PGT-M for Premature Ovarian Failure Related to CGG Repeat Expansion of the FMR1 Gene
Abstract
1. Introduction
2. Premature Ovarian Failure
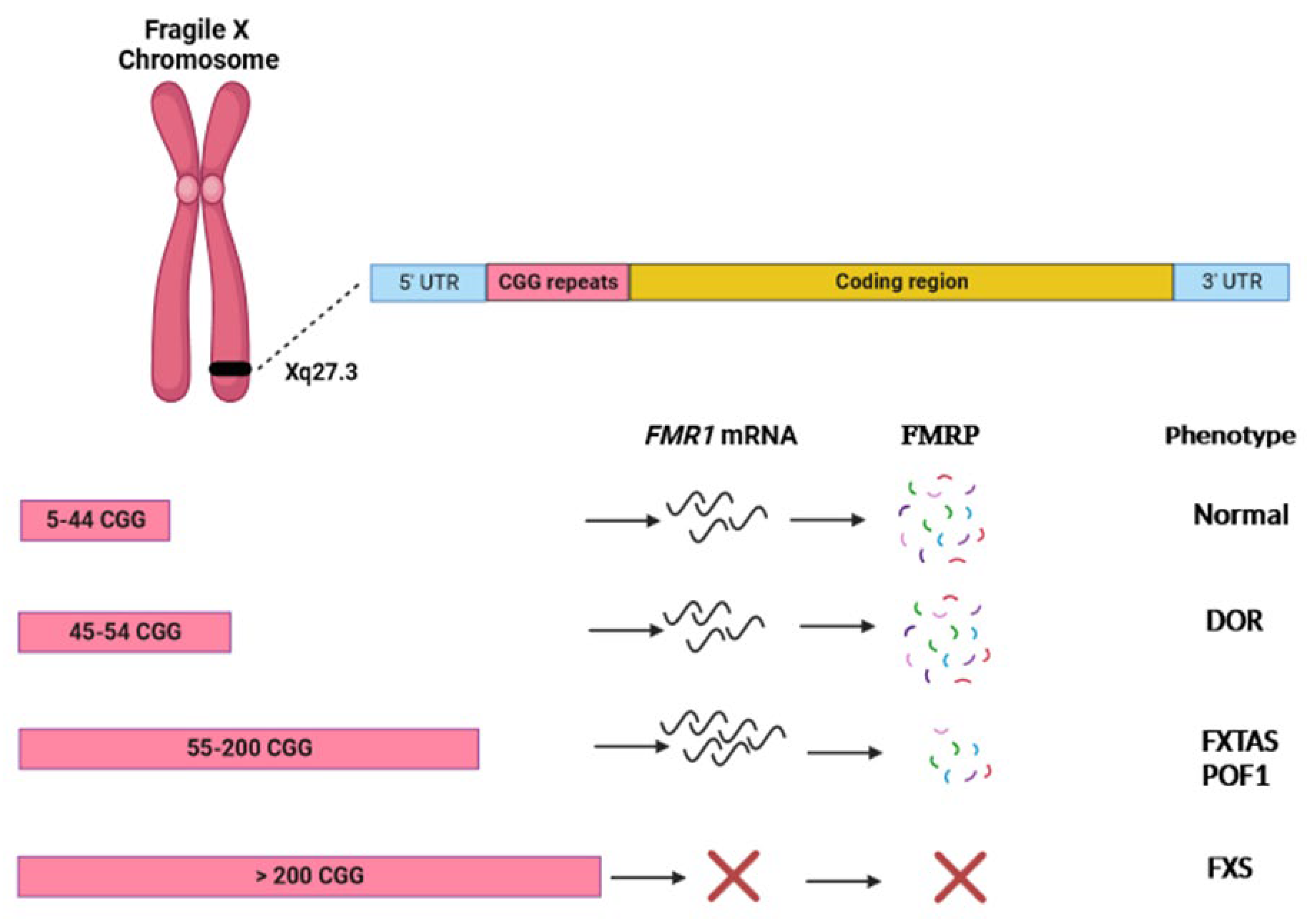
3. FMR1
4. POF1
5. FMR1 Premutation and Ovarian Damage
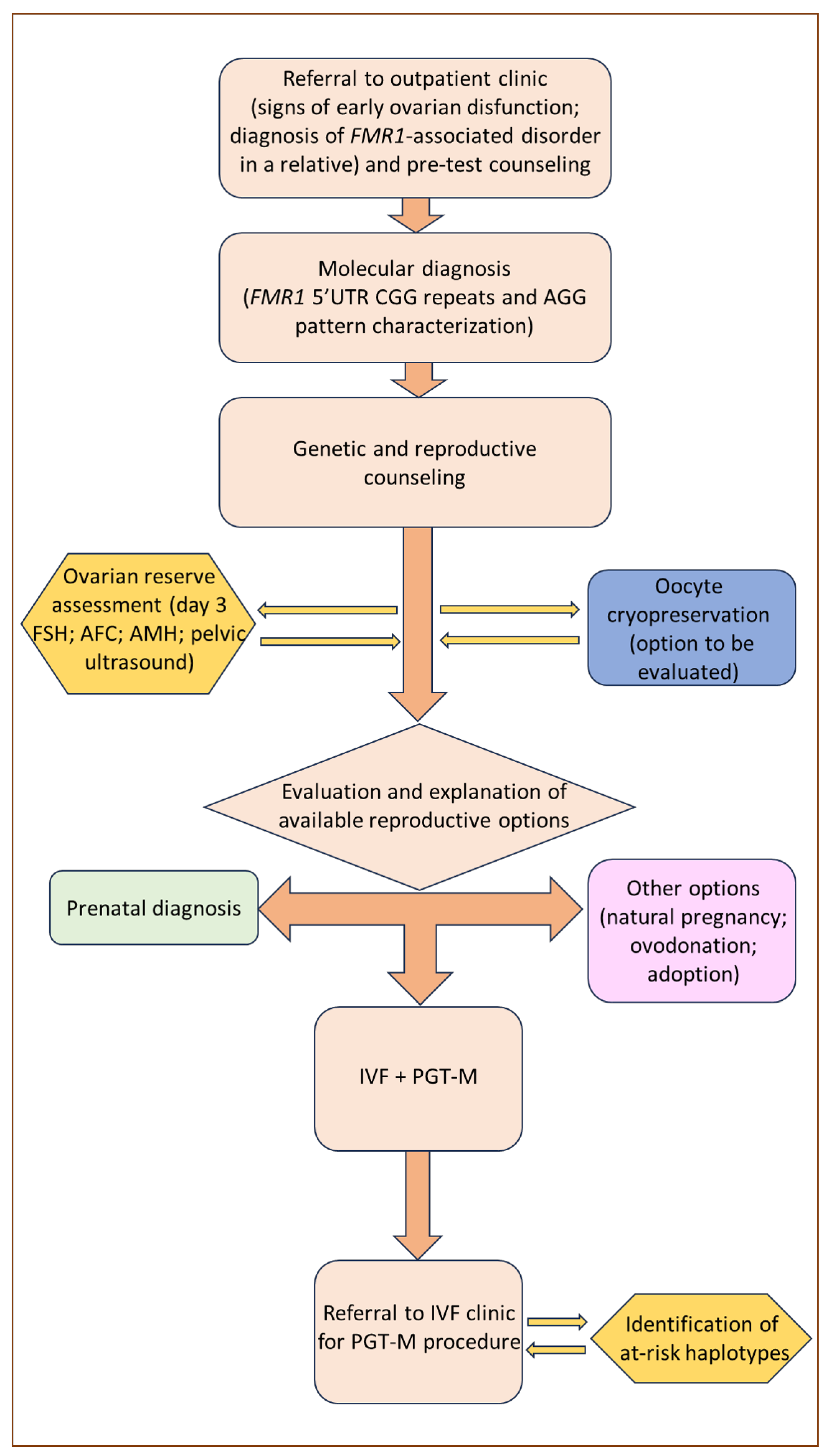
6. Reproductive Counseling and Fertility Preservation
7. PGT-M Strategies and Limitations in FMR1-Related Disorders
8. POF1 in the PGT-M Context
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists. Primary ovarian insufficiency in adolescents and young women. Obs. Gynecol. 2014, 124, 193–197. [Google Scholar] [CrossRef] [PubMed]
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI; Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; de Muinck Keizer-Schrama, S.; Hogervorst, E.; et al. Management of women with premature ovarian insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar] [PubMed]
- Ishizuka, B. Current Understanding of the Etiology, Symptomatology, and Treatment Options in Premature Ovarian Insufficiency (POI). Front. Endocrinol. 2021, 12, 626924. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Nelson, L.M. Mechanisms and models of immune tolerance breakdown in the ovary. Semin. Reprod. Med. 2011, 29, 308–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, Y.; Jiao, X.; Simpson, J.L.; Chen, Z.-J. Genetics of primary ovarian insufficiency: New developments and opportunities. Hum. Reprod. Update 2015, 21, 787–808. [Google Scholar] [CrossRef] [PubMed]
- Aittomäki, K.; Lucena, J.L.; Pakarinen, P.; Sistonen, P.; Tapanainen, J.; Gromoll, J.; Kaskikari, R.; Sankila, E.M.; Lehväslaiho, H.; Engel, A.R.; et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 1995, 82, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Conway, G.S.; Hettiarachchi, S.; Murray, A.; Jacobs, P.A. Fragile X premutation in familial premature ovarian failure. Lancet 1995, 346, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Dean, J.; Howard-Peebles, P.N.; Bugge, M.; Mikkelsen, M.; Tommerup, N.; Hull, C.; Hagerman, R.; Holden, J.J.; Stevenson, R.E. Obstetrical and gynecological complication in fragile X carriers: A multicenter study. Am. J. Med. Genet. 1994, 51, 400–402. [Google Scholar] [CrossRef]
- Verkerk, A.J.M.H.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.-H.; Kuhl, D.P.P.A.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.; et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Lubs, H.A.; Stevenson, R.E.; Schwartz, C.E. Fragile X and X-linked intellectual disability: Four decades of discovery. Am. J. Hum. Genet. 2012, 90, 579–590. [Google Scholar] [CrossRef]
- Vincent, A.; Heitz, D.; Petit, C.; Kretz, C.; Oberlé, I.; Mandel, J.L. Abnormal pattern detected in fragile-X patients by pulsed-field gel electrophoresis. Nature 1991, 349, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.V.; Hirst, M.C.; Nakahori, Y.; MacKinnon, R.N.; Roche, A.; Flint, T.J.; Jacobs, P.A.; Tommerup, N.; Tranebjaerg, L.; Froster-Iskenius, U.; et al. Physical mapping across the fragile X: Hypermethylation and clinical expression of the fragile X syndrome. Cell 1991, 64, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Kooy, R.F.; Willemsen, R.; Oosatra, B.A. Fragile X syndrome at turn of the century. Mol. Med. Today 2000, 6, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lisik, M.Z. Health problems in females carriers of premutation in the FMR1 gene. Psychiatr. Pol. 2017, 51, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Berry-Kravis, E.; Hipp, H.; Todd, P.K. FMR1 disorders. Gene Rev. 1993, 202. [Google Scholar]
- Yu, S.; Pritchard, M.; Kremer, E.; Lynch, L.; Nancarrow, J.; Baker, E.; Holman, K.; Mulley, J.C.; Warren, S.T.; Schlessinger, D. Fragile X genotype characterized by an unstable region of DNA. Science 1991, 252, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.R.; Pearson, C.E.; Sinden, R.R. Stability of triplet repeats of myotonic dystrophy and fragile X loci in human mutator mismatch repair cell lines. Hum. Genet. 1996, 98, 151–157. [Google Scholar] [CrossRef]
- Nelson, D.L.; Orr, H.T.; Warren, S.T. The unstable repeats three evolving faces of neurological disease. Neuron 2013, 77, 825–843. [Google Scholar] [CrossRef]
- Nolin, S.L.; Brown, W.T.; Glicksman, A.; Houck, G.E., Jr.; Gargano, A.D.; Sullivan, A.; Biancalana, V.; Bröndum-Nielsen, K.; Hjalgrim, H.; Holinski-Feder, E.; et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am. J. Hum. Genet. 2003, 72, 454–464. [Google Scholar] [CrossRef]
- Nolin, S.L.; Glicksman, A.; Ersalesi, N.; Dobkin, C.; Brown, W.T.; Cao, R.; Blatt, E.; Sah, S.; Latham, G.J.; Hadd, A.G. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet. Med. 2014, 17, 358–364. [Google Scholar] [CrossRef]
- Eichler, E.E.; Holden, J.J.; Popovich, B.W.; Reiss, A.L.; Snow, K.; Thibodeau, S.N.; Richards, C.S.; Ward, P.A.; Nelson, D.L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat. Genet. 1994, 8, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Yrigollen, C.M.; Durbin-Johnson, B.; Gane, L.; Nelson, D.L.; Hagerman, R.; Hagerman, P.J.; Tassone, F. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet. Med. 2012, 14, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Domniz, N.; Ries-Levavi, L.; Cohen, Y.; Marom-Haham, L.; Berkenstandt, M.; Pras, E.; Glicksman, A.; Tortora, N.; Latham, G.L.; Hadd, A.G.; et al. Absence of AGG interruptions is a risk factor for full mutation expansion among Israeli FMR1 premutation carriers. Front. Genet. 2018, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Wittenberger, M.D.; Hagerman, R.J.; Sherman, S.L.; McConkie-Rosell, A.; Welt, C.K.; Rebar, R.W.; Corrigan, E.C.; Simpson, J.L.; Nelson, L.M. The FMR1 premutation and reproduction. Fertil. Steril. 2007, 87, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Abrams, L.; Coffey, S.M.; Hall, D.A.; Greco, C.; Gane, L.W.; Grigsby, J.; Bourgeois, J.A.; Finucane, B.; Jacquemont, S. Fragile X-associated tremor/ataxia syndrome: Clinical features, genetics, and testing guidelines. Mov. Disord. 2007, 22, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Hangerman, R.J.; Hangerman, P. Fragile X -associated tremor/ataxia syndrome-features, mechanism and management. Nat. Rev. Neurol. 2016, 12, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.K.; Oh, S.Y.; Krans, A.; He, F.; Sellier, C.; Frazer, M.; Renoux, A.J.; Chen, K.C.; Scaglione, K.M.; Basrur, V.; et al. CGG repeated-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 2013, 78, 440–455. [Google Scholar] [CrossRef]
