Proteomics Analysis to Explore the Resistance Genes of Silkworm to Bombyx mori Nuclear Polyhedrosis Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silkworm Varieties and Virus Strains
2.2. Virus Inoculation and Sampling
2.3. Detection of BmNPV Proliferation in the Hemolymph
2.4. Protein Extraction and 2-DE
2.5. RNA Extraction and qRT-PCR
3. Results
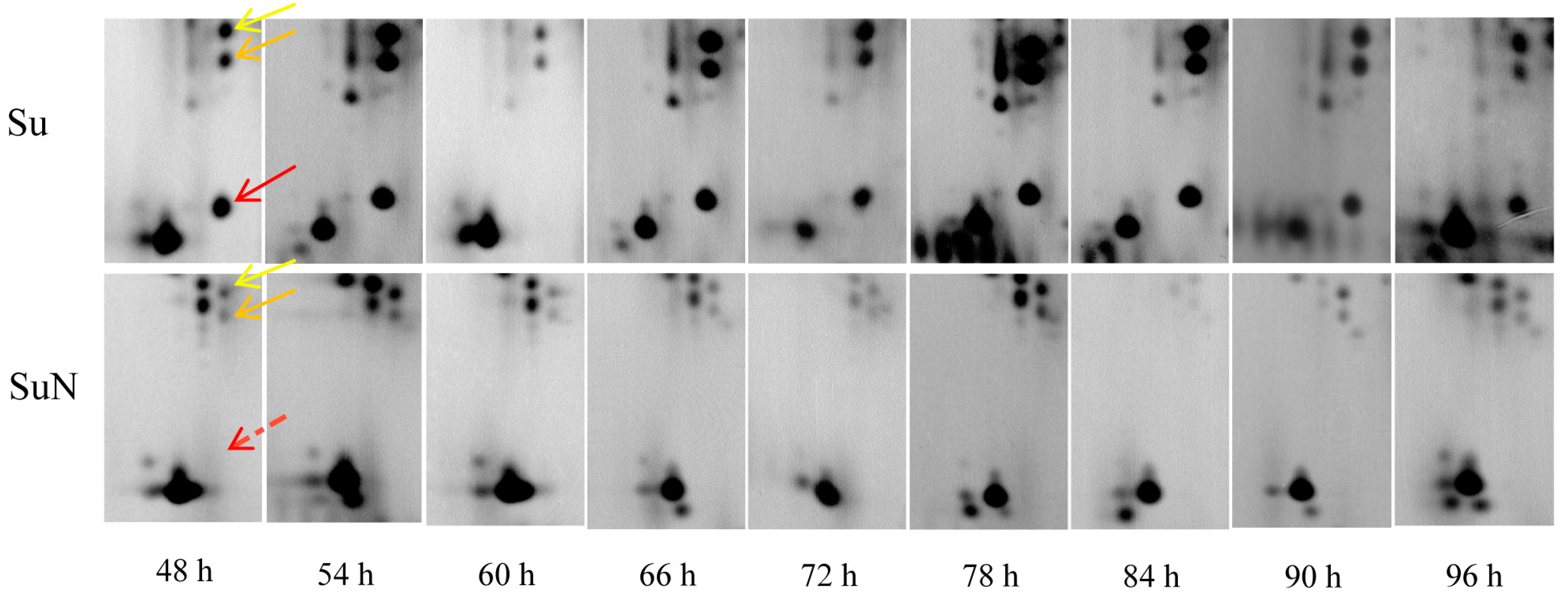
3.1. Midgut Protein of Su Was Degraded Greatly after BmNPV Inoculation
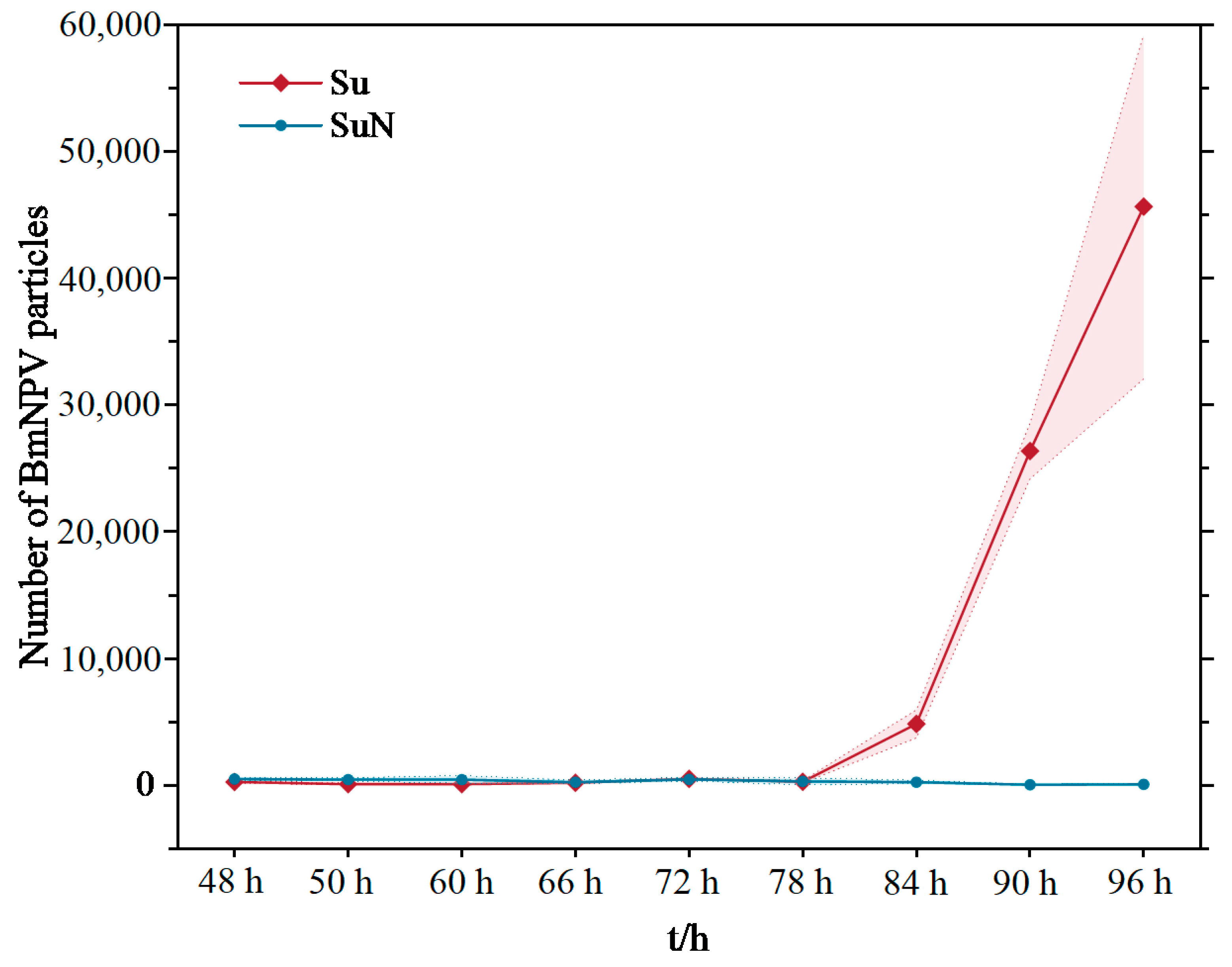
3.2. BmNPV Proliferated Rapidly in the Hemolymph of Su after Infection
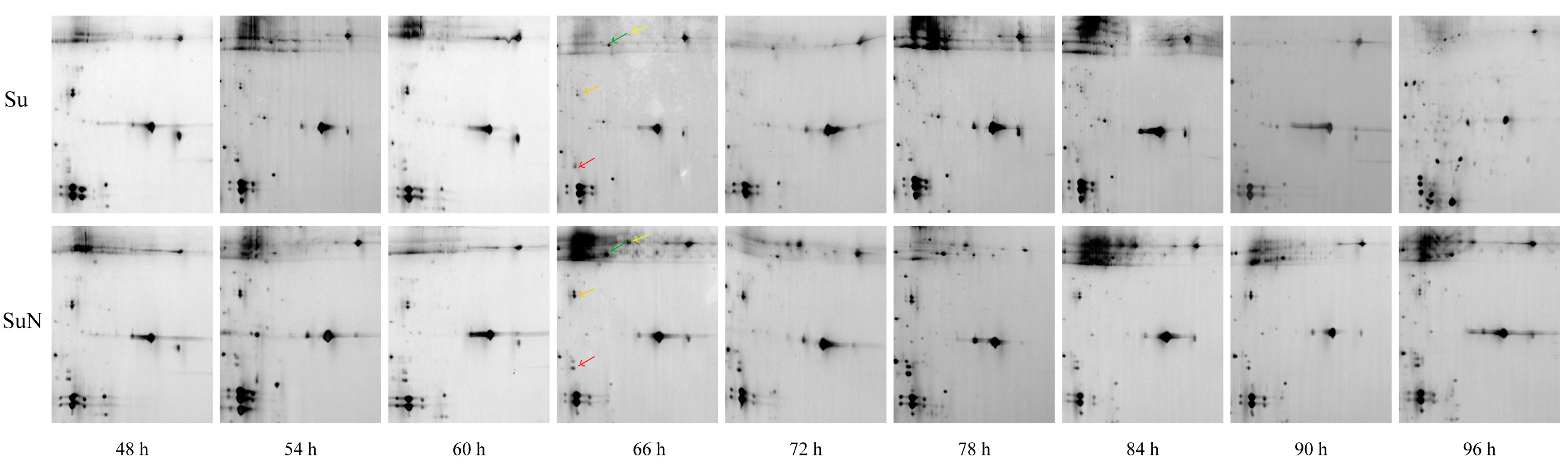
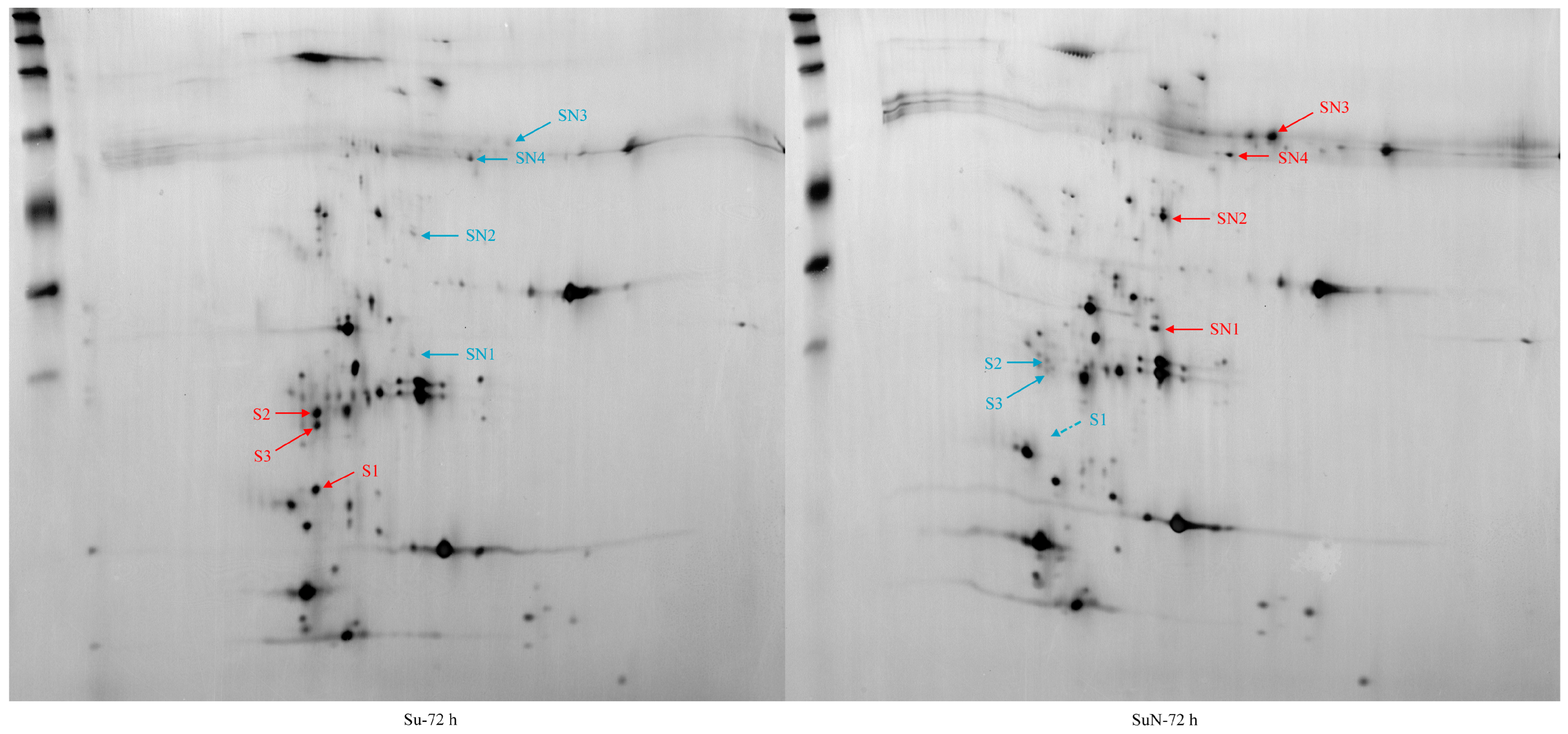
3.3. Expression of Hemolymph Proteins in Su and SuN after BmNPV Inoculation
3.4. Transcriptional Level Analysis of Genes Corresponding to DEPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldsmith, M.R.; Shimada, T.; Abe, H. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 2005, 50, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Mita, K.; Kasahara, M.; Sasaki, S.; Nagayasu, Y.; Yamada, T.; Kanamori, H.; Namiki, N.; Kitagawa, M.; Yamashita, H.; Yasukochi, Y.; et al. The genome sequence of silkworm, Bombyx mori. DNA Res. 2004, 11, 27–35. [Google Scholar] [CrossRef]
