Genetic Diversity and Sequence Conservation of Peptide-Binding Regions of MHC Class I Genes in Pig, Cattle, Chimpanzee, and Human
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of DNA Sequences
2.2. Sequence Alignment and Phylogenetic Analysis
2.3. Determination of Consensus Amino-Acid Sequences and Genetic Distance Analysis
2.4. Computation of Amino-Acid Conservation, Diversity, and Nucleotide Substitution Rate
2.5. Estimation of Gene Divergence Times
2.6. Statistical Analysis
3. Results
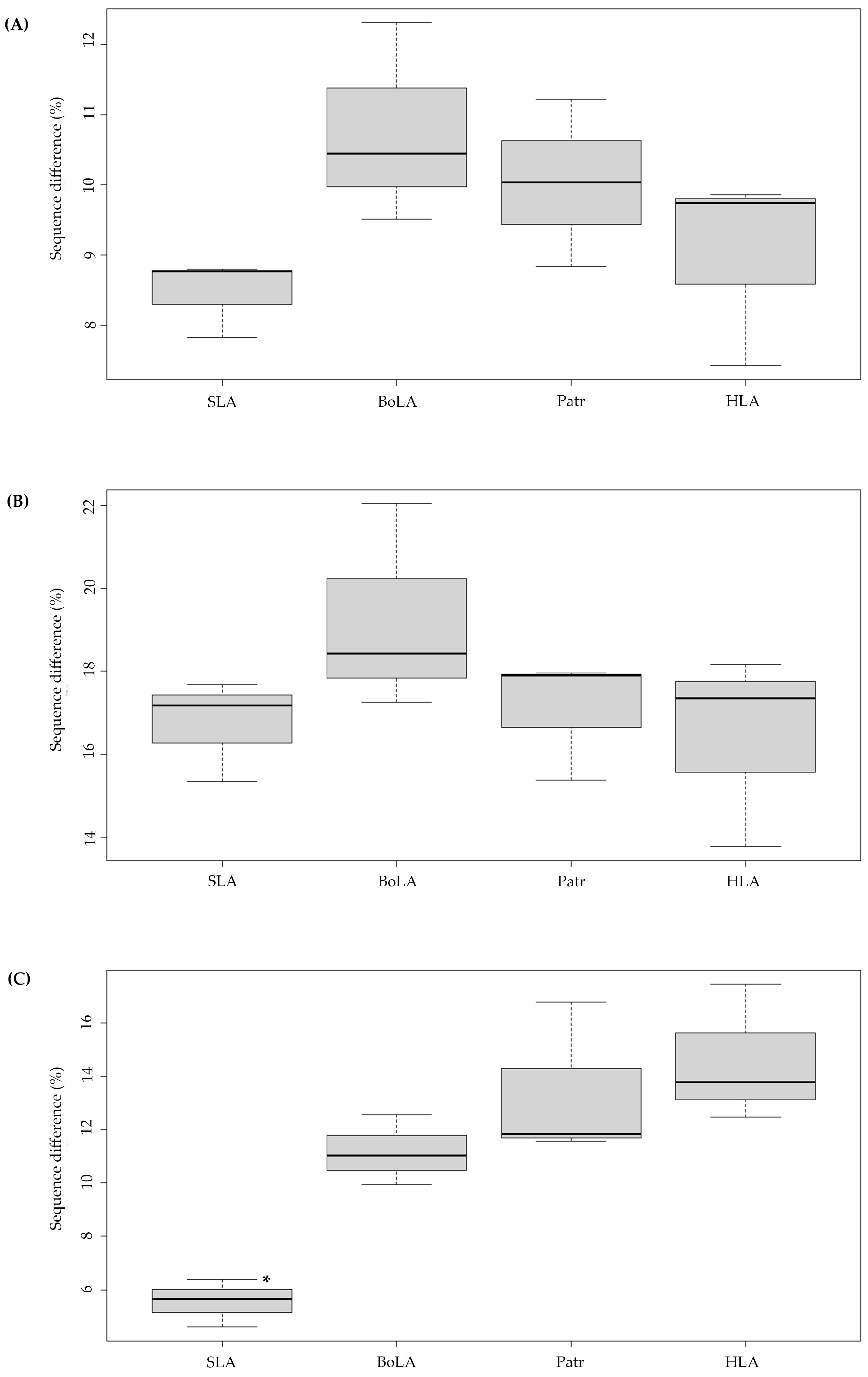
3.1. Intraspecies Variation of Nucleotide Diversity in the Peptide-Binding Region of Classical MHC Class I Genes
3.2. High Amino-Acid Sequence Diversity in the PBR of Pigs and Cattle MHC Class I Genes
3.3. Increased Nonsynonymous Substitutions in MHC Class I PBR in Artiodactyl Species than in Primates
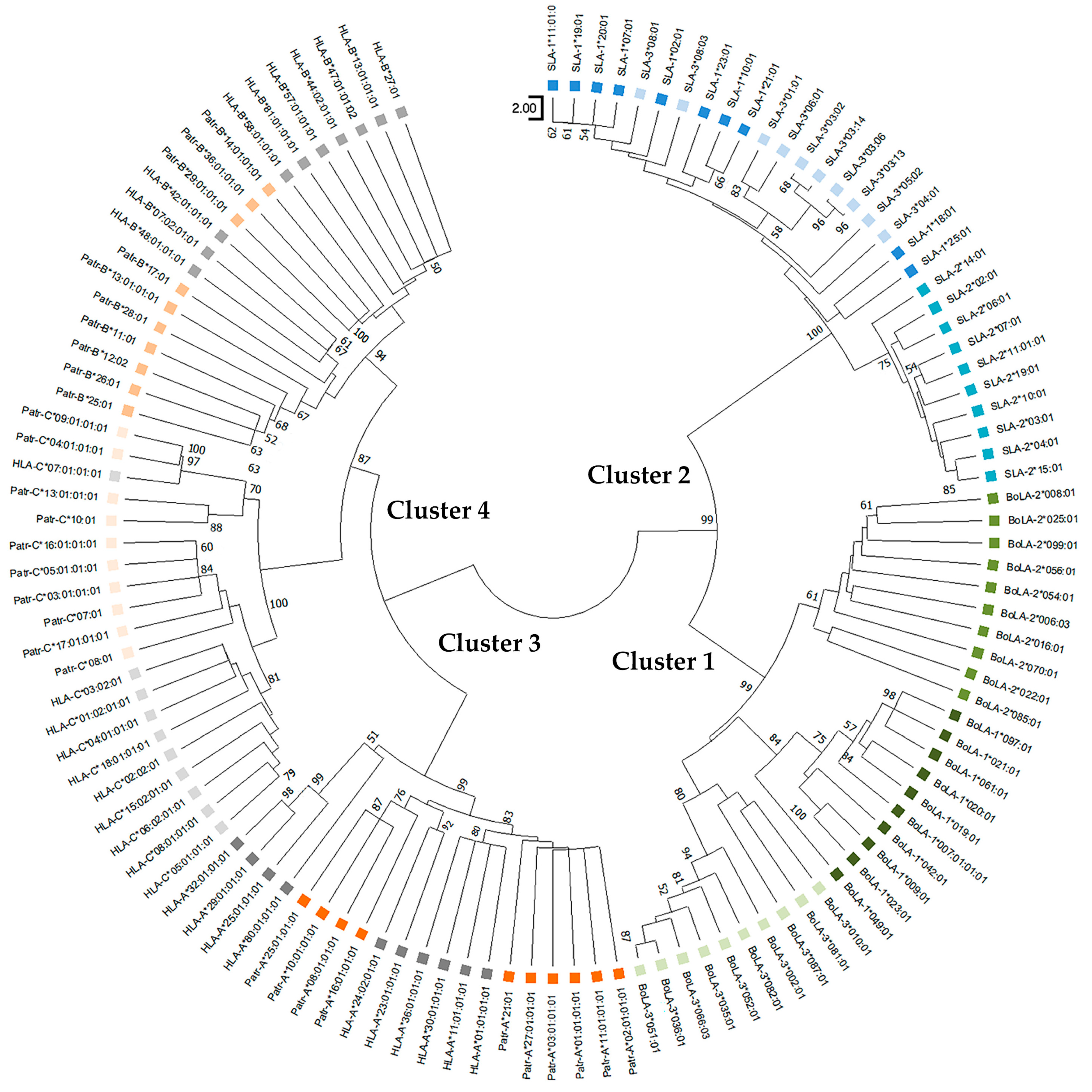
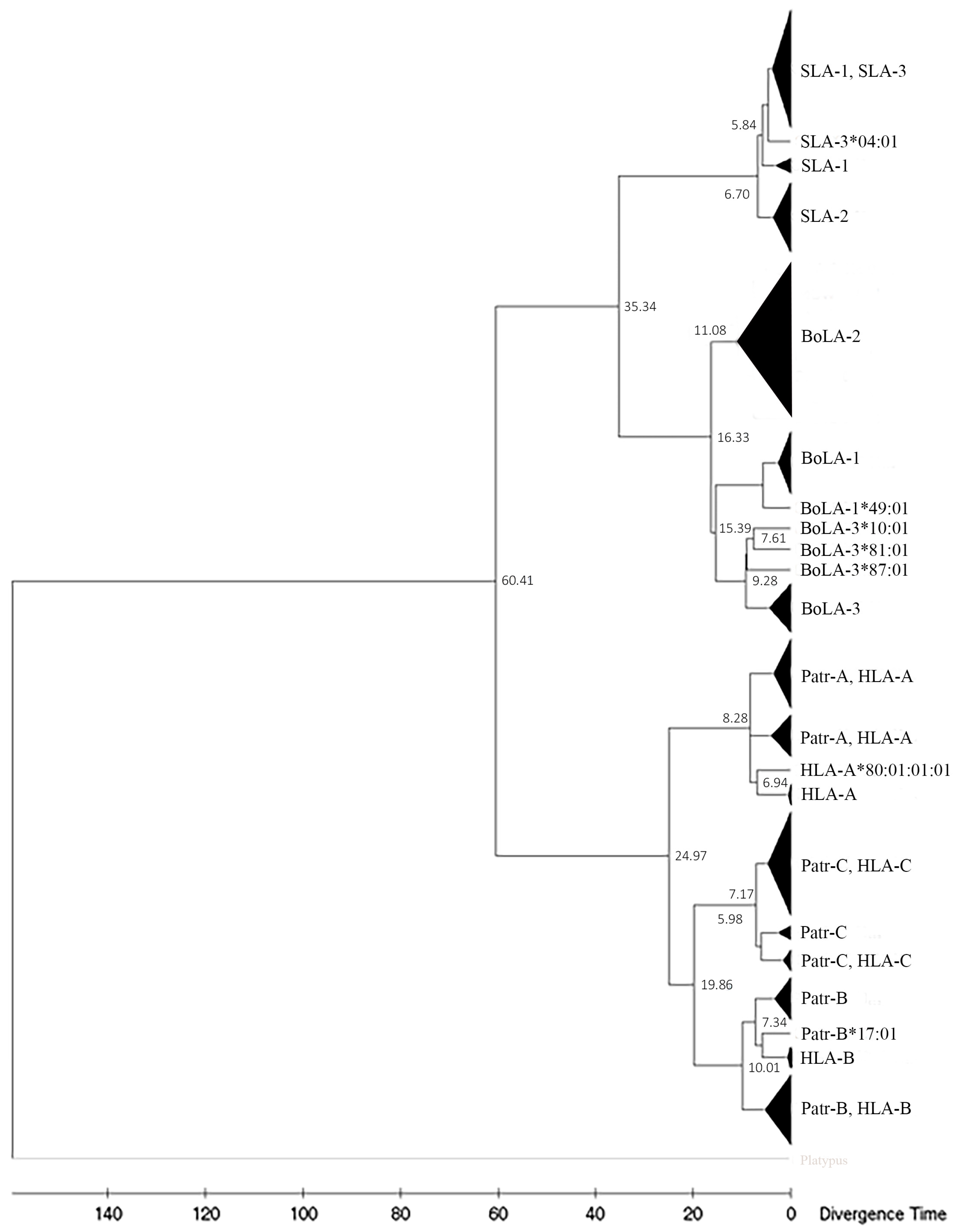
3.4. Phylogenetics Analysis
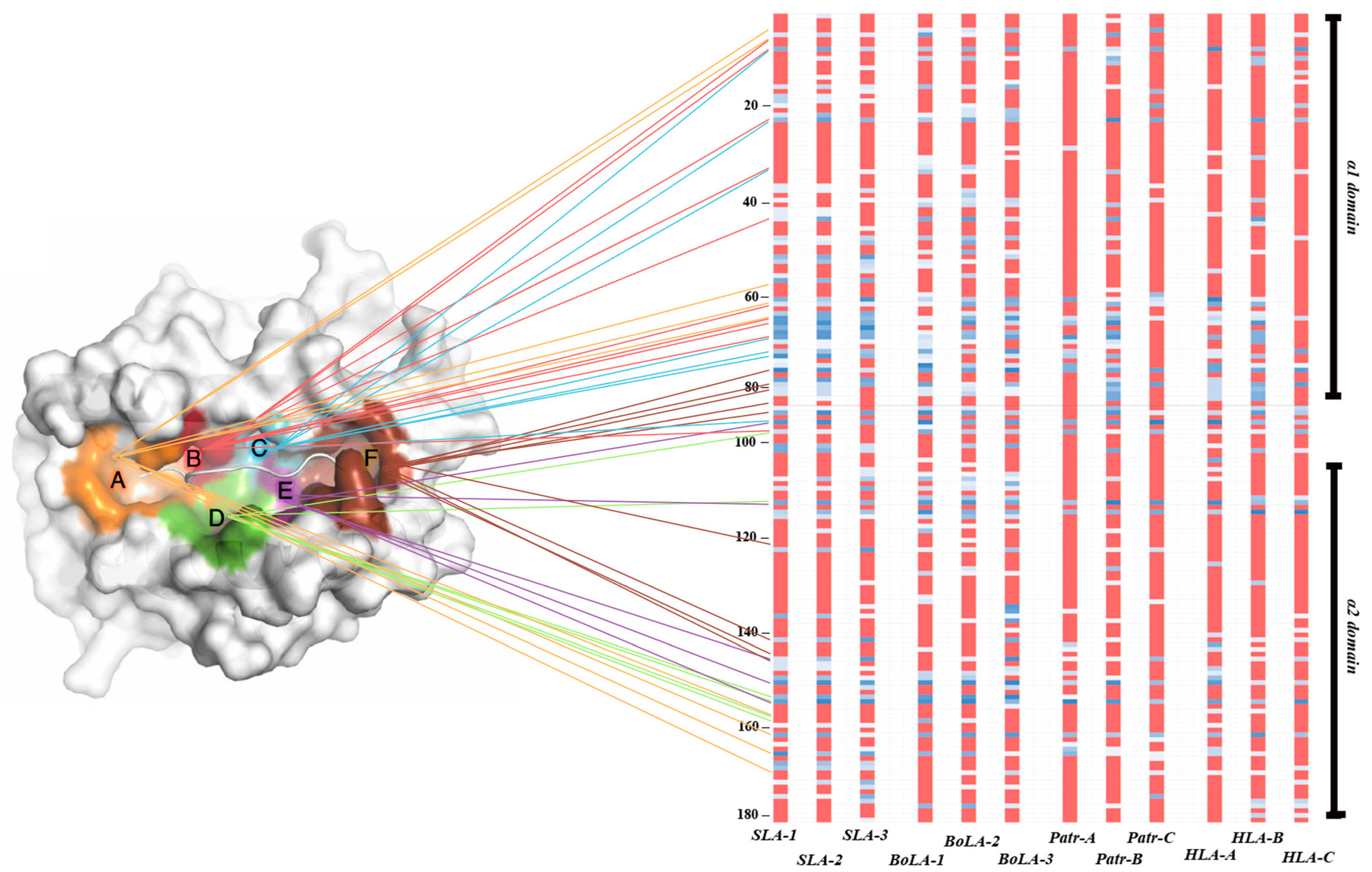
3.5. Intraspecies Conservation of the Genetic Diversity Level for Peptide-Binding Pockets A and E
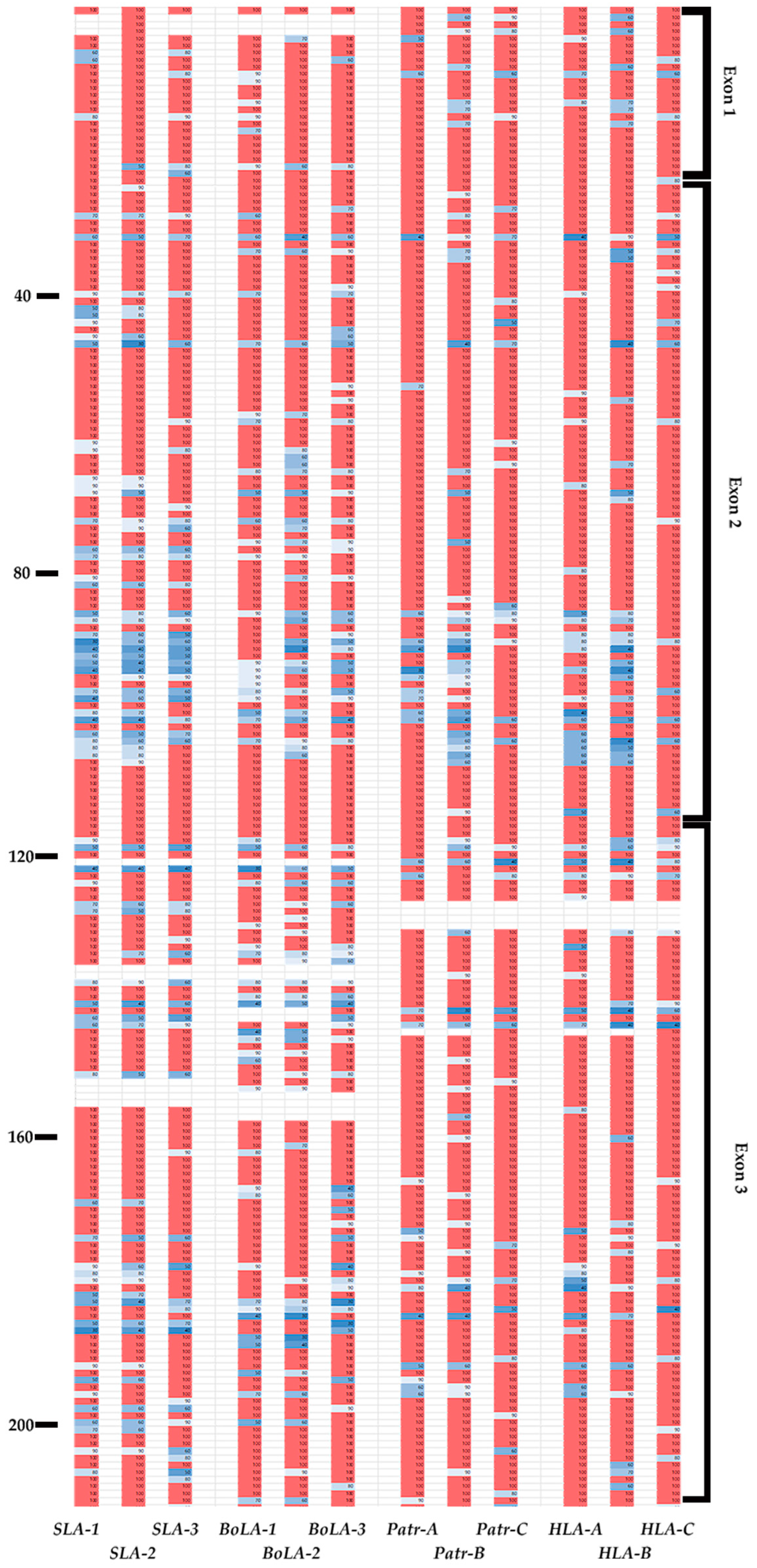
3.6. Presence of Conserved Sites in the Epitope-Binding Region of MHC Class I Genes across Different Species
3.7. Divergence Time of MHC Class I Genes between Artiodactyls and Primate Lineages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trowsdale, J. Both man & bird & beast: Comparative organization of MHC genes. Immunogenetics 1995, 41, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F.; Kasahara, M. Comparative genomics of the MHC: Glimpses into the evolution of the adaptive immune system. Immunity 2001, 15, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kulski, J.K.; Shiina, T.; Anzai, T.; Kohara, S.; Inoko, H. Comparative genomic analysis of the MHC: The evolution of class I duplication blocks, diversity and complexity from shark to man. Immunol. Rev. 2002, 190, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Benacerraf, B. Role of MHC gene products in immune regulation. Science 1981, 212, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Lechler, R.; Batchelor, R.; Lombardi, G. The relationship between MHC restricted and allospecific T cell recognition. Immunol. Lett. 1991, 29, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.P.-Y.; Trowsdale, J. Genetic Control of MHC Class II Expression. Cell 2002, 109, S21–S33. [Google Scholar] [CrossRef]
- Sette, A.; Sidney, J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and-B polymorphism. Immunogenetics 1999, 50, 201–212. [Google Scholar] [CrossRef]
- Sidney, J.; Peters, B.; Frahm, N.; Brander, C.; Sette, A. HLA class I supertypes: A revised and updated classification. BMC Immunol. 2008, 9, 1. [Google Scholar] [CrossRef]
- Trägärdh, L.; Rask, L.; Wiman, K.; Fohlman, J.; Peterson, P.A. Amino acid sequence of an immunoglobulin-like HLA antigen heavy chain domain. Proc. Natl. Acad. Sci. USA 1979, 76, 5839–5842. [Google Scholar] [CrossRef]
- Bjorkman, P.J.; Saper, M.A.; Samraoui, B.; Bennett, W.S.; Strominger, J.T.; Wiley, D.C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 1987, 329, 506–512. [Google Scholar] [CrossRef]
