Unveiling the Mechanism of Compound Ku-Shen Injection in Liver Cancer Treatment through an Ingredient–Target Network Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of the Active Components and Targets of CKI
2.2. Target Selection for Liver Cancer
2.3. Identifying the Potential Targets of CKI for Liver Cancer Treatment
2.4. Construction of Ingredient–Target Network
2.5. Construction of Protein–Protein Interaction (PPI) Network
2.6. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analyses
2.7. Molecular Docking
2.8. In Vitro Experiments
2.8.1. Cell Culture
2.8.2. Reagents and Instruments
2.8.3. Cell Proliferation Detection
2.8.4. Western Blotting
2.8.5. Statistical Methods
3. Results
3.1. Identification of Active Ingredients and Targets of CKI
3.2. Identification of Target for Liver Cancer
3.3. Potential Targets of CKI for Liver Cancer Treatment
3.4. Ingredient–Target Network Analyses
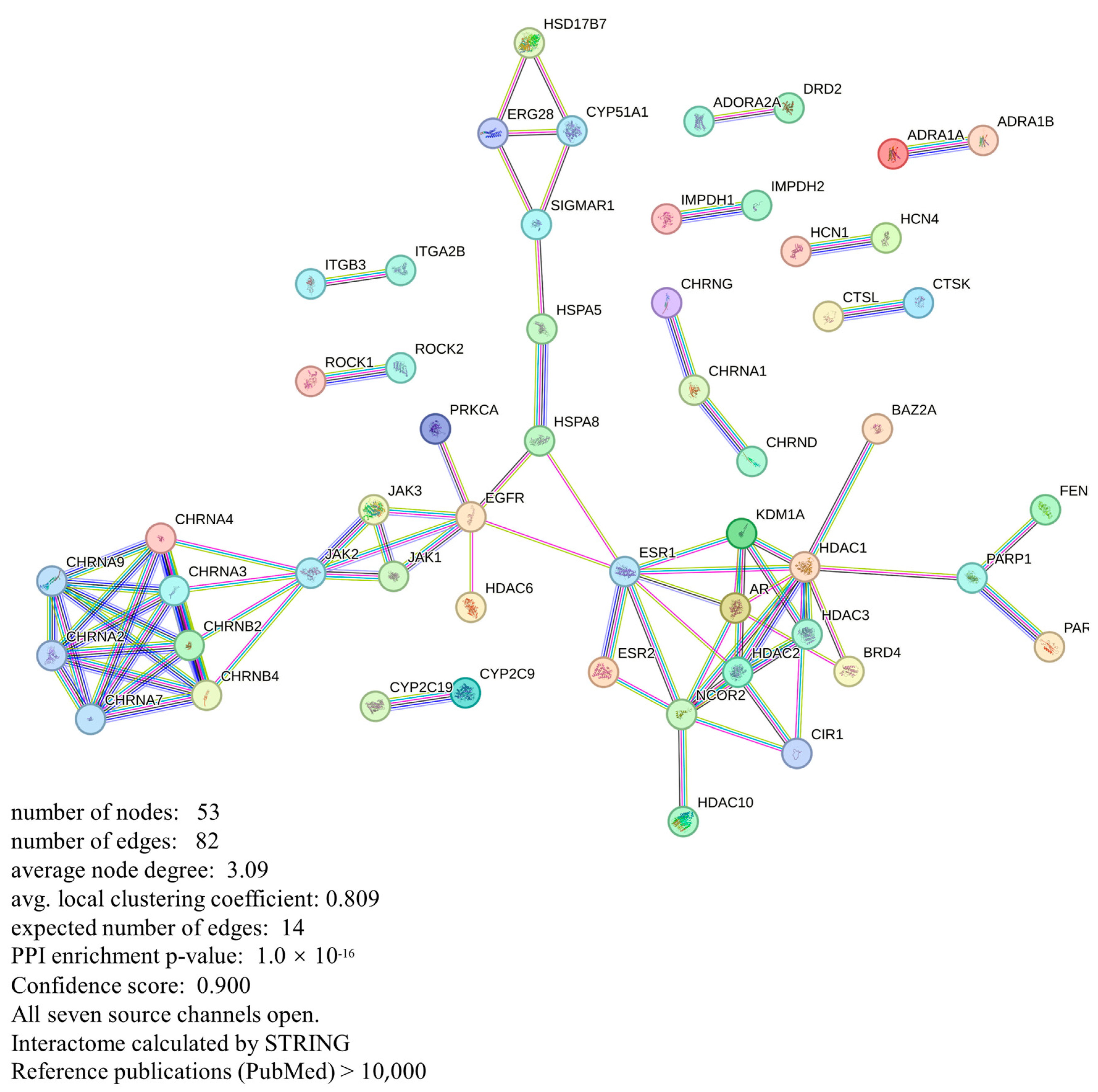
3.5. Protein–Protein Interaction (PPI) Network Analyses
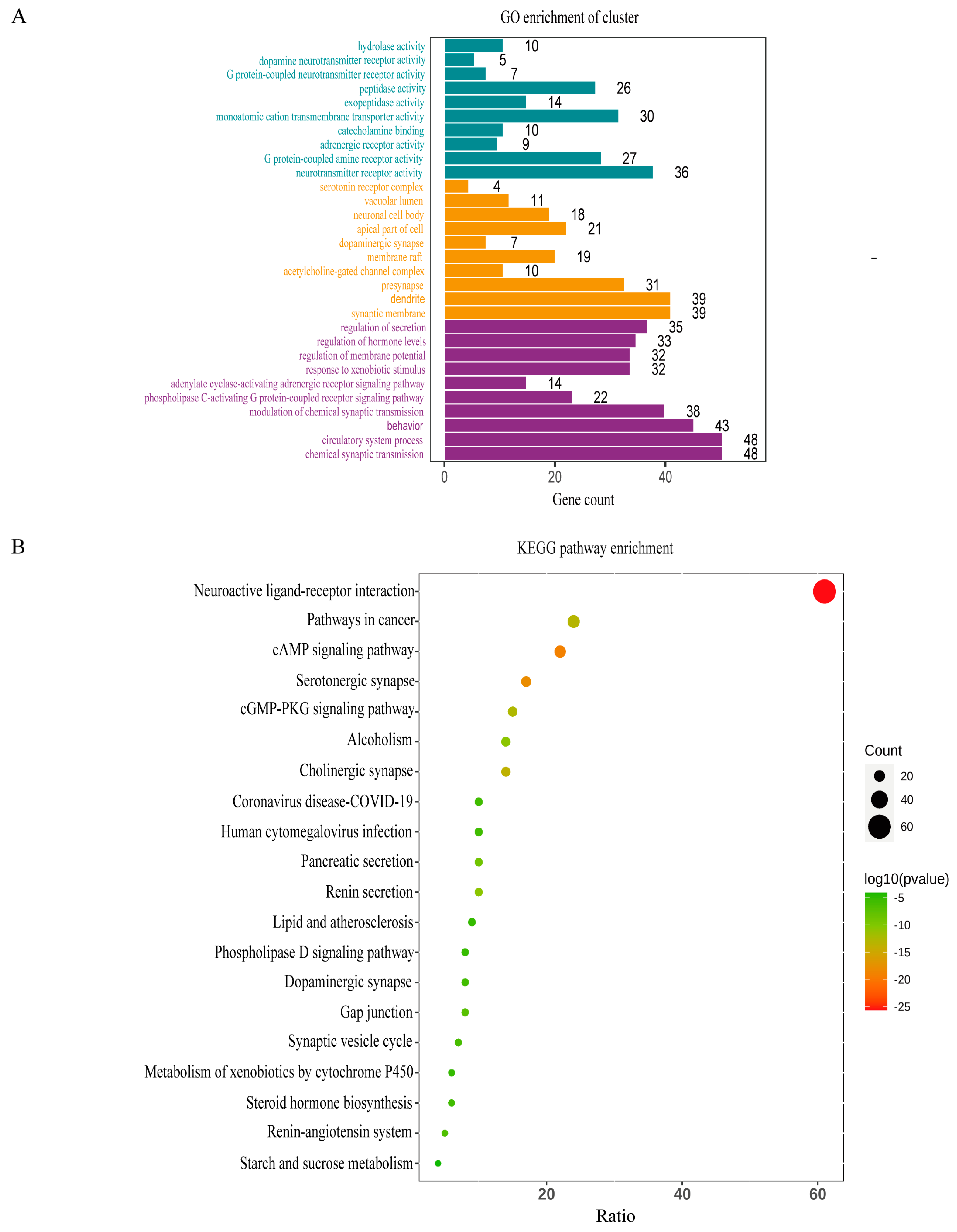
3.6. GO and KEGG Pathway Enrichment Analyses
