MicroRNA and Rare Human Diseases
Highlights
Abstract
1. Introduction
2. Biogenesis of MiRNA
2.1. Transcription and Primary miRNA (pri-miRNA) Formation
2.2. Nuclear Processing: From pri-miRNA to pre-miRNA
2.3. Cytoplasmic Processing: From pre-miRNA to Mature miRNA
2.4. Incorporation into RISC and Target Regulation
2.5. Seed Sequence
3. MicroRNA and Human Diseases
3.1. Mendelian Disorders Related to miRNA Biogenesis and Function
3.1.1. DROSHA and Tumor Predisposition
3.1.2. DGCR8 and Thyroid Cancer
3.1.3. DICER1 Syndrome
3.1.4. Argonaute (AGO) Proteins in NDD
3.2. The Role of miRNA Gene Mutations in Mendelian and Inherited Diseases
3.2.1. MIR96 and Hearing Loss
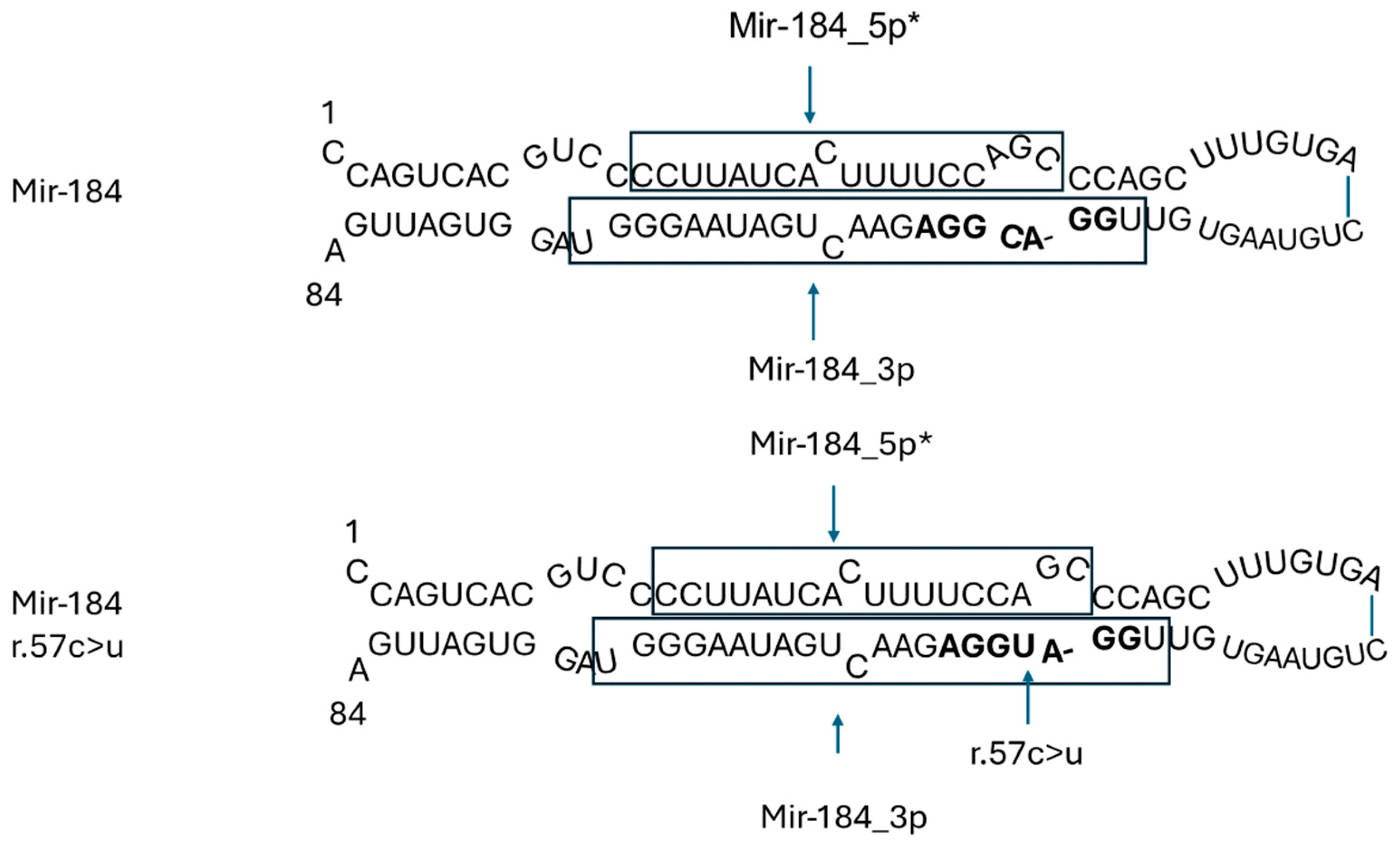
3.2.2. MIR184 in Eye Diseases
3.2.3. MIR204 in Inherited Retinal Dystrophy
3.2.4. MIR140 in Skeletal Dysplasia
3.2.5. MIR17HG: Feingold Syndrome Type 2
3.2.6. Copy Number Variations in MIR9-3 and MIR1299 Congenital Anomalies of the Kidney and Urinary Tract (CAKUTs)
3.3. Human Diseases Associated with Variants in miRNA-Binding Sites
3.3.1. The Variants in miR-189-Binding Site of SLITRK1 Gene Associated with Tourette’s Syndrome (TS)
3.3.2. Variants in miR-196-Binding Site of IRGM Gene Associated with Crohn’s Disease (CD)
3.3.3. Mutations in miR-433-Binding Site of HDAC6 Gene Associated with X-Linked Dominant Chondrodysplasia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| miRNAs | microRNAs |
| pri-miRNA | primary miRNA |
| DGCR8 | DiGeorge syndrome critical region 8 |
| RISC | RNA-induced silencing complex |
| NDDs | neurodevelopmental disorders |
| ncRNAs | non-coding RNAs |
| mRNAs | messenger RNAs |
| snoRNAs | small nucleolar RNAs |
| circRNAs | circular RNAs |
| lncRNAs | long non-coding RNAs |
| dsRBD | dsRNA-binding domain |
| dsRNA | double-stranded RNA |
| pre-miRNA | precursor miRNA |
| AGO | argonaute AGO |
| 3′ UTR | 3′ untranslated region |
| EDICT syndrome | endothelial dystrophy, iris hypoplasia, congenital cataracts, and stromal thinning |
References
- International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature 2004, 431, 931–945. [Google Scholar] [CrossRef]
- Eddy, S.R. Non-coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2001, 2, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Leitao, A.L.; Enguita, F.J. A Structural View of miRNA Biogenesis and Function. Noncoding RNA 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Machowska, M.; Galka-Marciniak, P.; Kozlowski, P. Consequences of genetic variants in miRNA genes. Comput. Struct. Biotechnol. J. 2022, 20, 6443–6457. [Google Scholar] [CrossRef]