- Tassone, F.; Hagerman, P. The fragile X-associated tremor ataxia syndrome. Results Probl. Cell Differ. 2012, 54, 337–357. [Google Scholar]
- Hangerman, R.J.; Hangerman, P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile-X-associated tremor/ataxia syndrome. Lancet Neurol. 2013, 12, 786–798. [Google Scholar] [CrossRef]
- Hagerman, P.J.; Hagerman, R.J. The fragile-X premutation: A maturing perspective. Am. J. Hum. Genet. 2004, 74, 805–816. [Google Scholar] [CrossRef]
- Jacquemont, S.; Hagerman, R.J.; Leehey, M. Fragile X premutation tremor/ataxia syndrome: Molecular, clinical and neuroimaging correlates. Am. J. Hum. Genet. 2003, 72, 869–878. [Google Scholar] [CrossRef]
- Tassone, F.; Protic, D.; Allen, E.G.; Archibald, A.D.; Baud, A.; Brown, T.W.; Budimirovic, D.B.; Cohen, J.; Dufour, B.; Eiges, R.; et al. Insight and Recommendations for Fragile X-Premutation-Associated Conditions from the Fifth International Conference on FMR1 Premutation. Cells 2023, 12, 2330. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.M. Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 2009, 360, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Marcus, M.; Epstein, M.; Allen, E.; Anido, A.; Paquin, J.; Yadav-Shah, M.; Sherman, S. Association of FMR1 repeat size with ovarian dysfunction. Hum. Reprod. 2005, 20, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Allingham-Hawkins, D.J.; Babul-Hirji, R.; Chitayat, D.; Holden, J.J.A.; Yang, K.T.; Lee, C.; Hudson, R.; Gorwill, H.; Nolin, S.R.; Glicksman, A.; et al. Fragile X Premutation Is a Significant Risk Factor for Premature Ovarian Failure. Am. J. Med. Genet. 1999, 83, 322–325. [Google Scholar] [CrossRef]
- Cronister, A.; Schreiner, R.; Wittenberger, M.; Amiri, K.; Harris, K.; Hagerman, R.J. Heterozygous fragile X female: Historical, physical, cognitive, and cytogenetic features. Am. J. Med. Genet. 1991, 38, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Welt, C.K. Primary ovarian insufficiency: A more accurate term for premature ovarian failure. Clin. Endocrinol. 2008, 68, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Bodega, B.; Bione, S.; Dalprà, L.; Toniolo, D.; Ornaghi, F.; Vegetti, W.; Ginelli, E.; Marozzi, A. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum. Reprod. 2006, 21, 952–957. [Google Scholar] [CrossRef]
- Streuli, I.; Fraisse, T.; Ibecheole, V.; Moix, I.; Morris, M.A.; de Ziegler, D. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil. Steril. 2009, 92, 464–470. [Google Scholar] [CrossRef]
- Barasoain, M.; Barrenetxea, G.; Huerta, I.; Télez, M.; Criado, B.; Arrieta, I. Study of the Genetic Etiology of Primary Ovarian Insufficiency: FMR1 Gene. Genes 2016, 7, 123. [Google Scholar] [CrossRef]