- Nikolich-Zugich, J.; Fremont, D.H.; Miley, M.J.; Messaoudi, I. The role of mhc polymorphism in anti-microbial resistance. Microbes Infect. 2004, 6, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Das, S.; Guo, P.; Cooper, M.D. Chapter 4—The Evolution of Adaptive Immunity in Vertebrates. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 109, pp. 125–157. [Google Scholar]
- Lu, X.; Wu, S.; Blackwell, C.E.; Humphreys, R.E.; Von Hofe, E.; Xu, M. Suppression of major histocompatibility complex class II-associated invariant chain enhances the potency of an HIV gp120 DNA vaccine. Immunology 2007, 120, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kubinak, J.L.; Ruff, J.S.; Hyzer, C.W.; Slev, P.R.; Potts, W.K. Experimental viral evolution to specific host MHC genotypes reveals fitness and virulence trade-offs in alternative MHC types. Proc. Natl. Acad. Sci. USA 2012, 109, 3422–3427. [Google Scholar] [CrossRef]
- MacKay, K.; Eyre, S.; Myerscough, A.; Milicic, A.; Barton, A.; Laval, S.; Barrett, J.; Lee, D.; White, S.; John, S.; et al. Whole-genome linkage analysis of rheumatoid arthritis susceptibility loci in 252 affected sibling pairs in the United Kingdom. Arthritis Rheum. 2002, 46, 632–639. [Google Scholar] [CrossRef]
- Takeshima, S.-N.; Corbi-Botto, C.; Giovambattista, G.; Aida, Y. Genetic diversity of BoLA-DRB3 in South American Zebu cattle populations. BMC Genet. 2018, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Satapornpong, P.; Jinda, P.; Jantararoungtong, T.; Koomdee, N.; Chaichan, C.; Pratoomwun, J.; Na Nakorn, C.; Aekplakorn, W.; Wilantho, A.; Ngamphiw, C.; et al. Genetic Diversity of HLA Class I and Class II Alleles in Thai Populations: Contribution to Genotype-Guided Therapeutics. Front. Pharmacol. 2020, 11, 78. [Google Scholar] [CrossRef]
- Le, M.T.; Choi, H.; Lee, H.; Le, V.C.Q.; Ahn, B.; Ho, C.-S.; Hong, K.; Song, H.; Kim, J.-H.; Park, C. SLA-1 Genetic Diversity in Pigs: Extensive Analysis of Copy Number Variation, Heterozygosity, Expression, and Breed Specificity. Sci. Rep. 2020, 10, 743. [Google Scholar] [CrossRef]
- Tsuji, K.; Aizawa, M.; Sasazuki, T. HLA 1991: In Proceedings of the Eleventh International Histocompatibility Workshop and Conference, Yokohama, Japan, 6–13 November 1991; Oxford University Press: Oxford, UK, 1992; Volume 2. [Google Scholar]
- Nei, M.; Gu, X.; Sitnikova, T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc. Natl. Acad. Sci. USA 1997, 94, 7799–7806. [Google Scholar] [CrossRef]
- Basham, T.Y.; Nickoloff, B.J.; Merigan, T.C.; Morhenn, V.B. Recombinant gamma interferon induces HLA-DR expression on cultured human keratinocytes. J. Investig. Dermatol. 1984, 83, 88–90. [Google Scholar] [CrossRef]
- Palankar, R.; Skirtach, A.G.; Kreft, O.; Bedard, M.; Garstka, M.; Gould, K.; Mohwald, H.; Sukhorukov, G.B.; Winterhalter, M.; Springer, S. Controlled intracellular release of peptides from microcapsules enhances antigen presentation on MHC class I molecules. Small 2009, 5, 2168–2176. [Google Scholar] [CrossRef]