- Pelletier, D.; Rivera, B.; Fabian, M.R.; Foulkes, W.D. miRNA biogenesis and inherited disorders: Clinico-molecular insights. Trends Genet. 2023, 39, 401–414. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Laloo, B.; Barillot, M.; Barnetche, T.; Blanchard, C.; Rooryck, C.; Marche, M.; Burgelin, I.; Coupry, I.; Chassaing, N.; et al. A mutation in the 3’-UTR of the HDAC6 gene abolishing the post-transcriptional regulation mediated by hsa-miR-433 is linked to a new form of dominant X-linked chondrodysplasia. Hum. Mol. Genet. 2010, 19, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Lessel, D.; Zeitler, D.M.; Reijnders, M.R.F.; Kazantsev, A.; Hassani Nia, F.; Bartholomaus, A.; Martens, V.; Bruckmann, A.; Graus, V.; McConkie-Rosell, A.; et al. Germline AGO2 mutations impair RNA interference and human neurological development. Nat. Commun. 2020, 11, 5797. [Google Scholar] [CrossRef]
- Schalk, A.; Cousin, M.A.; Dsouza, N.R.; Challman, T.D.; Wain, K.E.; Powis, Z.; Minks, K.; Trimouille, A.; Lasseaux, E.; Lacombe, D.; et al. De novo coding variants in the AGO1 gene cause a neurodevelopmental disorder with intellectual disability. J. Med. Genet. 2022, 59, 965–975. [Google Scholar] [CrossRef]
- Torrezan, G.T.; Ferreira, E.N.; Nakahata, A.M.; Barros, B.D.; Castro, M.T.; Correa, B.R.; Krepischi, A.C.; Olivieri, E.H.; Cunha, I.W.; Tabori, U.; et al. Recurrent somatic mutation in DROSHA induces microRNA profile changes in Wilms tumour. Nat. Commun. 2014, 5, 4039. [Google Scholar] [CrossRef] [PubMed]
- Rakheja, D.; Chen, K.S.; Liu, Y.; Shukla, A.A.; Schmid, V.; Chang, T.C.; Khokhar, S.; Wickiser, J.E.; Karandikar, N.J.; Malter, J.S.; et al. Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nat. Commun. 2014, 2, 4802. [Google Scholar] [CrossRef] [PubMed]
- Barish, S.; Senturk, M.; Schoch, K.; Minogue, A.L.; Lopergolo, D.; Fallerini, C.; Harland, J.; Seemann, J.H.; Stong, N.; Kranz, P.G.; et al. The microRNA processor DROSHA is a candidate gene for a severe progressive neurological disorder. Hum. Mol. Genet. 2022, 31, 2934–2950. [Google Scholar] [CrossRef]
- Paulsson, J.O.; Rafati, N.; DiLorenzo, S.; Chen, Y.; Haglund, F.; Zedenius, J.; Juhlin, C.C. Whole-genome Sequencing of Follicular Thyroid Carcinomas Reveal Recurrent Mutations in MicroRNA Processing Subunit DGCR8. J. Clin. Endocrinol. Metab. 2021, 106, 3265–3282. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.C.; Jorcyk, C.L.; Oxford, J.T. DICER1 Syndrome: DICER1 Mutations in Rare Cancers. Cancers 2018, 10, 143. [Google Scholar] [CrossRef]
- Friedman, L.M.; Avraham, K.B. MicroRNAs and epigenetic regulation in the mammalian inner ear: Implications for deafness. Mamm. Genome 2009, 20, 581–603. [Google Scholar] [CrossRef]
- Solda, G.; Robusto, M.; Primignani, P.; Castorina, P.; Benzoni, E.; Cesarani, A.; Ambrosetti, U.; Asselta, R.; Duga, S. A novel mutation within the MIR96 gene causes non-syndromic inherited hearing loss in an Italian family by altering pre-miRNA processing. Hum. Mol. Genet. 2012, 21, 577–585. [Google Scholar] [CrossRef]