- Mao, F.X.; Lei, J.H.; Enoch, O.; Wei, M.; Zhao, C.; Quan, Y.P.; Yu, W. Quantitative proteomics of Bombyx mori after BmNPV challenge. J. Proteom. 2011, 81, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Long, Y.Y.; Qu, D.C.; Xu, K.Z.; Zhu, F.R.; Chen, C.R.; Liang, X. Genetic polymorphism and evolution of gp64 gene from Bombyx mori nucleopolyhedrovirus in Guangxi. J. South. Agric. 2021, 52, 448–456. [Google Scholar]
- Tang, F.F.; Chen, S.; Shao, Y.F.; Zhu, F.; Zhang, Y.H.; Bai, X.R. Infection and Pathogenicity of BmNPV-YN1 Isolated from Yunnan to Bombyx mori Larvae of Different Instars. Sci. Seric. 2018, 44, 692–697. [Google Scholar]
- Li, W.X.; Qing, X.G.; Liu, J.F.; Liu, G.; Xiao, J.S.; Xu, A.Y. Appraisal study on Bombyx mori breed “871C×872C” resistance to BmNPV. Southwest China J. Agric. Sci. 2011, 24, 779–781. [Google Scholar]
- Xu, A.Y.; Lin, C.Q.; Qian, H.Y.; Sun, P.J.; Zhang, Y.H.; Liu, M.Z.; Li, L. Breeding of a new silkworm variety “Huakang 2” with tolerance to Bombyx mori nucleopolyhedrovirus disease. Sci. Seric. 2013, 39, 275–282. [Google Scholar]
- Xu, A.Y.; Qian, H.Y.; Sun, P.J.; Liu, M.Z.; Lin, C.Q.; Li, G.; Li, L.; Zhang, Y.H.; Zhao, G.D. Breeding of a new silkworm variety Huakang 3 with resistance to Bombyx mori nucleopolyhedrosis. Sci. Seric. 2019, 45, 201–211. [Google Scholar]