- Takahata, N.; Nei, M. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics 1990, 124, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Nei, M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 1988, 335, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Nei, M. Evolution of the major histocompatibility complex: Independent origin of nonclassical class I genes in different groups of mammals. Mol. Biol. Evol. 1989, 6, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.-Q.; He, K.; Sun, D.-D.; Ma, M.-Y.; Ge, Y.-F.; Fang, S.-G.; Wan, Q.-H. Balancing selection and recombination as evolutionary forces caused population genetic variations in golden pheasant MHC class I genes. BMC Evol. Biol. 2016, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Moroldo, M.; Perdomo-Sabogal, A.; Mach, N.; Marthey, S.; Lecardonnel, J.; Wahlberg, P.; Chong, A.Y.; Estellé, J.; Ho, S.Y.W.; et al. Inferring the evolution of the major histocompatibility complex of wild pigs and peccaries using hybridisation DNA capture-based sequencing. Immunogenetics 2018, 70, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Babiuk, S.; Horseman, B.; Zhang, C.; Bickis, M.; Kusalik, A.; Schook, L.B.; Abrahamsen, M.S.; Pontarollo, R. BoLA class I allele diversity and polymorphism in a herd of cattle. Immunogenetics 2007, 59, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sckisel, G.D.; Bouchlaka, M.N.; Monjazeb, A.M.; Crittenden, M.; Curti, B.D.; Wilkins, D.E.; Alderson, K.A.; Sungur, C.M.; Ames, E.; Mirsoian, A.; et al. Out-of-Sequence Signal 3 Paralyzes Primary CD4(+) T-Cell-Dependent Immunity. Immunity 2015, 43, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Svitek, N.; Nzau, B.; Steinaa, L.; Nene, V. A method to discriminate between closely related bovine major histocompatibility complex class I alleles by combining established PCR-SSP assays with RFLPs. Tissue Antigens 2015, 85, 278–282. [Google Scholar] [CrossRef]
- Adams, E.J.; Cooper, S.; Parham, P. A novel, nonclassical MHC class I molecule specific to the common chimpanzee. J. Immunol. 2001, 167, 3858–3869. [Google Scholar] [CrossRef]
- Anzai, T.; Shiina, T.; Kimura, N.; Yanagiya, K.; Kohara, S.; Shigenari, A.; Yamagata, T.; Kulski, J.K.; Naruse, T.K.; Fujimori, Y. Comparative sequencing of human and chimpanzee MHC class I regions unveils insertions/deletions as the major path to genomic divergence. Proc. Natl. Acad. Sci. USA 2003, 100, 7708–7713. [Google Scholar] [CrossRef]
- Geller, R.; Adams, E.J.; Guethlein, L.A.; Little, A.-M.; Madrigal, A.J.; Parham, P. Linkage of Patr-AL to Patr-A and-B in the major histocompatibility complex of the common chimpanzee (Pan troglodytes). Immunogenetics 2002, 54, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Le, M.T.; Lee, H.; Choi, M.K.; Cho, H.S.; Nagasundarapandian, S.; Kwon, O.J.; Kim, J.H.; Seo, K.; Park, J.K.; et al. Sequence variations of the locus-specific 5’ untranslated regions of SLA class I genes and the development of a comprehensive genomic DNA-based high-resolution typing method for SLA-2. Tissue Antigens 2015, 86, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Le, M.; Choi, H.; Choi, M.K.; Cho, H.; Kim, J.H.; Seo, H.G.; Cha, S.Y.; Seo, K.; Dadi, H.; Park, C. Development of a simultaneous high resolution typing method for three SLA class II genes, SLA-DQA, SLA-DQB1, and SLA-DRB1 and the analysis of SLA class II haplotypes. Gene 2015, 564, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Youk, S.; Le, M.T.; Kang, M.; Ahn, B.; Choi, M.; Kim, K.; Kim, T.H.; Kim, J.H.; Ho, C.S.; Park, C. Development of a high-resolution typing method for SLA-3, swine MHC class I antigen 3. Anim. Genet. 2022, 53, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, S.M.S.; Taylor, D.W.; Russell, G.C. Polymorphism of bovine major histocompatibility complex (MHC) class I genes revealed by polymerase chain reaction (PCR) and restriction enzyme analysis. Anim. Genet. 2001, 32, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.A.; Staines, K.A.; Stear, M.J.; Hensen, E.J.; Morrison, W.I. DNA typing for BoLA class I using sequence-specific primers (PCR-SSP). Eur. J. Immunogenet. 1998, 25, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.A.; Holmes, E.C.; Staines, K.A.; Smith, K.B.; Stear, M.J.; McKeever, D.J.; MacHugh, N.D.; Morrison, W.I. Variation in the number of expressed MHC genes in different cattle class I haplotypes. Immunogenetics 1999, 50, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Boyson, J.E.; Shufflebotham, C.; Cadavid, L.F.; Urvater, J.A.; Knapp, L.A.; Hughes, A.L.; Watkins, D.I. The MHC class I genes of the rhesus monkey. Different evolutionary histories of MHC class I and II genes in primates. J. Immunol. 1996, 156, 4656–4665. [Google Scholar] [CrossRef]
- Wroblewski, E.E.; Parham, P.; Guethlein, L.A. Two to tango: Co-evolution of hominid natural killer cell receptors and MHC. Front. Immunol. 2019, 10, 177. [Google Scholar] [CrossRef]
- Klein, J.; Figueroa, F. Evolution of the major histocompatibility complex. Crit. Rev. Immunol. 1986, 6, 295–386. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Ward, F.E.; Ennis, P.D.; Jackson, A.P.; Parham, P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature 1988, 335, 268–271. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.N.; Boyson, J.E.; Liu, X.; Garber, T.L.; Hughes, A.L.; Bontrop, R.E.; Watkins, D.I. A uniquely high level of recombination at the HLA-B locus. Proc. Natl. Acad. Sci. USA 1994, 91, 5893–5897. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.J.; Thomson, G.; Parham, P. Evidence for an HLA-C-like locus in the orangutan Pongo pygmaeus. Immunogenetics 1999, 49, 865–871. [Google Scholar] [PubMed]
- Fukami-Kobayashi, K.; Shiina, T.; Anzai, T.; Sano, K.; Yamazaki, M.; Inoko, H.; Tateno, Y. Genomic evolution of MHC class I region in primates. Proc. Natl. Acad. Sci. USA 2005, 102, 9230–9234. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Franzo-Romain, M.H.; Lee, Y.J.; Lee, J.H.; Smith, D.M. Sequence-based characterization of swine leucocyte antigen alleles in commercially available porcine cell lines. Int. J. Immunogenet. 2009, 36, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.J.; Andersson, L.; Mikko, S.; Ellis, S.A.; Hensen, E.J.; Lewin, H.A.; Muggli-Cockett, N.E.; Poel, J.J.V.D.; Russell, G.C. Nomenclature for factors of the BoLA system, 1996: Report of the ISAG BoLA Nomenclature Committee. Anim. Genet. 1997, 28, 159–168. [Google Scholar] [CrossRef]