- Giovannucci Uzielli, M.L.; Guarducci, S.; Lapi, E.; Cecconi, A.; Ricci, U.; Ricotti, G.; Biondi, C.; Scarselli, B.; Vieri, F.; Scarnato, P.; et al. Premature ovarian failure (POF) and fragile X premutation females: From POF to fragile X carrier identification, from fragile X carrier diagnosis to POF association data. Am. J. Med. Genet. 1999, 84, 300–303. [Google Scholar] [CrossRef]
- Ennis, S.; Ward, D.; Murray, A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur. J. Hum. Genet. 2006, 14, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.S.; Charen, K.; Hipp, H.S.; Shubeck, L.; Amin, A.; He, W.; Nolin, S.L.; Glicksman, A.; Tortora, N.; McKinnon, B.; et al. Refining the risk for fragile X-associated primary ovarian insufficiency (POF1) by FMR1 CGG repeat size. Genet. Med. 2021, 23, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Iong, K.P.; Tong, T.H.; Lo, J.; Gane, L.W.; Berry-Kravis, E.; Nguyen, D.; Mu, L.Y.; Laffin, J.; Bailey, D.B.; et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012, 4, 100. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, B.; Okamoto, N.; Hamada, N.; Sugishita, Y.; Saito, J.; Takahashi, N.; Ogata, T.; Itho, M.T. Number of CGG repeats in the FMR1 gene of Japanese patients with primary ovarian insufficiency. Fertil. Steril. 2011, 96, 1170–1174. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Apilot study of premature ovarian senescence: Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Mullerian hormone. Fertil. Steril. 2009, 91, 1700–1706. [Google Scholar] [CrossRef]
- Bretherick, K.L.; Fluker, M.R.; Robinson, W.P. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum. Genet. 2005, 117, 376–382. [Google Scholar] [CrossRef]
- Pastore, L.M.; Young, S.L.; Baker, V.L.; Karns, L.B.; Williams, C.D.; Silverman, L.M. Elevated prevalence of 35-44 FMR1 trinucleotide repeats in women with diminished ovarian reserve. Reprod. Sci. 2012, 19, 1226–1231. [Google Scholar] [CrossRef]
- Bennet, C.E.; Conway, G.S.; Macpherson, J.N.; Jacobs, P.A.; Murray, A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum. Reprod. 2010, 25, 1335–1338. [Google Scholar] [CrossRef]
- Voorhuis, M.; Onland-Moret, N.C.; Janse, F.; Ploos van Amstel, H.K.; Govede, A.J.; Lambalk, C.B.; Laven, J.S.E.; van der Schouw, Y.T.; Broekmans, F.J.M.; Fauser, B.C.J.M.; et al. The significance of fragile X mental retardation gene I CGG repeats sizes in the normal and intermediate range in women with primary ovarian insufficiency. Hum. Reprod. 2014, 29, 1585–1593. [Google Scholar] [CrossRef]
- Sherman, S.L. Premature ovarian failure among fragile X premutation carriers: Parent of origin effect? Am. J. Hum. Genet. 2000, 67, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Vianna-Morgante, A.M.; Costa, S.S.; Pavanello, R.C.; Otto, P.A.M.; Mingroni-Netto, R.C. Premature ovarian failure (POF) in Brazilian fragile X carriers. Genet. Mol. Biol. 1999, 22, 471–474. [Google Scholar] [CrossRef]