- Chen, T.T.; Tan, L.R.; Hu, N.; Dong, Z.Q.; Hu, Z.G.; Qin, Q.; Long, J.-Q.; Chen, P.; Xu, A.-Y.; Pan, M.-H.; et al. Specific genes related to nucleopolyhedrovirus in Bombyx mori susceptible and near-isogenic resistant strains through comparative transcriptome analysis. Insect Mol. Biol. 2019, 28, 473–484. [Google Scholar] [CrossRef]
- Chen, T.T.; Hu, N.; Tan, L.R.; Xiao, Q.; Dong, Z.Q.; Chen, P.; Xu, A.-Y.; Pan, M.-H.; Lu, C. Resistant silkworm strain block viral infection independent of melanization. Pestic. Biochem. Physiol. 2019, 154, 88–96. [Google Scholar] [CrossRef]
- Shi, M.N.; Bi, L.H.; Gu, J.D.; Fei, M.H.; Qi, G.J.; Wei, B.Y.; Huang, J.T.; Huang, L.L.; Su, H.M.; Meng, Y.Y. Breeding of new highly resistant nuclear ployhedrosis disease silkworm variety Guican N2. Guangxi Seric. 2012, 49, 1–12. [Google Scholar]
- Chen, K.P.; Lin, C.Q.; Yao, Q. Studies on the resistance to NPV and its hereditary regularity in the silkworm (Bombyx mori L.). Acta Sericologica Sin. 1996, 22, 160–164. [Google Scholar]
- Qian, H.Y.; Xu, A.Y.; Lin, C.Q. Studies on the resistance to nuclear polyhedrosis virus (NPV) and its inheritance law in silkworm Bombyx mori. J. Hebei Agric. Univ. 2006, 29, 77–91. [Google Scholar]
- Lv, P. Molecular Tagging and Mapping of Resistance Gene of Bombyx mori to B. mori Nucleopolyhedrovirus and Function of Some Related Genes. Ph.D. Thesis, Jiangsu University, Zhenjiang, China, 2014. [Google Scholar]
