Alternative Traits for Genetic Evaluation of Mastitis Based on Lifetime Merit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1: Single Herd Follow Up
2.2. Experiment 2: Analysis of Israeli Herd Book Data
2.3. Statistical Analysis
3. Results
3.1. Experiment 1: Single Herd Follow Up
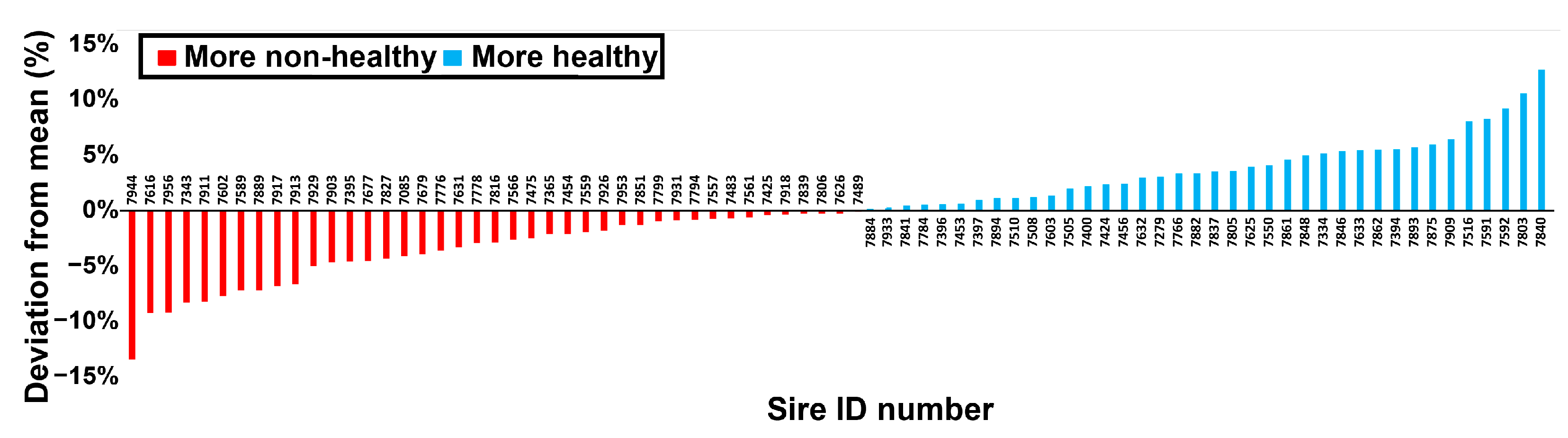
3.2. Experiment 2: Analysis of Israeli Herd Book Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halasa, T.; Huijps, K.; Osteras, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Quart. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Losinger, W.C. Economic impacts of reduced milk production associated with an increase in bulk-tank somatic cell count on US dairies. J. Am. Vet. Med. Assoc. 2005, 226, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- The Cattle Site. How Much Does Mastitis Cost Dairy Producers Annually? Available online: https://www.thecattlesite.com/focus/thermo-fisher-scientific/2335/bovine-diagnostics-how-much-does-mastitis-cost-dairy-producers-annually (accessed on 9 January 2024).
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The Role of Streptococcus spp. in Bovine Mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Gabler, M.T.; Tozer, P.R.; Heinrichs, A.J. Development of a cost analysis spreadsheet for calculating the costs to raise a replacement dairy heifer. J. Dairy Sci. 2000, 83, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.C.; Rushton, J.; Wathes, D.C. An empirical analysis of the cost of rearing dairy heifers from birth to first calving and the time taken to repay these costs. Animal 2017, 11, 1372–1380. [Google Scholar] [CrossRef]
- Hawkins, A.C. Evaluating Costs Associated with Management Decisions of Replacement Dairy Heifers and Their Impact on the Total Rearing Investment. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2019. [Google Scholar]
- National Animal Health Monitoring, S. Dairy 2014. Health and Management Practices on US. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_Mastitis.pdf (accessed on 9 January 2024).
- CDIC. Culling and Replacement Rates in Dairy Herds in Canada. Available online: https://agriculture.canada.ca/en/sector/animal-industry/canadian-dairy-information-centre/statistics-market-information/dairy-animal-genetics/culling-replacement (accessed on 9 January 2024).
