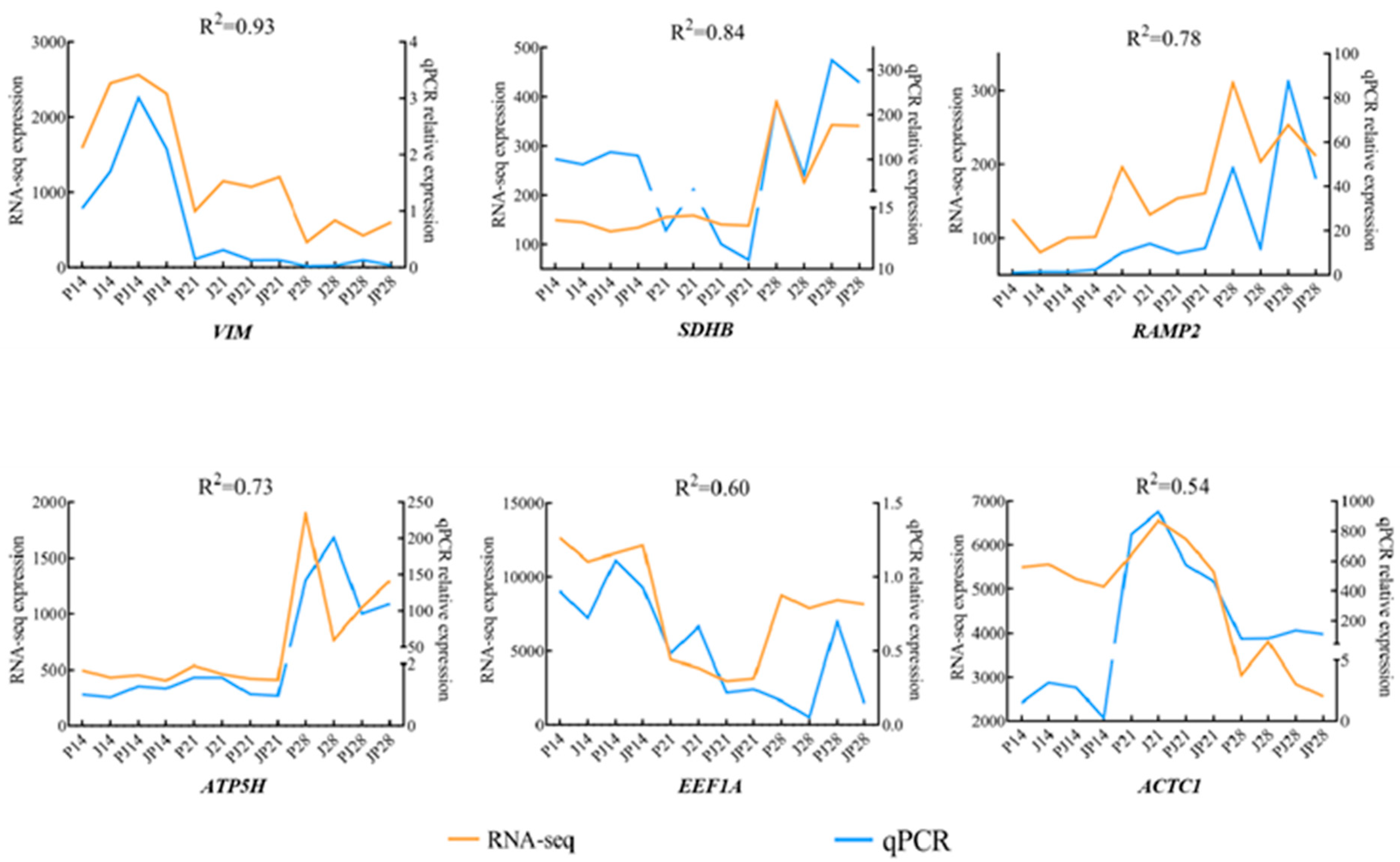
3.5. Validation of RNA-Seq Data Using qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Relaix, F.; Rocancourt, D.; Mansouri, A.; Buckingham, M. A Pax3/Pax7-Dependent Population of Skeletal Muscle Progenitor Cells. Nature 2005, 435, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Hirsinger, E.; Malapert, P.; Dubrulle, J.; Delfini, M.-C.; Duprez, D.; Henrique, D.; Ish-Horowicz, D.; Pourquié, O. Notch Signalling Acts in Postmitotic Avian Myogenic Cells to Control MyoD Activation. Development 2001, 128, 107–116. [Google Scholar] [CrossRef]
- Hu, Z.; Cao, J.; Ge, L.; Zhang, J.; Zhang, H.; Liu, X. Characterization and Comparative Transcriptomic Analysis of Skeletal Muscle in Pekin Duck at Different Growth Stages Using Rna-Seq. Animals 2021, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic Regulatory Factors: The Orchestrators of Myogenesis after 30 Years of Discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.H.; Xu, T.S.; Huang, W.; Xie, M.; Shi, W.B.; Sun, S.D.; Hou, S.S. Developmental Characteristics of Pectoralis Muscle in Pekin Duck Embryos. Genet. Mol. Res. 2013, 12, 6733–6742. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, C.; Tao, Z.; Xu, W.; Song, W.; Hu, Y.; Zhu, W.; Song, C. MyoD and Myf6 Gene Expression Patterns in Skeletal Muscle during Embryonic and Posthatch Development in the Domestic Duck (Anas Platyrhynchos Domestica). J. Anim. Breed. Genet. 2014, 131, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gi, G.; Tao, Z.; Song, C.; Zhu, W.; Song, W.; Li, H. Development of Skeletal Muscle and Expression of Myogenic Regulatory Factors during Embryonic Development in Jinding Ducks (Anas Platyrhynchos Domestica). Poult. Sci. 2014, 93, 1211–1216. [Google Scholar] [CrossRef]
- Abmayr, S.M.; Pavlath, G.K. Myoblast Fusion: Lessons from Flies and Mice. Development 2012, 139, 641–656. [Google Scholar] [CrossRef]
- Xu, T.-S.; Gu, L.-H.; Huang, W.; Xia, W.-L.; Zhang, Y.-S.; Zhang, Y.-G.; Rong, G.; Schachtschneider, K.; Hou, S.-S. Gene Expression Profiling in Pekin Duck Embryonic Breast Muscle. PLoS ONE 2017, 12, e0174612. [Google Scholar] [CrossRef]
- Shu, J.; Li, H.; Shan, Y.; Xu, W.; Chen, W.; Song, C.; Song, W. Expression Profile of IGF-I-Calcineurin-NFATc3-Dependent Pathway Genes in Skeletal Muscle during Early Development between Duck Breeds Differing in Growth Rates. Dev. Genes. Evol. 2015, 225, 139–148. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Garber, M.; Grabherr, M.G.; Guttman, M.; Trapnell, C. Computational Methods for Transcriptome Annotation and Quantification Using RNA-Seq. Nat. Methods 2011, 8, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-P.; Liu, H.-H.; Liu, J.-Y.; Hu, J.-W.; Yan, X.-P.; Wang, D.-M.-C.; Li, L.; Wang, J.-W. Transcriptional Profiling Identifies Location-Specific and Breed-Specific Differentially Expressed Genes in Embryonic Myogenesis in Anas Platyrhynchos. PLoS ONE 2015, 10, e0143378. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate Alignment of Transcriptomes in the Presence of Insertions, Deletions and Gene Fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Xu, T.; Huang, W.; Zhang, X.; Ye, B.; Zhou, H.; Hou, S. Identification and Characterization of Genes Related to the Development of Breast Muscles in Pekin Duck. Mol. Biol. Rep. 2012, 39, 7647–7655. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, Y.; Ma, J.; Hu, S.; Hu, J.; Hu, B.; Liu, H.; Li, L.; He, H.; Wang, J. Effects of Different Breeds/Strains on Fatty Acid Composition and Lipid Metabolism-Related Genes Expression in Breast Muscle of Ducks. Poult. Sci. 2022, 101, 101813. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tangara, M.; Xu, J.; Peng, J. Developmental Transition of Pectoralis Muscle from Atrophy in Late-Term Duck Embryos to Hypertrophy in Neonates: Muscle Development in Ducks. Exp. Physiol. 2012, 97, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Xu, T.; Huang, W.; Xie, M.; Sun, S.; Hou, S. Identification and Profiling of MicroRNAs in the Embryonic Breast Muscle of Pekin Duck. PLoS ONE 2014, 9, e86150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Song, W.; Tao, Z.; Liu, H.; Xu, W.; Zhang, S.; Li, H. Deep RNA Sequencing of Pectoralis Muscle Transcriptomes during Late-Term Embryonic to Neonatal Development in Indigenous Chinese Duck Breeds. PLoS ONE 2017, 12, e0180403. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.O.; Mills, S.T.; Pavlath, G.K. Calcineurin Differentially Regulates Maintenance and Growth of Phenotypically Distinct Muscles. Am. J. Physiol. Cell Physiol. 2002, 282, C984–C992. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial Proton and Electron Leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [PubMed]
- Brand-Saberi, B. Genetic and Epigenetic Control of Skeletal Muscle Development. Ann. Anat. Anat. Anz. 2005, 187, 199–207. [Google Scholar] [CrossRef]
- Stewart, C.E.H.; Rittweger, J. Adaptive Processes in Skeletal Muscle: Molecular Regulators and Genetic Influences. J. Musculoskelet. Neuronal Interact. 2006, 6, 73. [Google Scholar]
- Le Grand, F.; Rudnicki, M.A. Skeletal Muscle Satellite Cells and Adult Myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Te Pas, M.F.W.; Wimmers, K.; Brameld, J.M.; Nissen, P.M.; Berri, C.; Valente, L.M.P.; Power, D.M.; Picard, B.; Stickland, N.C. Advances in Research on the Prenatal Development of Skeletal Muscle in Animals in Relation to the Quality of Muscle-Based Food. I. Regulation of Myogenesis and Environmental Impact. Animal 2011, 5, 703–717. [Google Scholar] [CrossRef]
- Yin, H.; Li, D.; Wang, Y.; Zhao, X.; Liu, Y.; Yang, Z.; Zhu, Q. Myogenic Regulatory Factor (MRF) Expression Is Affected by Exercise in Postnatal Chicken Skeletal Muscles. Gene 2015, 561, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, D.; Dubińska-Magiera, M.; Migocka-Patrza\lek, M.; Niedbalska-Tarnowska, J.; Haczkiewicz-Leśniak, K.; Dzięgiel, P.; Daczewska, M. Everybody Wants to Move—Evolutionary Implications of Trunk Muscle Differentiation in Vertebrate Species. In Proceedings of the Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 104, pp. 3–13. [Google Scholar]