- Gao, R.; Li, C.L.; Tong, X.L. lnsight into genetic basis of Bombyx mori resistant strains with resistance to BmNPV by molecular linkage analysis. Sci. Agric. Sin. 2017, 50, 195–204. [Google Scholar]
- Wu, S.S.; Xu, Q.; Yu, J.J.; Zhang, Y.S.; He, X.B.; Qin, G.X.; Xu, A.Y.; Zhang, G.Z. Linkage analysis and mapping of BmNPV resistant gene in AN strain of silkworm (Bombyx mori). Acta Sericologica Sin. 2017, 43, 209–216. [Google Scholar]
- Shao, L.L. Fine Mapping of BmNPV Resistance in Silkworm AN Strain and Functional Identification Analysis of Bmvncp Gene. Master’ Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2020. [Google Scholar]
- Zhao, Y. Molecular Tagging and Mapping in Bombyx mori against BmNPV and the Differential Protein Expression Profiling in the Midgut Tissue of Silkworm Infected by BmNPV. Ph.D. Thesis, Jiangsu University, Zhenjiang, China, 2007. [Google Scholar]
- Bao, Y.Y.; Tang, X.D.; Lv, Z.Y.; Wang, X.Y.; Tian, C.H.; Xu, Y.P.; Zhang, C.X. Gene expression profiling of resistant and susceptible Bombyx mori strains reveals nucleopolyhedrovirus-associated variations in host gene transcript levels. Genomics 2009, 94, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.Y.; Lv, Z.Y.; Liu, Z.B.; Xue, J.; Xu, Y.P.; Zhang, C.X. Comparative analysis of Bombyx mori nucleopolyhedrovirus responsive genes in fat body and haemocyte of B. mori resistant