- Maccari, G.; Robinson, J.; Bontrop, R.E.; Otting, N.; de Groot, N.G.; Ho, C.-S.; Ballingall, K.T.; Marsh, S.G.E.; Hammond, J.A. IPD-MHC: Nomenclature requirements for the non-human major histocompatibility complex in the next-generation sequencing era. Immunogenetics 2018, 70, 619–623. [Google Scholar] [CrossRef]
- Marsh, S.G.E.; Albert, E.D.; Bodmer, W.F.; Bontrop, R.E.; Dupont, B.; Erlich, H.A.; Fernández-Viña, M.; Geraghty, D.E.; Holdsworth, R.; Hurley, C.K.; et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens 2010, 75, 291–455. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Antao, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef]
- Garcia-Boronat, M.; Diez-Rivero, C.M.; Reinherz, E.L.; Reche, P.A. PVS: A web server for protein sequence variability analysis tuned to facilitate conserved epitope discovery. Nucleic Acids Res. 2008, 36, W35–W41. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.K.M.F.; Delhomme, N.; Nandi, S.; Fällman, M. ProkSeq for complete analysis of RNA-Seq data from prokaryotes. Bioinformatics 2021, 37, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Chujoh, Y.; Sobao, Y.; Miwa, K.; Kaneko, Y.; Takiguchi, M. The role of anchor residues in the binding of peptides to HLA-A* 1101 molecules. Tissue Antigens 1998, 52, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Szeto, C.; Gras, S. The pockets guide to HLA class I molecules. Biochem. Soc. Trans. 2021, 49, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front. Zool. 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Apanius, V.; Penn, D.; Slev, P.R.; Ruff, L.R.; Potts, W.K. The Nature of Selection on the Major Histocompatibility Complex. Crit. Rev. Immunol. 1997, 17, 179–224. [Google Scholar] [CrossRef]
- Reche, P.A.; Reinherz, E.L. Sequence Variability Analysis of Human Class I and Class II MHC Molecules: Functional and Structural Correlates of Amino Acid Polymorphisms. J. Mol. Biol. 2003, 331, 623–641. [Google Scholar] [CrossRef]
- Bernatchez, L.; Landry, C. MHC studies in nonmodel vertebrates: What have we learned about natural selection in 15 years? J. Evol. Biol. 2003, 16, 363–377. [Google Scholar] [CrossRef]
- Gaudieri, S.; Dawkins, R.L.; Habara, K.; Kulski, J.K.; Gojobori, T. SNP Profile within the Human Major Histocompatibility Complex Reveals an Extreme and Interrupted Level of Nucleotide Diversity. Genome Res. 2000, 10, 1579–1586. [Google Scholar] [CrossRef][Green Version]
- Cruz-López, M.; Fernández, G.; Hipperson, H.; Palacios, E.; Cavitt, J.; Galindo-Espinosa, D.; Gómez del Angel, S.; Pruner, R.; Gonzalez, O.; Burke, T.; et al. Allelic diversity and patterns of selection at the major histocompatibility complex class I and II loci in a threatened shorebird, the Snowy Plover (Charadrius nivosus). BMC Evol. Biol. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.R.; Hein, W.R. A large proportion of bovine T cells express the γδ T cell receptor and show a distinct tissue distribution and surface phenotype. Int. Immunol. 