- Pinedo, P.J.; De Vries, A.; Webb, D.W. Dynamics of culling risk with disposal codes reported by Dairy Herd Improvement dairy herds. J. Dairy Sci. 2010, 93, 2250–2261. [Google Scholar] [CrossRef]
- Weller, J.I.; Ezra, E.; Seroussi, E.; Gershoni, M. Genetic and Genomic Analysis of Cow Mortality in the Israeli Holstein Population. Genes 2023, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Yanga, D.S.; Jaja, I.F. Culling and mortality of dairy cows: Why it happens and how it can be mitigated. F1000Research 2017, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Carlen, E.; Strandberg, E.; Roth, A. Genetic parameters for clinical mastitis, somatic cell score, and production in the first three lactations of Swedish holstein cows. J. Dairy Sci. 2004, 87, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Windig, J.J.; Ouweltjes, W.; Ten Napel, J.; de Jong, G.; Veerkamp, R.F.; De Haas, Y. Combining somatic cell count traits for optimal selection against mastitis. J. Dairy Sci. 2010, 93, 1690–1701. [Google Scholar] [CrossRef]
- Urioste, J.I.; Franzen, J.; Windig, J.J.; Strandberg, E. Genetic relationships among mastitis and alternative somatic cell count traits in the first 3 lactations of Swedish Holsteins. J. Dairy Sci. 2012, 95, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Koeck, A.; Miglior, F.; Kelton, D.F.; Schenkel, F.S. Alternative somatic cell count traits to improve mastitis resistance in Canadian Holsteins. J. Dairy Sci. 2011, 95, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Kirsanova, E.; Heringstad, B.; Lewandowska-Sabat, A.; Olsaker, I. Alternative subclinical mastitis traits for genetic evaluation in dairy cattle. J. Dairy Sci. 2019, 102, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.E.; Heller, E.D.; Leitner, G. Long term effects of Escherichia coli mastitis. Vet. J. 2014, 201, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lavon, Y.; Ezra, E.; Leitner, G.; Wolfenson, D. Association of conception rate with pattern and level of somatic cell count elevation relative to time of insemination in dairy cows. J. Dairy Sci. 2011, 94, 4538–4545. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.P.; Gonzalez, R.N.; Hogan, J.S.; Jayarao, B.M.; Owens, W.E. Microbiological Procedures for the Diagnosis of Bovine udder Infection and Determination of Milk Quality, 4th ed.; The National Mastitis Council, Inc.: Verona, WI, USA, 2004. [Google Scholar]
- Seroussi, E.; Blum, S.E.; Krifucks, O.; Shirak, A.; Jacoby, S.; Leitner, G. Basal levels of CD18 antigen presenting cells in cow milk associate with copy number variation of Fc Gamma Receptors. Genes 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.C.; Barkema, H.W.; De Vries, A.; Kelton, D.F.; Orsel, K. Invited review: Academic and applied approach to evaluating longevity in dairy cows. J. Dairy Sci. 2020, 103, 11008–11024. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A. Symposium review: Why revisit dairy cattle productive lifespan? J. Dairy Sci. 2020, 103, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Sekyere, E.; Nyman, A.K.; Lindberg, M.; Adamie, B.A.; Agenas, S.; Hansson, H. Dairy cow longevity: Impact of animal health and farmers’ investment decisions. J. Dairy Sci. 2023, 106, 3509–3524. [Google Scholar] [CrossRef] [PubMed]
- VanRaden, P.M.; Cole, J.B.; Gaddis, K.L.P. Net Merit as a Measure of Lifetime Profit: 2014 Revision; Animal Genomics and Improvement Laboratory, Agricultural Research Service, USDA: Beltsville, MD, USA, 2018; pp. 20705–22350. [Google Scholar]
- Weller, J.I.; Ezra, E. The Israeli Selection Index. Available online: https://www.israeldairy.com/israeli-selection-index-2/ (accessed on 9 January 2024).
- Israel Cattle Breeders Association (ICBA). Sire Test 2023-11. Available online: https://akol.co.il/icbaapp/mivhanparim/ (accessed on 9 January 2024).