- Scicchitano, B.M.; Rizzuto, E.; Musarò, A. Counteracting Muscle Wasting in Aging and Neuromuscular Diseases: The Critical Role of IGF-1. Aging 2009, 1, 451. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Zhang, J.; Gao, B.; Bai, X.; Lan, Y.; Lin, P.; Guo, H.; Gao, Y.; Xing, B. Castration-Induced Changes in the Expression Profiles and Promoter Methylation of the GHR Gene in Huainan Male Pigs. Anim. Sci. J. 2017, 88, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Robaszkiewicz, K.; Ostrowska, Z.; Cyranka-Czaja, A.; Moraczewska, J. Impaired Tropomyosin–Troponin Interactions Reduce Activation of the Actin Thin Filament. Biochim. Biophys. Acta BBA Proteins Proteom. 2015, 1854, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, C.A.; Dalecki, D.; Hocking, D.C. Extracellular Matrix Fibronectin Stimulates the Self-Assembly of Microtissues on Native Collagen Gels. Tissue Eng. Part. A 2010, 16, 3805–3819. [Google Scholar] [CrossRef] [PubMed]
- Dalton, C.J.; Lemmon, C.A. Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells 2021, 10, 2443. [Google Scholar] [CrossRef] [PubMed]
- George, E.L.; Georges-Labouesse, E.N.; Patel-King, R.S.; Rayburn, H.; Hynes, R.O. Defects in Mesoderm, Neural Tube and Vascular Development in Mouse Embryos Lacking Fibronectin. Development 1993, 119, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Georges-Labouesse, E.N.; George, E.L.; Rayburn, H.; Hynes, R.O. Mesodermal Development in Mouse Embryos Mutant for Fibronectin. Dev. Dyn. 1996, 207, 145–156. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Xue, P.; Ma, X.; Li, J.; Zhang, J. FN1 Promotes Chondrocyte Differentiation and Collagen Production via TGF-β/PI3K/Akt Pathway in Mice with Femoral Fracture. Gene 2021, 769, 145253. [Google Scholar] [CrossRef]
- Chen, J.L.; Colgan, T.D.; Walton, K.L.; Gregorevic, P.; Harrison, C.A. The TGF-β Signalling Network in Muscle Development, Adaptation and Disease. In Growth Factors and Cytokines in Skeletal Muscle Development, Growth, Regeneration and Disease; White, J., Smythe, G., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 97–131. ISBN 978-3-319-27511-6. [Google Scholar]
- Yue, C.; Wang, J.; Shen, Y.; Zhang, J.; Liu, J.; Xiao, A.; Liu, Y.; Eer, H.; Zhang, Q. Whole-Genome DNA Methylation Profiling Reveals Epigenetic Signatures in Developing Muscle in Tan and Hu Sheep and Their Offspring. Front. Vet. Sci. 2023, 10, 1186040. [Google Scholar] [CrossRef]