1989, 1, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Šinkora, M.; Butler, J.E. The ontogeny of the porcine immune system. Dev. Comp. Immunol. 2009, 33, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Born, W.; Cady, C.; Jones-Carson, J.; Mukasa, A.; Lahn, M.; O’brien, R. Immunoregulatory Functions of γδ T Cells. In Advances in Immunology; Dixon, F.J., Ed.; Academic Press: Cambridge, MA, USA, 1998; Volume 71, pp. 77–144. [Google Scholar]
- Sommer, S.; Hommen, U. Modelling the effects of life-history traits and changing ecological conditions on the population dynamics and persistence of the endangered Malagasy giant jumping rat (Hypogeomys antimena). Anim. Conserv. Forum 2000, 3, 333–343. [Google Scholar] [CrossRef]
- Sommer, S.; Volahy, A.T.; Seal, U.S. A population and habitat viability assessment for the highly endangered giant jumping rat (Hypogeomys antimena), the largest extant endemic rodent of Madagascar. Anim. Conserv. Forum 2002, 5, 263–273. [Google Scholar] [CrossRef]
- Sommer, S. Effects of habitat fragmentation and changes of dispersal behaviour after a recent population decline on the genetic variability of noncoding and coding DNA of a monogamous Malagasy rodent. Mol. Ecol. 2003, 12, 2845–2851. [Google Scholar] [CrossRef]
- Chappell, P.E.; Meziane, E.K.; Harrison, M.; Magiera, Ł.; Hermann, C.; Mears, L.; Wrobel, A.G.; Durant, C.; Nielsen, L.L.; Buus, S.; et al. Expression levels of MHC class I molecules are inversely correlated with promiscuity of peptide binding. eLife 2015, 4, e05345. [Google Scholar] [CrossRef]
- Kaufman, J. Generalists and Specialists: A New View of How MHC Class I Molecules Fight Infectious Pathogens. Trends Immunol. 2018, 39, 367–379. [Google Scholar] [CrossRef]
- Manczinger, M.; Boross, G.; Kemény, L.; Müller, V.; Lenz, T.L.; Papp, B.; Pál, C. Pathogen diversity drives the evolution of generalist MHC-II alleles in human populations. PLoS Biol. 2019, 17, e3000131. [Google Scholar] [CrossRef]
- Leakey, M.G.; Feibel, C.S.; McDougall, I.; Ward, C.; Walker, A. New specimens and confirmation of an early age for Australopithecus anamensis. Nature 1998, 393, 62–66. [Google Scholar] [CrossRef]
- Glazko, G.V.; Nei, M. Estimation of Divergence Times for Major Lineages of Primate Species. Mol. Biol. Evol. 2003, 20, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Filipski, A.; Swarna, V.; Walker, A.; Hedges, S.B. Placing confidence limits on the molecular age of the human–chimpanzee divergence. Proc. Natl. Acad. Sci. USA 2005, 102, 18842–18847. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Pevzner, P.A.; O’Brien, S.J. Mammalian phylogenomics comes of age. Trends Genet. 2004, 20, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.-L.; Postlethwait, J. Preservation of Duplicate Genes by Complementary, Degenerative Mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [CrossRef]
- van Oosterhout, C. A new theory of MHC evolution: Beyond selection on the immune genes. Proc. R. Soc. B Biol. Sci. 2008, 276, 657–665. [Google Scholar] [CrossRef]