- Murray, A.; Ennis, S.; MacSwiney, F.; Webb, J.; Morton, N.E. Reproductive and menstrual history of females with fragile X expansions. Eur. J. Hum. Genet. 2000, 8, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Mallolas, J.; Duran, M.; Sánchez, A.; Jimenez, D.; Castellvi-Bel, S.; Rife, M.; Milá, M. Implications of the FMR1 gene in menopause: Study of 147 Spanish women. Menopause 2001, 8, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, R.D.; Smits, A.O.; Thomas, C.M.; Kiemeney, L.A.; Braat, D.D. Female carriers of fragile X premutations have no increased risk for additional diseases other than premature ovarian failure. Am. J. Med. Genet. 2003, 117A, 6–9. [Google Scholar] [CrossRef]
- Lekovich, J.; Man, L.; Xu, K.; Canon, C.; Lilienthal, D.; Stewart, J.D.; Pereira, N.; Rosenwaks, Z.; Gerhardt, J. CGG repeat length and AGG interruptions as indicators of fragile X-associated diminished ovarian reserve. Genet. Med. 2018, 20, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Murray, A. Premature ovarian failure and the FMR1 gene. Semin. Reprod. Med. 2000, 18, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C. Effect of early menopause on bone mineral density and fractures. Menopause 2007, 14, 567–571. [Google Scholar] [CrossRef]
- Kalantaridou, S.N.; Naka, K.K.; Papanikolaou, E.; Kazakos, N.; Kravariti, M.; Calis, K.A.; Paraskevaidis, E.A.; Sideris, D.A.; Tsatsoulis, A.; Chrousos, G.P.; et al. Impaired endothelial function in young women with premature ovarian failure: Normalization with hormone therapy. J. Clin. Endocrinol. Metab. 2004, 89, 3907–3913. [Google Scholar] [CrossRef]
- Atsma, F.; Bartelink, M.L.; Grobbee, D.E.; van der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006, 13, 265–279. [Google Scholar] [CrossRef]
- Allen, E.G.; He, W.; Yadav-Shah, M.; Sherman, S.L. A Study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum. Genet. 2004, 114, 439–447. [Google Scholar]
- Tassone, F.; Bellina, A.; Carosi, C.; Albertosi, S.; Bagni, C.; Li, L.; Glover, K.; Bentley, D.; Hanerman, P.J. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 2007, 13, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Kenneson, A.; Zhang, F.; Hagedorn, C.H.; Warren, S.T. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet. 2001, 10, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Peprah, E.; He, W.; Allen, E.; Oliver, T.; Boyne, A.; Sherman, S.L. Examination of FMR1 transcript and protein levels among 74 premutation carriers. J. Hum. Genet. 2010, 55, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alegria, E.; Ibanez, B.; Minguez, M.; Poch, M.; Valiente, A.; Sanz-Parra, A.; Martinez-Bouzas, C.; Beristain, E.; Tejada, M.I. Analysis of FMR1 gene expression in female premutation carriers using robust segmented linear regression models. RNA 2007, 13, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Leehey, M.; Heinrichs, W.; Tassone, F.; Wilson, R.; Hills, J.; Grigsby, J.; Gage, B.; Hagerman, P.J. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001, 57, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Elizur, S.E.; Lebovitz, O.; Derech-Haim, S.; Dratviman-Storobinsky, O.; Feldman, B.; Dor, J.; Orvieto, T.; Cohen, Y. Elevated levels of FMR1 mRNA in granulosa cells are associated with low ovarian reserve in FMR1 premutation carriers. PLoS ONE 2014, 9, e105121. [Google Scholar] [CrossRef]