and susceptible strains. Insect Mol. Biol. 2010, 19, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qian, H.Y.; Luo, X.F.; Xu, P.Z.; Yang, J.H.; Liu, M.Z.; Xu, A. Transcriptomic analysis of resistant and susceptible Bombyx mori strains following BmNPV infection provides insights into the antiviral mechanisms. Int. J. Genom. 2016, 2016, 2086346. [Google Scholar]
- Qian, H.Y.; Li, G.; Zhao, G.D.; Liu, M.; Xu, A. Metabolic characterisation of the midgut of Bombyx mori varieties after BmNPV infection using GC-MS-based metabolite profiling. Int. J. Mol. Sci. 2020, 21, 4707. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, K.; Yang, B.; Huang, W.; Dobens, L.; Song, H.; Ling, E. Gut immunity in Lepidopteran insects. Dev. Comp. Immunol. 2016, 64, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Day, M.F. Midgut epithelial regeneration as a means of studying insect digestion. Nature 1949, 164, 878. [Google Scholar] [CrossRef] [PubMed]
- Washburn, J.O.; Kirkpatrick, B.A.; Volkman, L.E. Comparative pathogenesis of Autographa californica M nuclear polyhedrosis virus in larvae of Trichoplusia ni and Heliothis virescens. Virology 1995, 209, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Washburn, J.O.; Wong, J.F.; Volkman, L.E. Comparative pathogenesis of Helicoverpa zea S nucleopolyhedrovirus in noctuid larvae. J. Gen. Virol. 