- Chen, J.; Johnson, S.L.; Lewis, M.A.; Hilton, J.M.; Huma, A.; Marcotti, W.; Steel, K.P. A reduction in Ptprq associated with specific features of the deafness phenotype of the miR-96 mutant mouse diminuendo. Eur. J. Neurosci. 2014, 39, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Iliff, B.W.; Riazuddin, S.A.; Gottsch, J.D. Documenting the corneal phenotype associated with the MIR184 c.57C>T mutation. Am. J. Hum. Genet. 2012, 90, 934. [Google Scholar] [CrossRef][Green Version]
- Bykhovskaya, Y.; Caiado Canedo, A.L.; Wright, K.W.; Rabinowitz, Y.S. C.57 C > T Mutation in MIR 184 is Responsible for Congenital Cataracts and Corneal Abnormalities in a Five-generation Family from Galicia, Spain. Ophthalmic Genet. 2015, 36, 244–247. [Google Scholar] [CrossRef]
- Hughes, A.E.; Bradley, D.T.; Campbell, M.; Lechner, J.; Dash, D.P.; Simpson, D.A.; Willoughby, C.E. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 2011, 89, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.; Bae, H.A.; Guduric-Fuchs, J.; Rice, A.; Govindarajan, G.; Siddiqui, S.; Abi Farraj, L.; Yip, S.P.; Yap, M.; Das, M.; et al. Mutational analysis of MIR184 in sporadic keratoconus and myopia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5266–5272. [Google Scholar] [CrossRef] [PubMed]
- Starega-Roslan, J.; Koscianska, E.; Kozlowski, P.; Krzyzosiak, W.J. The role of the precursor structure in the biogenesis of microRNA. Cell. Mol. Life. Sci. 2011, 68, 2859–2871. [Google Scholar] [CrossRef]
- Li, W.B.; Zhang, Y.S.; Lu, Z.Y.; Dong, L.J.; Wang, F.E.; Dong, R.; Li, X.R. Development of retinal pigment epithelium from human parthenogenetic embryonic stem cells and microRNA signature. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5334–5343. [Google Scholar] [CrossRef]
- Wang, F.E.; Zhang, C.; Maminishkis, A.; Dong, L.; Zhi, C.; Li, R.; Zhao, J.; Majerciak, V.; Gaur, A.B.; Chen, S.; et al. MicroRNA-204/211 alters epithelial physiology. FASEB J. 2010, 24, 1552–1571. [Google Scholar] [CrossRef]
- Deo, M.; Yu, J.Y.; Chung, K.H.; Tippens, M.; Turner, D.L. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev. Dyn. 2006, 235, 2538–2548. [Google Scholar] [CrossRef]
- Conte, I.; Carrella, S.; Avellino, R.; Karali, M.; Marco-Ferreres, R.; Bovolenta, P.; Banfi, S. miR-204 is required for lens and retinal development via Meis2 targeting. Proc. Natl. Acad. Sci. USA 2010, 107, 15491–15496. [Google Scholar] [CrossRef]
- Lei, Z.; He-Lin, Z.; Hai-Yan, W.; Wei, J.; Ru, W.; Zhi-Li, C.; Qian-Feng, W. Retinitis pigmentosa with iris coloboma due to miR-204 gene variant in a Chinese family. Mol. Genet. Genom. Med. 2024, 12, e2481. [Google Scholar] [CrossRef] [PubMed]