- Wheatley, S.C.; Isacke, C.M.; Crossley, P.H. Restricted Expression of the Hyaluronan Receptor, CD44, during Postimplantation Mouse Embryogenesis Suggests Key Roles in Tissue Formation and Patterning. Development 1993, 119, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Abdullah, A.; Wendt, M.K.; Calve, S. Hyaluronic Acid, CD44 and RHAMM Regulate Myoblast Behavior during Embryogenesis. Matrix Biol. 2019, 78–79, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a Tumor Biomarker and Therapeutic Target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Rahman, M.A.; Nazim, M.; Nasrin, F.; Lin, Y.; Takeda, J.; Masuda, A. Splicing Regulation and Dysregulation of Cholinergic Genes Expressed at the Neuromuscular Junction. J. Neurochem. 2017, 142, 64–72. [Google Scholar] [CrossRef]
- Oury, J.; Zhang, W.; Leloup, N.; Koide, A.; Corrado, A.D.; Ketavarapu, G.; Hattori, T.; Koide, S.; Burden, S.J. Mechanism of Disease and Therapeutic Rescue of Dok7 Congenital Myasthenia. Nature 2021, 595, 404–408. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) Family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Dancevic, C.M.; McCulloch, D.R.; Ward, A.C. The ADAMTS Hyalectanase Family: Biological Insights from Diverse Species. Biochem. J. 2016, 473, 2011–2022. [Google Scholar] [CrossRef]
- Glickman, R.M.; Rogers, M.; Glickman, J.N. Apolipoprotein B Synthesis by Human Liver and Intestine in Vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 5296–5300. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, H.; Li, H. Cloning and Tissue Expression Characterization of the Chicken APOB Gene. Anim. Biotechnol. 2007, 18, 243–250. [Google Scholar] [CrossRef]
- Naruo, K.-I.; Seko, C.; Kuroshima, K.; Matsutani, E.; Sasada, R.; Kondo, T.; Kurokawa, T. Novel Secretory Heparin-Binding Factors from Human Glioma Cells (Glia-Activating Factors) Involved in Glial Cell Growth. Purification and Biological Properties. J. Biol. Chem. 1993, 268, 2857–2864. [Google Scholar] [CrossRef]
- Itoh, N.; Ohta, H.; Nakayama, Y.; Konishi, M. Roles of FGF Signals in Heart Development, Health, and Disease. Front. Cell Dev. Biol. 2016, 4, 110. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, K.; Shiflett, L.A.; Brotto, L.; Bonewald, L.F.; Wacker, M.J.; Dallas, S.L.; Brotto, M. Fibroblast Growth Factor 9 (FGF9) Inhibits Myogenic Differentiation of C2C12 and Human Muscle Cells. Cell Cycle 2019, 18, 3562–3580. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The Extracellular Matrix in Development and Morphogenesis: A Dynamic View. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix Nanotopography as a Regulator of Cell Function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef]
- Yang, H.S.; Lee, B.; Tsui, J.H.; Macadangdang, J.; Jang, S.; Im, S.G.; Kim, D. Electroconductive Nanopatterned Substrates for Enhanced Myogenic Differentiation and Maturation. Adv. Healthc. Mater. 2016, 5, 137–145. [Google Scholar] [CrossRef]
- Hirate, Y.; Hirahara, S.; Inoue, K.; Kiyonari, H.; Niwa, H.; Sasaki, H. Par- aPKC -dependent and -independent Mechanisms Cooperatively Control Cell Polarity, Hippo Signaling, and Cell Positioning in 16-cell Stage Mouse Embryos. Develop. Growth Differ. 2015, 57, 544–556. [Google Scholar] [CrossRef]