- Allen, E.G.; Sullivan, A.K.; Marcus, M.; Small, C.; Dominguez, C.; Epstein, M.P.; Charen, K.; He, W.; Taylor, K.C.; Sherman, S.L. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum. Reprod. 2007, 22, 2142–2152. [Google Scholar] [CrossRef]
- Buijsen, R.A.; Visser, J.A.; Kramer, P.; Severijnen, E.A.; Gearing, M.; Charlet-Berguerand, N.; Sherman, S.L.; Berman, R.F.; Willemsen, R.; Hukema, R.K. Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency. Hum. Reprod. 2016, 31, 158–168. [Google Scholar] [CrossRef]
- Napierala, M.; Michalowski, D.; de Mezer, M.; Krzyzosiak, W.J. Facile FMR1 mRNA structure regulation by interruptions in CGG repeats. Nucleic Acids Res. 2005, 33, 451–463. [Google Scholar] [CrossRef]
- Sonigo, C.; Mayeur, A.; Sadoun, M.; Pinto, M.; Benguigui, J.; Frydman, N.; Monnot, S.; Benachi, A.; Steffann, J.; Grynberg, M. What is the threshold of mature oocytes to obtain at least one healthy transferable cleavage-stage embryo after preimplantation genetic testing for fragile X syndrome? Hum. Reprod. 2021, 36, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Mastellari, E. Fertility preservation for genetic diseases leading to premature ovarian insufficiency (POI). J. Assist. Reprod. Genet. 2021, 38, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Vandervorst, M.; Liebaers, I.; Sermon, K.; Staessen, C.; De Vos, A.; Van de Velde, H.; Van Assche, E.; Joris, H.; Van Steirteghem, A.; Devroey, P. Successful preimplantation genetic diagnosis is related to the number of available cumulus-oocyte complexes. Hum. Reprod. 1998, 13, 3169–3176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wheeler, A.C.; Raspa, M.; Green, A.; Bishop, E.; Bann, C.; Edwards, A.; Bailey, D.B., Jr. Health and reproductive experiences of women with an FMR1 premutation with and without fragile X premature ovarian insufficiency. Front. Genet. 2014, 5, 300. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, G.J.; Broekmans, F.J.M.; Dorland, M.; Habbema, J.D.F.; Looman, C.W.N.; te Velde, E.R. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil. Steril. 1999, 72, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.T.; Hofmann, G.E.; Oehninger, S.; Muasher, S.J. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil. Steril. 1990, 54, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Seifer, D.B.; Baker, V.L.; Leader, B. Age-specific serum anti-Müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil. Steril. 2011, 95, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.; de Faoite, E. Psychological Impact of Abortion due to Fetal Anomaly: A Review of Published Research. Issues Law. Med. 2017, 32, 19–30. [Google Scholar]
- Ranisavljevic, N.; Hess, M.; Castelli, C.; Willems, M.; Ferrieres-Hoa, A.; Girardet, A.; Anahory, T. Are ovarian response and pregnancy rates similar in selected FMR1 premutated and mutated patients undergoing preimplantation genetic testing? J. Assist. Reprod. Genet. 2020, 37, 1675–1683. [Google Scholar] [CrossRef]