- Pua, H.H.; Krishnamurthi, S.; Farrell, J.; Margeta, M.; Ursell, P.C.; Powers, M.; Slavotinek, A.M.; Jeng, L.J. Novel interstitial 2.6 Mb deletion on 9q21 associated with multiple congenital anomalies. Am. J. Med. Genet. A 2014, 164A, 237–242. [Google Scholar] [CrossRef]
- Baglietto, M.G.; Caridi, G.; Gimelli, G.; Mancardi, M.; Prato, G.; Ronchetto, P.; Cuoco, C.; Tassano, E. RORB gene and 9q21.13 microdeletion: Report on a patient with epilepsy and mild intellectual disability. Eur. J. Med. Genet. 2014, 57, 44–46. [Google Scholar] [CrossRef]
- Boudry-Labis, E.; Demeer, B.; Le Caignec, C.; Isidor, B.; Mathieu-Dramard, M.; Plessis, G.; George, A.M.; Taylor, J.; Aftimos, S.; Wiemer-Kruel, A.; et al. A novel microdeletion syndrome at 9q21.13 characterised by mental retardation, speech delay, epilepsy and characteristic facial features. Eur. J. Med. Genet. 2013, 56, 163–170. [Google Scholar] [CrossRef]
- Bartnik, M.; Szczepanik, E.; Derwinska, K.; Wisniowiecka-Kowalnik, B.; Gambin, T.; Sykulski, M.; Ziemkiewicz, K.; Kedzior, M.; Gos, M.; Hoffman-Zacharska, D.; et al. Application of array comparative genomic hybridization in 102 patients with epilepsy and additional neurodevelopmental disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 760–771. [Google Scholar] [CrossRef]
- Conte, I.; Hadfield, K.D.; Barbato, S.; Carrella, S.; Pizzo, M.; Bhat, R.S.; Carissimo, A.; Karali, M.; Porter, L.F.; Urquhart, J.; et al. MiR-204 is responsible for inherited retinal dystrophy associated with ocular coloboma. Proc. Natl. Acad. Sci. USA 2015, 112, E3236–E3245. [Google Scholar] [CrossRef] [PubMed]
- Wienholds, E.; Kloosterman, W.P.; Miska, E.; Alvarez-Saavedra, E.; Berezikov, E.; de Bruijn, E.; Horvitz, H.R.; Kauppinen, S.; Plasterk, R.H. MicroRNA expression in zebrafish embryonic development. Science 2005, 309, 310–311. [Google Scholar] [CrossRef]
- Tuddenham, L.; Wheeler, G.; Ntounia-Fousara, S.; Waters, J.; Hajihosseini, M.K.; Clark, I.; Dalmay, T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006, 580, 4214–4217. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.; Charlton, S.; Cheung, K.; Hao, Y.; Soul, J.; Reynard, L.N.; Crowe, N.; Swingler, T.E.; Skelton, A.J.; Pirog, K.A.; et al. microRNA-seq of cartilage reveals an overabundance of miR-140-3p which contains functional isomiRs. RNA 2020, 26, 1575–1588. [Google Scholar] [CrossRef]
- Grigelioniene, G.; Suzuki, H.I.; Taylan, F.; Mirzamohammadi, F.; Borochowitz, Z.U.; Ayturk, U.M.; Tzur, S.; Horemuzova, E.; Lindstrand, A.; Weis, M.A.; et al. Gain-of-function mutation of microRNA-140 in human skeletal dysplasia. Nat. Med. 2019, 25, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Hemmat, M.; Rumple, M.J.; Mahon, L.W.; Strom, C.M.; Anguiano, A.; Talai, M.; Nguyen, B.; Boyar, F.Z. Short stature, digit anomalies and dysmorphic facial features are associated with the duplication of miR-17 ~ 92 cluster. Mol. Cytogenet. 2014, 7, 27. [Google Scholar] [CrossRef]