| Group 1 | Sample | Clean Reads 2 | Clean Bases (Gb) 3 | Q20 (%) 4 | Q30 (%) 5 | GC Content (%) 6 |
|---|---|---|---|---|---|---|
| J14 | 1 | 54,964,154 | 8.15 | 98.16 | 94.76 | 52.32 |
| 2 | 66,766,480 | 9.89 | 98.19 | 94.85 | 52.47 | |
| 3 | 55,963,296 | 8.28 | 98.23 | 94.91 | 52.24 | |
| J21 | 1 | 49,696,672 | 7.27 | 98.29 | 94.96 | 50.12 |
| 2 | 59,501,280 | 8.80 | 98.15 | 94.77 | 53.51 | |
| 3 | 63,014,954 | 9.32 | 98.16 | 94.80 | 53.69 | |
| J28 | 1 | 66,050,398 | 9.78 | 98.20 | 94.89 | 54.06 |
| 2 | 61,710,802 | 9.14 | 98.11 | 94.67 | 54.11 | |
| 3 | 62,580,976 | 9.26 | 98.22 | 94.92 | 53.48 | |
| JP14 | 1 | 58,135,548 | 8.61 | 98.11 | 94.66 | 53.24 |
| 2 | 59,868,580 | 8.90 | 98.18 | 94.82 | 52.97 | |
| 3 | 55,533,882 | 8.25 | 98.06 | 94.56 | 52.43 | |
| JP21 | 1 | 54,631,272 | 8.08 | 98.12 | 94.72 | 52.82 |
| 2 | 61,418,642 | 9.11 | 98.08 | 94.60 | 53.06 | |
| 3 | 58,556,596 | 8.68 | 98.15 | 94.68 | 52.89 | |
| JP28 | 1 | 58,369,278 | 8.67 | 98.17 | 94.79 | 52.64 |
| 2 | 53,300,948 | 7.91 | 98.09 | 94.59 | 52.49 | |
| 3 | 56,088,598 | 8.33 | 98.09 | 94.59 | 52.74 | |
| P14 | 1 | 46,643,606 | 6.90 | 98.10 | 94.66 | 52.85 |
| 2 | 49,462,680 | 7.28 | 98.23 | 95.03 | 53.73 | |
| 3 | 57,653,512 | 8.53 | 98.07 | 94.60 | 53.78 | |
| P21 | 1 | 54,928,900 | 8.12 | 98.02 | 94.46 | 53.18 |
| 2 | 49,144,136 | 7.26 | 98.07 | 94.60 | 54.26 | |
| 3 | 46,298,258 | 6.84 | 97.97 | 94.45 | 54.26 | |
| P28 | 1 | 52,808,292 | 7.80 | 98.09 | 94.66 | 53.29 |
| 2 | 49,559,050 | 7.31 | 97.99 | 94.39 | 53.52 | |
| 3 | 42,368,078 | 6.27 | 98.06 | 94.63 | 53.80 | |
| PJ14 | 1 | 49,781,428 | 7.39 | 98.07 | 94.65 | 52.82 |
| 2 | 57,148,852 | 8.47 | 98.10 | 94.59 | 52.48 | |
| 3 | 56,494,950 | 8.41 | 98.16 | 94.73 | 52.80 | |
| PJ21 | 1 | 60,868,366 | 9.06 | 98.14 | 94.71 | 53.55 |
| 2 | 58,061,018 | 8.62 | 98.00 | 94.43 | 53.52 | |
| 3 | 58,194,764 | 8.63 | 98.20 | 94.88 | 52.65 | |
| PJ28 | 1 | 55,547,062 | 8.22 | 98.16 | 94.82 | 54.27 |
| 2 | 52,360,940 | 7.75 | 98.19 | 94.87 | 53.51 | |
| 3 | 56,167,902 | 8.30 | 98.13 | 94.76 | 52.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lu, Y.; Yu, M.; Wang, J.; Du, X.; Zhao, D.; Pian, H.; He, Z.; Wu, G.; Li, S.; et al. Transcriptome Profiling Identifies Differentially Expressed Genes in Skeletal Muscle Development in Native Chinese Ducks. Genes 2024, 15, 52. https://doi.org/10.3390/genes15010052
Zhang Y, Lu Y, Yu M, Wang J, Du X, Zhao D, Pian H, He Z, Wu G, Li S, et al. Transcriptome Profiling Identifies Differentially Expressed Genes in Skeletal Muscle Development in Native Chinese Ducks. Genes. 2024; 15(1):52. https://doi.org/10.3390/genes15010052
Chicago/Turabian StyleZhang, Yuchen, Yinglin Lu, Minli Yu, Jin Wang, Xubin Du, Dong Zhao, Huifang Pian, Zongliang He, Guansuo Wu, Shiwei Li, and et al. 2024. "Transcriptome Profiling Identifies Differentially Expressed Genes in Skeletal Muscle Development in Native Chinese Ducks" Genes 15, no. 1: 52. https://doi.org/10.3390/genes15010052