- Platteau, P.; Sermon, K.; Seneca, S.; Van Steirteghem, A.; Devroey, P.; Liebaers, I. Preimplantation genetic diagnosis for fragile X syndrome: Difficult but not impossible. Hum. Reprod. 2002, 17, 2807–2812. [Google Scholar] [CrossRef]
- Harton, G.L.; Magli, M.C.; Lundin, K.; Montag, M.; Lemmen, J.; Harper, J.C. ESHRE PGD Consortium/Embryology Special Interest Group–Best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (PGD/PGS). Hum. Reprod. 2011, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sermon, K.; Seneca, S.; Vanderfaeillie, A.; Lissens, W.; Joris, H.; Vandervorst, M.; Van Steirteghem, A.; Liebaers, I. Preimplantation diagnosis for fragile X syndrome based on the detection of the non-expanded paternal and maternal CGG. Prenat. Diagn. 1999, 19, 1223–1226. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, M.; Lee, C.G.; Chong, S.S. Identification of microsatellite markers <1 Mb from the FMR1 CGG repeat and development of a single-tube tetradecaplex PCR panel of highly polymorphic markers for preimplantation genetic diagnosis of fragile X syndrome. Genet. Med. 2016, 18, 869–875. [Google Scholar] [PubMed]
- Dreesen, J.C.; Jacobs, L.J.; Bras, M.; Herbergs, J.; Dumoulin, J.C.; Geraedts, J.P.; Evers, J.L.; Smeets, H.J. Multiplex PCR of polymorphic markers flanking the CFTR gene; a general approach for preimplantation genetic diagnosis of cystic fibrosis. Mol. Hum. Reprod. 2000, 6, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Apessos, A.; Abou-Sleiman, P.M.; Harper, J.C.; Delhanty, J.D.A. Preimplantation genetic diagnosis of the fragile X syndrome by use of linked polymorphic markers. Prenat. Diagn. 2001, 21, 504–511. [Google Scholar] [CrossRef]
- Malcov, M.; Naiman, T.; Ben Yosef, D.; Carmon, A.; Mey-Raz, N.; Amit, A.; Vagman, I.; Yaron, Y. Preimplantation genetic diagnosis for fragile X syndrome using multiplex nested PCR. Reprod. Biomed. Online 2007, 14, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, E.; Nicod, J.C.; Gardes, N.; Kastner, C.; Becker, N.; Celebi, C.; Pirrello, O.; Rongières, C.; Koscinski, I.; Gosset, P.; et al. Improving preimplantation genetic diagnosis for Fragile X syndrome: Two new powerful single-round multiplex indirect and direct tests. Eur. J. Hum. Genet. 2016, 24, 221–227. [Google Scholar] [CrossRef]
- Rajan-Babu, I.-S.; Lian, M.; Cheah, F.S.H.; Chen, M.; Tan, A.S.C.; Ethiraj, B.; Prasath; Loh, S.F.; Chong, S.S. FMR1 CGG repeat expansion mutation detection and linked haplotype analysis for reliable and accurate preimplantation genetic diagnosis of fragile X syndrome. Expert Rev. Mol. Med. 2017, 19, e10. [Google Scholar] [CrossRef]
- Sihombing, N.R.B.; Cai, S.; Wong, D.P.W.; Guan, M.; Chong, S.S.-C.; Winarni, T.I. Repeat expansion and methylation-sensitive triplet-primed polymerase chain reaction for fragile X mental retardation 1 gene screening in institutionalised intellectually disabled individuals. Singap. Med. J. 2021, 62, 143–148. [Google Scholar] [CrossRef]
- Discenza, M.; Nusblat, D.; Goodall, N.N.; McWilliams, K. Observed outcomes of fmr1 pgt-m analysis with incorporation ofCGG repeat expansion can lead to extra embryos suitable for transfer. Fertil. Steril. 2021, 116, e99. [Google Scholar] [CrossRef]