- Sharaidin, S.; Knipe, S.; Bain, N.; Goel, H. Clinical features associated with a 15.41 Mb deletion of chromosome 13q encompassing the MIR17HG locus. Clin. Dysmorphol. 2013, 22, 68–70. [Google Scholar] [CrossRef]
- de Pontual, L.; Yao, E.; Callier, P.; Faivre, L.; Drouin, V.; Cariou, S.; Van Haeringen, A.; Genevieve, D.; Goldenberg, A.; Oufadem, M.; et al. Germline deletion of the miR-17 approximately 92 cluster causes skeletal and growth defects in humans. Nat. Genet. 2011, 43, 1026–1030. [Google Scholar] [CrossRef]
- Siavriene, E.; Preiksaitiene, E.; Maldziene, Z.; Mikstiene, V.; Rancelis, T.; Ambrozaityte, L.; Gueneau, L.; Reymond, A.; Kucinskas, V. A de novo 13q31.3 microduplication encompassing the miR-17 ~ 92 cluster results in features mirroring those associated with Feingold syndrome 2. Gene 2020, 753, 144816. [Google Scholar] [CrossRef] [PubMed]
- Zivotic, I.; Kolic, I.; Cvetkovic, M.; Spasojevic-Dimitrijeva, B.; Zivkovic, M.; Stankovic, A.; Jovanovic, I. Copy number variation analysis identifies MIR9-3 and MIR1299 as novel miRNA candidate genes for CAKUT. Pediatr. Nephrol. 2024, 39, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Abelson, J.F.; Kwan, K.Y.; O’Roak, B.J.; Baek, D.Y.; Stillman, A.A.; Morgan, T.M.; Mathews, C.A.; Pauls, D.L.; Rasin, M.R.; Gunel, M.; et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005, 310, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Shumway, A.J.; Shanahan, M.T.; Hollville, E.; Chen, K.; Beasley, C.; Villanueva, J.W.; Albert, S.; Lian, G.; Cure, M.R.; Schaner, M.; et al. Aberrant miR-29 is a predictive feature of severe phenotypes in pediatric Crohn’s disease. JCI Insight 2024, 9, e168800. [Google Scholar] [CrossRef]
- Chassaing, N.; Siani, V.; Carles, D.; Delezoide, A.L.; Alberti, E.M.; Battin, J.; Chateil, J.F.; Gilbert-Dussardier, B.; Coupry, I.; Arveiler, B.; et al. X-linked dominant chondrodysplasia with platyspondyly, distinctive brachydactyly, hydrocephaly, and microphthalmia. Am. J. Med. Genet. A 2005, 136A, 307–312. [Google Scholar] [CrossRef]
- Sheng, N.; Xie, X.; Wang, Y.; Huang, L.; Zhang, S.; Gao, L.; Wang, H. A Survey of Deep Learning for Detecting miRNA-Disease Associations: Databases, Computational Methods, Challenges, and Future Directions. IEEE/ACM Trans. Comput. Biol. Bioinform. 2024, 21, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zhong, B.; Fan, R.; Cui, Q. HMDD v4.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 2024, 52, D1327–D1332. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Ling, Y.; Zhou, C.; Wang, H.; Teschendorff, A.E.; Zhao, Y.; Zhao, H.; He, Y.; Zhang, G.; et al. dbDEMC 3.0: Functional Exploration of Differentially Expressed miRNAs in Cancers of Human and Model Organisms. Genom. Proteom. Bioinform. 2022, 20, 446–454. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Y.; Hao, Y.; Juan, L.; Teng, M.; Zhang, X.; Li, M.; Wang, G.; Liu, Y. miR2Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009, 37, D98–D104. [Google Scholar] [CrossRef]