2001, 82, 1777–1784. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yu, H.Z.; Geng, L.; Xu, J.P.; Yu, D.; Zhang, S.Z.; Ma, Y.; Fei, D.Q. Comparative transcriptome analysis of Bombyx mori (Lepidoptera) larval midgut response to BmNPV in susceptible and near-isogenic resistant strains. PLoS ONE 2016, 11, e0155341. [Google Scholar] [CrossRef]
- Shen, Y.; Zeng, X.; Chen, G.; Wu, X. Comparative transcriptome analysis reveals regional specialization of gene expression in larval silkworm (Bombyx mori) midgut. Insect Sci. 2022, 29, 1329–1345. [Google Scholar] [CrossRef]
- Vermunt, A.M.; Kamimura, M.; Hirai, M.; Kiuchi, M.; Shiotsuki, T. The juvenile hormone binding protein of silkworm haemolymph: Gene and functional analysis. Insect Mol. Biol. 2001, 10, 147–154. [Google Scholar] [CrossRef]
- Zhang, J.J. Protein-Protein Interaction Networks among Structural Proteins of Bombyx mori Nucleopolyhedrovirus (Budded Virus) and Characterization of Interactions between GP64 and Its Associated Proteins. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2018. [Google Scholar]
- Hossen, M.S.; Shapla, U.M.; Gan, S.H.; Khalil, M.I. Impact of bee venom enzymes on diseases and immune responses. Molecules 2016, 22, 25. [Google Scholar] [CrossRef]
- Seiler, N. Polyamine oxidase, properties and functions. Prog. Brain Res. 1995, 106, 333–344. [Google Scholar]