- Hutchinson, A.P.; Pereira, N.; Lilienthal, D.P.; Coveney, S.; Lekovich, J.P.; Elias, R.T.; Rosenwaks, Z. Impact of FMR1 Pre-Mutation Status on Blastocyst Development in Patients Undergoing Pre-Implantation Genetic Diagnosis. Gynecol. Obs. Investig. 2018, 83, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tsafrir, A.; Altarescu, G.; Margalioth, E.; Brooks, B.; Renbaum, P.; Levy-Lahad, E.; Rabinowitz, R.; Varshaver, I.; Eldar-Geva, T. PGD for fragile X syndrome: Ovarian function is the main determinant of success. Hum. Reprod. 2010, 10, 2629–2636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avraham, S.; Almog, B.; Reches, A.; Zakar, L.; Malcov, M.; Sokolov, A.; Alpern, S.; Azem, F. The ovarian response in fragile X patients and premutation carriers undergoing IVF-PGD: Reappraisal. Hum. Reprod. 2017, 32, 1508–1511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friedman-Gohas, M. Does the presence of AGG interruptions within the CGG repeat tract have a protective effect on the fertility phenotype of female FMR1 premutation carriers? J. Assist. Reprod. Genet. 2020, 37, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Bibi, G.; Malcov, M.; Yuval, Y.; Reches, A.; ben-Yosef, D.; Almog, B.; Amit, A.; Azem, F. The effect of CGG repeat number on ovarian response among fragile X premutation carriers undergoing preimPlantation genetic diagnosis. Fertil. Steril. 2010, 94, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Burlet, P.; Frydman, N.; Gigarel, N.; Kerbrat, V.; Tachdjian, G.; Feyereisen, E.; Bonnefont, J.-P.; Frydman, R.; Munnich, A.; Steffann, J. Multiple displacement amplification improves PGD for fragile X syndrome. Mol. Hum. Reprod. 2006, 12, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Nayot, D.; Chung, J.T.; Son, W.Y.; Ao, A.; Hughes, M.; Dahan, M.H. Live birth following serial vitrification of embryos and PGD for fragile X syndrome in a patient with the premutation and decreased ovarian reserve. J. Assist. Reprod. Genet. 2013, 30, 1439–1444. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persico, T.; Tranquillo, M.L.; Seracchioli, R.; Zuccarello, D.; Sorrentino, U. PGT-M for Premature Ovarian Failure Related to CGG Repeat Expansion of the FMR1 Gene. Genes 2024, 15, 6. https://doi.org/10.3390/genes15010006
Persico T, Tranquillo ML, Seracchioli R, Zuccarello D, Sorrentino U. PGT-M for Premature Ovarian Failure Related to CGG Repeat Expansion of the FMR1 Gene. Genes. 2024; 15(1):6. https://doi.org/10.3390/genes15010006
Chicago/Turabian StylePersico, Tiziana, Maria Lucrezia Tranquillo, Renato Seracchioli, Daniela Zuccarello, and Ugo Sorrentino. 2024. "PGT-M for Premature Ovarian Failure Related to CGG Repeat Expansion of the FMR1 Gene" Genes 15, no. 1: 6. https://doi.org/10.3390/genes15010006
APA StylePersico, T., Tranquillo, M. L., Seracchioli, R., Zuccarello, D., & Sorrentino, U. (2024). PGT-M for Premature Ovarian Failure Related to CGG Repeat Expansion of the FMR1 Gene. Genes, 15(1), 6. https://doi.org/10.3390/genes15010006