- Xie, B.; Ding, Q.; Han, H.; Wu, D. miRCancer: A microRNA-cancer association database constructed by text mining on literature. Bioinformatics 2013, 29, 638–644. [Google Scholar] [CrossRef]



| miRNA Gene | Associated Disease | Mutation Type | Mechanism |
|---|---|---|---|
| DROSHA | Wilms’ tumor | Nonsense/missense | Defective miRNA processing |
| DGCR8 | Thyroid tumor | Missense and second hit mutation | Decreased levels of mature miRNA |
| DICER1 | Rare tumors like pleuropulmonary blastoma | Germline nonsense/frameshift leading to loss of function with second hit mutation | Decrease in mature miRNA |
| AGO1 | NDDs | Missense and small deletions | Defecting RISC processing |
| AGO2 | NDDs | Missense variants | Defecting RISC processing |
| MIR96 | Nonsyndromic hearing loss (NSHL) | Point mutations (n.13G>A, n.14C>A) | Disrupts miRNA seed sequence, affecting mRNA regulation |
| MIR184 | Keratoconus and cataracts | n.57C>T | Alters seed sequence, disrupts competition with miR-205-5p for target mRNAs |
| MIR204 | Inherited retinal dystrophy, iris coloboma | n.37C>T | Gain-of-function mutation alters recognition of target genes |
| MIR140 | Skeletal dysplasia | n.24A>G | Seed sequence mutation affects cartilage development |
| MIR17HG | Feingold syndrome type 2 | Deletion or microduplication | Alters miR-17–92 cluster expression, impacting developmental processes |
| MIR9-3, MIR1299 | Congenital Anomalies of Kidney and Urinary Tract (CAKUTs) | Copy number variations | Affects kidney development through altered miRNA-mediated gene regulation |
| SLITRK1 (miR-189-binding site) | Tourette’s syndrome | Single-base variant in 3′UTR | Disrupts miRNA binding, altering SLITRK1 expression |
| IRGM (miR-196-binding site) | Crohn’s disease | Synonymous variant (c.313C>T) | Reduces miR-196 binding, increasing IRGM expression, affecting autophagy |
| HDAC6 (miR-433-binding site) | X-linked chondrodysplasia | c.*281A>T in 3′UTR | Disrupts miR-433 binding, leading to overexpression of HDAC6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goel, H.; Goel, A. MicroRNA and Rare Human Diseases. Genes 2024, 15, 1243. https://doi.org/10.3390/genes15101243
Goel H, Goel A. MicroRNA and Rare Human Diseases. Genes. 2024; 15(10):1243. https://doi.org/10.3390/genes15101243
Chicago/Turabian StyleGoel, Himanshu, and Amy Goel. 2024. "MicroRNA and Rare Human Diseases" Genes 15, no. 10: 1243. https://doi.org/10.3390/genes15101243
APA StyleGoel, H., & Goel, A. (2024). MicroRNA and Rare Human Diseases. Genes, 15(10), 1243. https://doi.org/10.3390/genes15101243