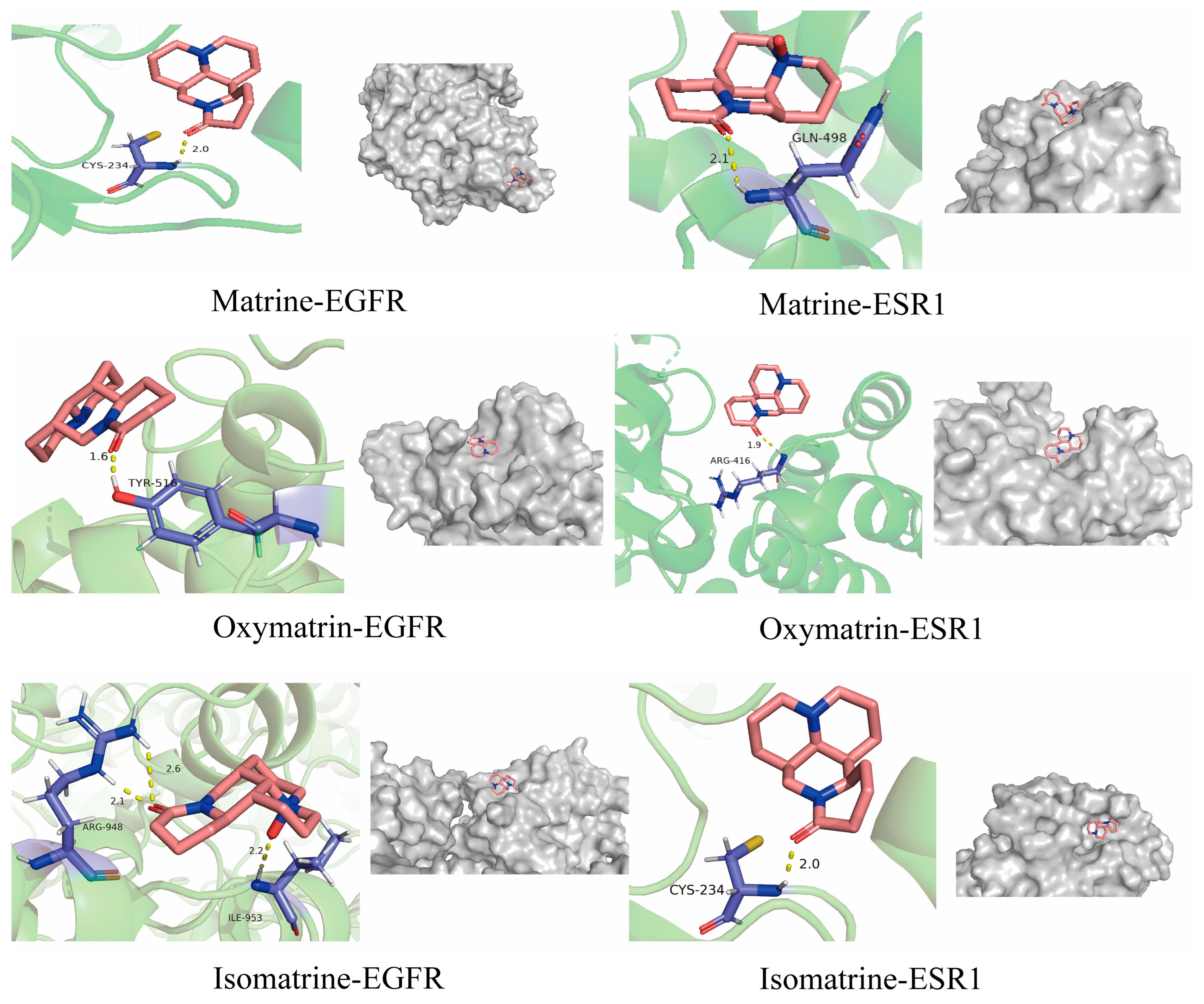
3.7. Molecular Docking Results
3.8. Effect of Oxymatrine and Matrine on Cell Proliferation
3.9. Effect of Oxymatrine on the Expression of EGFR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCM | Traditional Chinese Medicine |
| CKI | Compound Ku-Shen Injection |
| TCMSP | The Chinese Medicine System Pharmacology Database |
| OB | Oral Bioavailability |
| DL | Drug Similarity |
| PPI | Protein–Protein Interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BP | Biological Process |
| CC | Cellular Component |
| MF | Molecular Function |
| EGFR | Epidermal Growth Factor Receptor |
| p-EGFR | Phosphorylated EGFR |
| ESR1 | Estrogen Receptor 1 |
| HDAC1 | Histone Deacetylase 1 |
| IC50 | The 50% Inhibitory Concentration |
| RMSD | Root Mean Square Deviation |
| BC | Betweenness Centrality |
References
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Chang, D. Tumor Markers Research Focus; Nova Biomedical Books: New York, NY, USA, 2007; 300p. [Google Scholar]