- Salter, R.D.; Benjamin, R.J.; Wesley, P.K.; Buxton, S.E.; Garrett, T.P.J.; Clayberger, C.; Krensky, A.M.; Norment, A.M.; Littman, D.R.; Parham, P. A binding site for the T-cell co-receptor CD8 on the α3 domain of HLA-A2. Nature 1990, 345, 41–46. [Google Scholar] [CrossRef]


| Sequence Differences (%) a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BoLA | HLA | Patr | SLA | ||||||||||
| 1 | 2 | 3 | A | B | C | A | B | C | 1 | 2 | 3 | ||
| BoLA-1 | |||||||||||||
| BoLA-2 | non-PBR b | 9.94 | |||||||||||
| PBR c | 18.43 | ||||||||||||
| BoLA-3 | non-PBR | 12.57 | 11.02 | ||||||||||
| PBR | 22.04 | 17.25 | |||||||||||
| HLA-A | non-PBR | 27.66 | 25.84 | 29.94 | |||||||||
| PBR | 25.10 | 23.69 | 26.72 | ||||||||||
| HLA-B | non-PBR | 24.86 | 23.04 | 26.28 | 13.79 | ||||||||
| PBR | 22.40 | 23.06 | 25.46 | 18.16 | |||||||||
| HLA-C | non-PBR | 29.02 | 27.01 | 29.99 | 17.45 | 12.47 | |||||||
| PBR | 23.75 | 21.92 | 23.55 | 17.34 | 13.77 | ||||||||
| Patr-A | non-PBR | 26.20 | 24.39 | 28.62 | 4.26 | 12.83 | 16.86 | ||||||
| PBR | 24.69 | 23.16 | 26.29 | 9.84 | 19.09 | 17.41 | |||||||
| Patr-B | non-PBR | 24.24 | 22.27 | 25.62 | 12.97 | 3.99 | 11.68 | 11.83 | |||||
| PBR | 22.70 | 23.26 | 25.63 | 17.34 | 12.51 | 13.86 | 17.90 | ||||||
| Patr-C | non-PBR | 29.26 | 27.21 | 30.18 | 17.27 | 12.22 | 5.32 | 16.77 | 11.56 | ||||
| PBR | 24.66 | 22.98 | 24.60 | 18.57 | 15.47 | 7.93 | 17.96 | 15.38 | |||||
| SLA-1 | non-PBR | 21.18 | 21.05 | 23.80 | 28.47 | 27.27 | 29.72 | 27.22 | 27.44 | 29.67 | |||
| PBR | 26.28 | 24.62 | 27.02 | 26.69 | 26.73 | 25.50 | 26.30 | 26.62 | 26.15 | ||||
| SLA-2 | non-PBR | 22.46 | 21.64 | 24.14 | 29.29 | 27.28 | 30.17 | 28.00 | 27.44 | 30.13 | 5.66 | ||
| PBR | 25.87 | 24.61 | 26.83 | 26.90 | 26.12 | 25.29 | 26.56 | 26.12 | 26.07 | 15.34 | |||
| SLA-3 | non-PBR | 21.57 | 21.12 | 23.60 | 29.17 | 27.78 | 29.82 | 27.82 | 27.92 | 29.81 | 4.59 | 6.38 | |
| PBR | 25.29 | 24.31 | 26.43 | 26.74 | 25.70 | 24.92 | 26.05 | 25.55 | 25.24 | 17.68 | 17.18 | ||
| Shannon Diversity Index (H) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | BoLA | HLA | Patr | SLA | |||||||||
| 1 * | 2 * | 3 * | A * | B * | C * | A * | B *,** | C | 1 * | 2 * | 3 * | ||
| PBR a | Entire region | 0.30 | 0.35 | 0.33 | 0.21 | 0.25 | 0.15 | 0.15 | 0.28 | 0.14 | 0.36 | 0.37 | 0.30 |
| α1 domain | 0.31 | 0.4 | 0.34 | 0.21 | 0.3 | 0.13 | 0.15 | 0.37 | 0.12 | 0.44 | 0.44 | 0.31 | |
| α2 domain | 0.33 | 0.34 | 0.35 | 0.23 | 0.22 | 0.18 | 0.16 | 0.21 | 0.16 | 0.33 | 0.34 | 0.3 | |
| Non-PBR b | 0.06 | 0.10 | 0.13 | 0.10 | 0.08 | 0.08 | 0.03 | 0.05 | 0.10 | 0.07 | 0.05 | 0.08 | |
| Species | Locus | No. of Variable Sites (%) a | No. of Synonymous Sites (%) b (A) | No. of Nonsynonymous Sites (%) b (B) | (A)/(B) Ratio | Ka/Ks Ratio | |
|---|---|---|---|---|---|---|---|
| α1 Domain | α2 Domain | ||||||
| Human | HLA-A | 71 (13.00) | 12 (2.20) | 43 (7.88) | 0.28 | 1.74 | 1.57 |
| HLA-B | 83 (15.20) | 17 (3.11) | 45 (8.24) | 0.38 | 1.40 | 1.20 | |
| HLA-C | 58 (10.62) | 13 (2.38) | 31 (5.68) | 0.42 | 0.75 | 1.18 | |
| Mean | 70.67 (12.94) | 14.00 (2.56) | 39.67 (7.26) | 0.36 | 1.30 | 1.32 | |