- Archer, S.C.; Mc Coy, F.; Wapenaar, W.; Green, M.J. Association between somatic cell count during the first lactation and the cumulative milk yield of cows in Irish dairy herds. J. Dairy Sci. 2014, 97, 2135–2144. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Intra-Mammary Infection Type | p [F] | ||
|---|---|---|---|---|
| Non-Infected (n = 65, 43.9%) | Subclinical (n = 64, 43.2%) | Clinical (n = 19, 12.8%) | ||
| Culled due to mastitis (%) | - | 7 (10.9%) | 11 (57.9%) | p < 0.001 |
| Culled due another reason | 3 (4.7%) | 6 (11%) | - | NS 1 |
| Finished first lactation | 62 (95.3%) | 50 (78.1%) | 8 (42.1%) | p < 0.001 2 |
| Mean SCC (×103 cells/mL) | 62 ± 6 | 263 ± 35 | 562 ± 114 | p < 0.001 |
| Parameter | Intra-Mammary Infection Type | Total (%) | ||
|---|---|---|---|---|
| Subclinical (n = 64, 77.1%) | Clinical (n = 19, 22.9%) | |||
| Estimated time of | Pre- or immediately post-partum (0–7 DIM) | 39 (69.6) | 15 (55.6) | 54 (65.1%) |
| Lactation (30–270 DIM) | 17 (30.4) | 12 (44.4) | 29 (34.9%) | |
| CoNS * (number per gland) | 46 (19/16/7/4) | 6 (4/2/-/-) | 52 (62.7%) | |
| Staphylococcus aureus | 1 | 2 | 3 (3.6%) | |
| Streptococcus spp. (number per gland) | 9 (8/1/-/-) | 12 (10/2/-/-) | 21 (25.3%) | |
| Escherichia coli | - | 7 | 7 (8.4%) | |
| Parameter | Intra-Mammary Infection Type | F | p [r] | |
|---|---|---|---|---|
| Non-Infected (n = 65) | Infected (n = 83) | |||
| Number of lactations | 3.65 ± 0.21 | 2.96 ± 0.19 | 7.26 | 0.017 |
| Productivity days | 1335 ± 80 | 889 ± 71 | 19.95 | <0.001 |
| Lifetime ECM | 49,470 ± 3346 | 37,729 ± 2961 | 8.16 | 0.0095 |
| Parameter | Intra-Mammary Infection Type | F | p [r] | |
|---|---|---|---|---|
| Sub-Clinical (n = 64) | Clinical (n = 19) | |||
| Number of lactations | 3.30 ± 0.21 | 1.84 ± 0.39 | 10.58 | 0.003 |
| Productivity days | 1043 ± 80 | 383 ± 141 | 13.35 | 0.0003 |
| Lifetime ECM | 42,830 ± 3432 | 20,544 ± 6299 | 9.89 | 0.0043 |
| Group | Cows 1 | Number of Lactations | Productivity Days | Lifetime ECM |
|---|---|---|---|---|
| Udder healthy | 18,165 | 3.24 ± 0.01 | 1019 ± 4.13 | 37,632 ± 158 |
| Udder non-healthy | 71,436 | 2.97± 0.005 | 1006 ± 2.1 | 35,896 ± 79 |
| Change | 0.27 | 13 | 1736 | |
| p [r] | <0.0001 | 0.008 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitner, G.; Blum, S.E.; Krifucks, O.; Lavon, Y.; Jacoby, S.; Seroussi, E. Alternative Traits for Genetic Evaluation of Mastitis Based on Lifetime Merit. Genes 2024, 15, 92. https://doi.org/10.3390/genes15010092
Leitner G, Blum SE, Krifucks O, Lavon Y, Jacoby S, Seroussi E. Alternative Traits for Genetic Evaluation of Mastitis Based on Lifetime Merit. Genes. 2024; 15(1):92. https://doi.org/10.3390/genes15010092
Chicago/Turabian StyleLeitner, Gabriel, Shlomo E. Blum, Oleg Krifucks, Yaniv Lavon, Shamay Jacoby, and Eyal Seroussi. 2024. "Alternative Traits for Genetic Evaluation of Mastitis Based on Lifetime Merit" Genes 15, no. 1: 92. https://doi.org/10.3390/genes15010092