- Kraus, D.; Yang, Q.; Kong, D.; Banks, A.S.; Zhang, L.; Rodgers, J.T.; Pirinen, E.; Pulinilkunnil, T.C.; Gong, F.; Wang, Y.-C.; et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 2014, 508, 258–262. [Google Scholar] [CrossRef]
- Li, R.; Hu, C.; Geng, T.; Lv, D.; Gao, K.; Guo, X.; Hou, C. Expressional analysis of the silkworm storage protein 1 and identification of its interacting proteins. Insect Mol. Biol. 2020, 29, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Daugavet, M.A.; Dobrynina, M.I.; Shaposhnikova, T.G.; Solovyeva, A.I.; Mittenberg, A.G.; Shabelnikov, S.V.; Babkina, I.Y.; Grinchenko, A.V.; Ilyaskina, D.V.; Podgornaya, O.I. New putative phenol oxidase in ascidian blood cells. Sci. Rep. 2022, 12, 14326. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.C. Bomyx mori carboxylesterase42 (Bmae42) against Bombyx mori Nuclear Polyhedrosis Virus Infection. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2017. [Google Scholar]


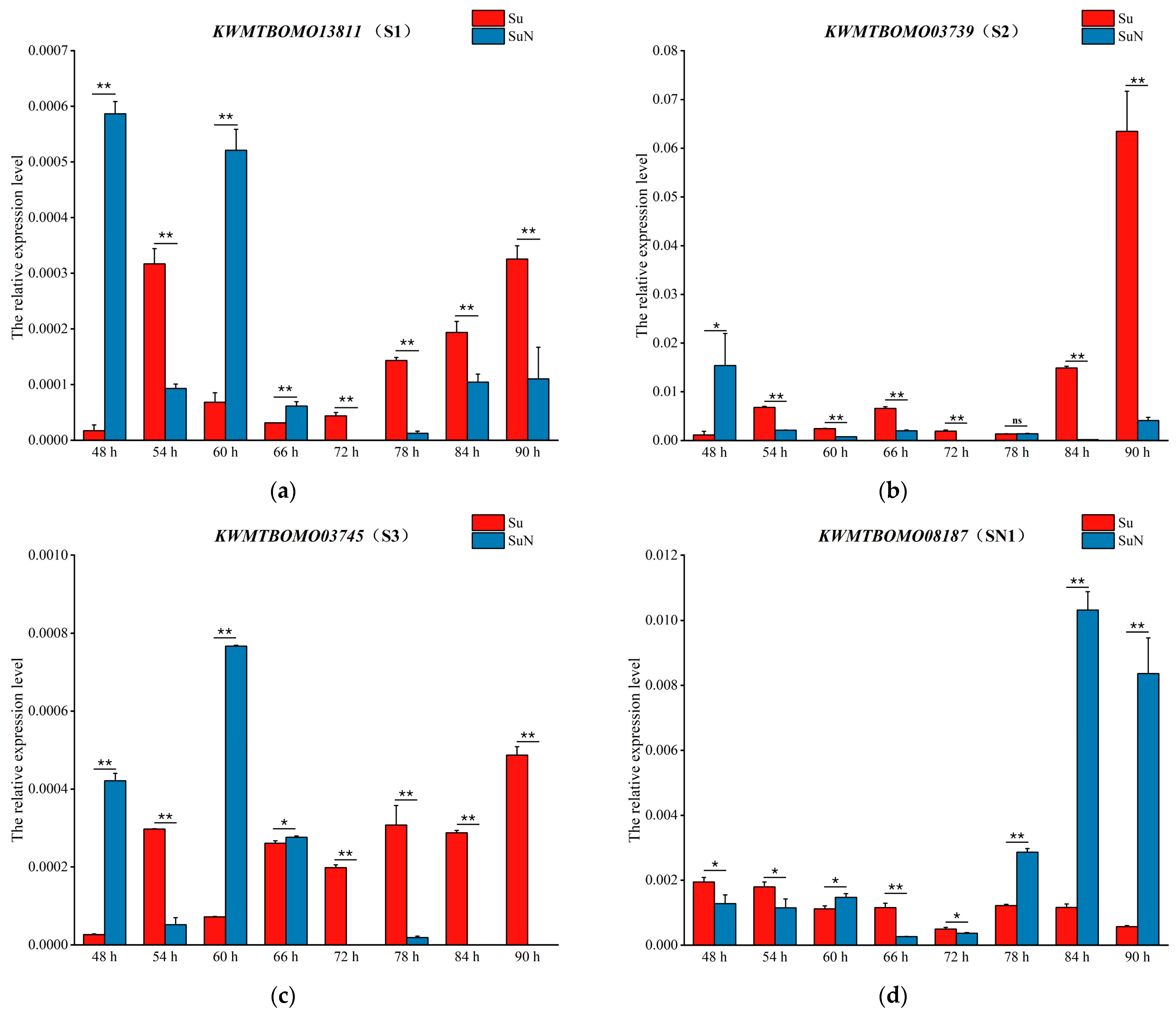
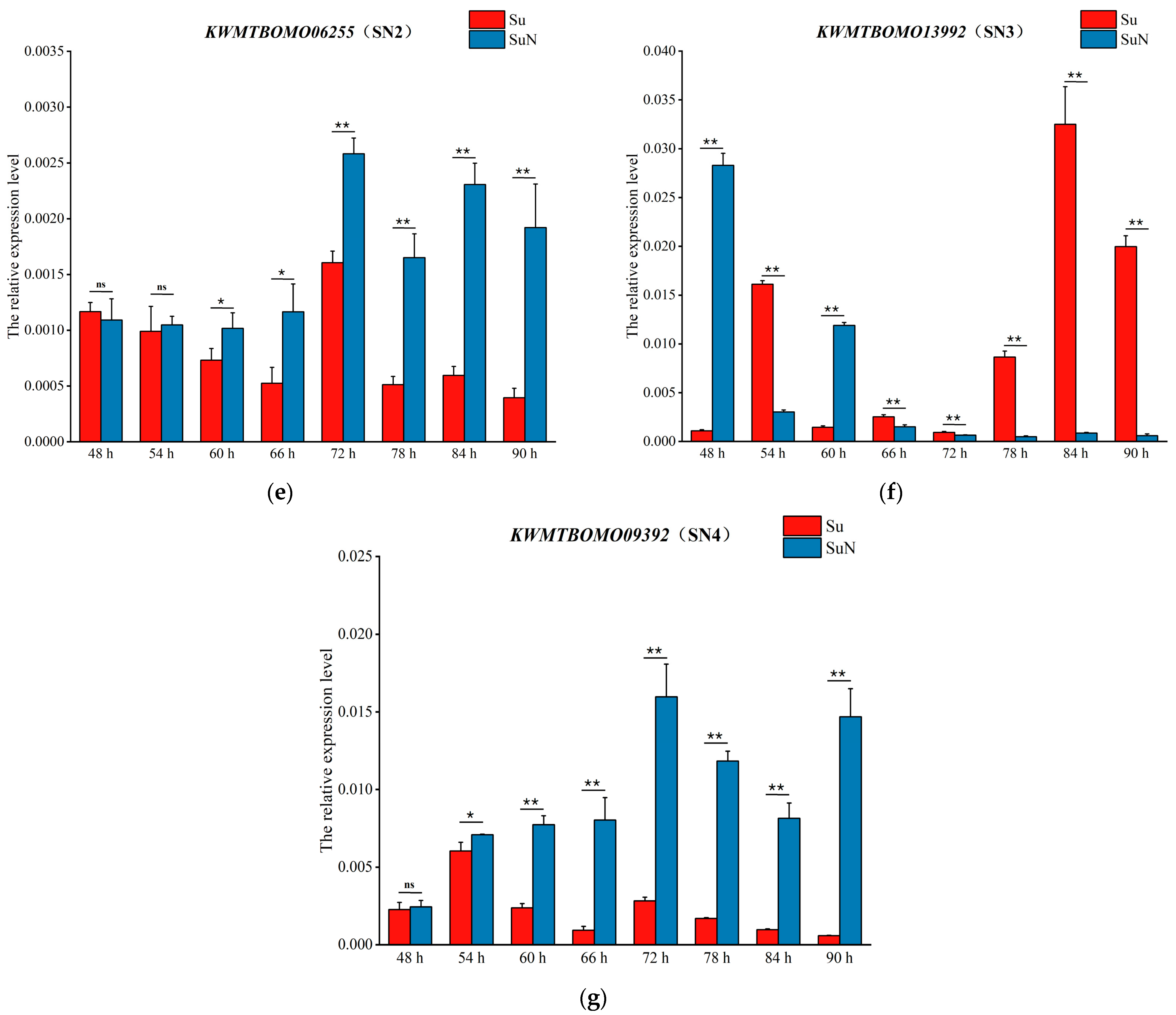
| DEPs | Genes | Chromosome Position | Gene Annotation in Silkworm Genome Database |
|---|---|---|---|
| S1 | KWMTBOMO13811 | Chr23: 10000380–10005184 (+) | hemolymph juvenile hormone binding protein precursor [Bombyx mori] |
| S2 | KWMTBOMO03739 | Chr7: 371497–372518 (−) | Uncharacterized protein [Bombyx mori] |