| Chimpanzee | Patr-A | 54 (9.89) | 16 (2.93) | 27 (4.95) | 0.59 | 0.89 | 1.12 |
| Patr-B | 100 (18.32) | 22 (4.03) | 58 (10.62) | 0.38 | 1.08 | 1.21 | |
| Patr-C | 52 (9.52) | 10 (1.83) | 30 (5.49) | 0.33 | 0.70 | 1.71 | |
| Mean | 68.67 (12.58) | 16.00 (2.93) | 38.33 (7.02) | 0.44 | 0.89 | 1.35 | |
| Cattle | BoLA-1 | 128 (23.44) | 30 (5.49) | 59 (10.81) | 0.51 | 0.63 | 1.63 |
| BoLA-2 | 124 (22.71) | 23 (4.21) | 55 (10.07) | 0.42 | 1.05 | 1.65 | |
| BoLA-3 | 119 (21.79) | 26 (4.76) | 60 (10.99) | 0.43 | 1.80 | 1.18 | |
| Mean | 123.67 (22.65) | 26.33 (4.82) | 58.00 (10.62) | 0.45 | 1.16 | 1.49 | |
| Pig | SLA-1 | 125 (22.89) | 17 (3.11) | 56 (10.26) | 0.30 | 1.54 | 1.66 |
| SLA-2 | 135 (24.73%) | 29 (5.31%) | 59 (10.81%) | 0.49 | 1.16 | 1.86 | |
| SLA-3 | 105 (19.23%) | 15 (2.75%) | 71 (13.00%) | 0.21 | 2.27 | 1.49 | |
| Mean | 121.67 (22.28%) | 20.33 (3.72%) | 62.00 (11.3%) | 0.34 | 1.66 | 1.67 | |
| Sheep | Ovar-N c | 142 (26.01) | 26 (4.76) | 61 (11.17) | 0.43 | 1.41 | 1.93 |
| Shannon Diversity Index (H) | ||||||
|---|---|---|---|---|---|---|
| Locus | Peptide-Binding Pockets a | |||||
| A | B | C | D | E * | F | |
| SLA-1 | 0.65 | 1.12 | 1.48 | 1.01 | 1.59 | 0.74 |
| SLA-2 | 0.58 | 1.18 | 1.55 | 1.16 | 1.75 | 0.88 |
| SLA-3 | 0.38 | 0.63 | 0.96 | 0.68 | 1.3 | 0.66 |
| Mean | 0.54 | 0.98 | 1.33 | 0.95 | 1.55 | 0.76 |
| BoLA-1 | 0.39 | 0.72 | 1.15 | 1.55 | 1.75 | 0.7 |
| BoLA-2 | 0.72 | 1.34 | 1.2 | 1.65 | 1.89 | 0.66 |
| BoLA-3 | 0.69 | 0.98 | 1.23 | 1.28 | 1.7 | 0.85 |
| Mean | 0.6 | 1.01 | 1.19 | 1.49 | 1.78 | 0.74 |
| Patr-A | 0.37 | 0.66 | 0.91 | 0.9 | 1.12 | 0.28 |
| Patr-B | 0.41 | 1.05 | 0.92 | 0.84 | 1.49 | 0.8 |
| Patr-C | 0.44 | 0.42 | 0.55 | 0.86 | 1.36 | 0.48 |
| Mean | 0.41 | 0.71 | 0.79 | 0.87 | 1.32 | 0.46 |
| HLA-A | 0.47 | 0.58 | 0.78 | 0.78 | 1.38 | 0.47 |
| HLA-B | 0.45 | 1.07 | 0.97 | 0.64 | 1.08 | 0.88 |
| HLA-C | 0.2 | 0.47 | 0.64 | 0.81 | 1.01 | 0.57 |
| Mean | 0.37 | 0.71 | 0.8 | 0.74 | 1.15 | 0.64 |
| Ovar-N | 0.89 | 1.13 | 1.19 | 1.24 | 1.81 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youk, S.; Kang, M.; Ahn, B.; Koo, Y.; Park, C. Genetic Diversity and Sequence Conservation of Peptide-Binding Regions of MHC Class I Genes in Pig, Cattle, Chimpanzee, and Human. Genes 2024, 15, 7. https://doi.org/10.3390/genes15010007
Youk S, Kang M, Ahn B, Koo Y, Park C. Genetic Diversity and Sequence Conservation of Peptide-Binding Regions of MHC Class I Genes in Pig, Cattle, Chimpanzee, and Human. Genes. 2024; 15(1):7. https://doi.org/10.3390/genes15010007
Chicago/Turabian StyleYouk, Seungyeon, Mingue Kang, Byeongyong Ahn, Yangmo Koo, and Chankyu Park. 2024. "Genetic Diversity and Sequence Conservation of Peptide-Binding Regions of MHC Class I Genes in Pig, Cattle, Chimpanzee, and Human" Genes 15, no. 1: 7. https://doi.org/10.3390/genes15010007
APA StyleYouk, S., Kang, M., Ahn, B., Koo, Y., & Park, C. (2024). Genetic Diversity and Sequence Conservation of Peptide-Binding Regions of MHC Class I Genes in Pig, Cattle, Chimpanzee, and Human. Genes, 15(1), 7. https://doi.org/10.3390/genes15010007