| S3 | KWMTBOMO03745 | Chr7: 422709–423596 (+) | Uncharacterized protein [Bombyx mori] |
| SN1 | KWMTBOMO08187 | Chr14: 1161961–1192896 (+) | Venom acid phosphatase Acph-1-like [Papilio xuthus] |
| SN2 | KWMTBOMO06255 | Chr11: 3382416–3398048 (+) | Probable polyamine oxidase 5 [Bombyx mori] |
| SN3 | KWMTBOMO13992 | Chr23: 15551943–15556861 (−) | sex-specific storage-protein 1 precursor [Bombyx mori] |
| SN4 | KWMTBOMO09392 | Chr16: 360567–373088 (+) | Pro-phenol oxidase [Bombyx mandarina] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, G.; Qian, H.; Sun, J.; Yang, R.; Gui, T.; Wang, W.; Liu, Q.; Chen, A. Proteomics Analysis to Explore the Resistance Genes of Silkworm to Bombyx mori Nuclear Polyhedrosis Virus. Genes 2024, 15, 59. https://doi.org/10.3390/genes15010059
Ouyang G, Qian H, Sun J, Yang R, Gui T, Wang W, Liu Q, Chen A. Proteomics Analysis to Explore the Resistance Genes of Silkworm to Bombyx mori Nuclear Polyhedrosis Virus. Genes. 2024; 15(1):59. https://doi.org/10.3390/genes15010059
Chicago/Turabian StyleOuyang, Gui, Heying Qian, Juan Sun, Runhuan Yang, Tao Gui, Wenbing Wang, Qiang Liu, and Anli Chen. 2024. "Proteomics Analysis to Explore the Resistance Genes of Silkworm to Bombyx mori Nuclear Polyhedrosis Virus" Genes 15, no. 1: 59. https://doi.org/10.3390/genes15010059