- Corti, C.; De Angelis, C.; Bianchini, G.; Malorni, L.; Giuliano, M.; Hamilton, E.; Jeselsohn, R.; Jhaveri, K.; Curigliano, G.; Criscitiello, C. Novel endocrine therapies: What is next in estrogen receptor positive, HER2 negative breast cancer? Cancer Treat. Rev. 2023, 117, 102569. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Y.; Cai, A.; Yu, Y.; Wang, X.; Lan, L.; Guo, X.; Yan, H.; Gao, X.; Li, H.; et al. Cannabidiol represses miR-143 to promote cardiomyocyte proliferation and heart regeneration after myocardial infarction. Eur. J. Pharmacol. 2024, 963, 176245. [Google Scholar] [CrossRef]
- Ling, C.-Q.; Fan, J.; Lin, H.-S.; Shen, F.; Xu, Z.-Y.; Lin, L.-Z.; Qin, S.-K.; Zhou, W.-P.; Zhai, X.-F.; Li, B.; et al. Clinical practice guidelines for the treatment of primary liver cancer with integrative traditional Chinese and Western medicine. J. Integr. Med. 2018, 16, 236–248. [Google Scholar] [CrossRef]
- Nourmohammadi, S.; Aung, T.N.; Cui, J.; Pei, J.V.; De Ieso, M.L.; Harata-Lee, Y.; Qu, Z.; Adelson, D.L.; Yool, A.J. Effect of Compound Kushen Injection, a Natural Compound Mixture, and Its Identified Chemical Components on Migration and Invasion of Colon, Brain, and Breast Cancer Cell Lines. Front. Oncol. 2019, 9, 314. [Google Scholar] [CrossRef]
- Li, C.; Niu, D.; Zhu, R.; Yan, X.; Qu, H.; Zhang, Y.; Zheng, Y. Adjunctive effect of compound Kushen injection for cancer: An overview of systematic reviews. J. Ethnopharmacol. 2023, 317, 116778. [Google Scholar] [CrossRef]
- Shen, H.; Qu, Z.; Harata-Lee, Y.; Aung, T.N.; Cui, J.; Wang, W.; Kortschak, R.D.; Adelson, D.L. Understanding the Mechanistic Contribution of Herbal Extracts in Compound Kushen Injection with Transcriptome Analysis. Front. Oncol. 2019, 9, 632. [Google Scholar] [CrossRef]
- Luan, X.; Zhang, L.-J.; Li, X.-Q.; Rahman, K.; Zhang, H.; Chen, H.-Z.; Zhang, W.-D. Compound-based Chinese medicine formula: From discovery to compatibility mechanism. J. Ethnopharmacol. 2020, 254, 112687. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, M.; Yao, W.; Wang, F.; Li, X.; Wang, W.; Li, J.; Gao, Z.; Qiu, L.; You, R.; et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J. Immunother. Cancer 2020, 8, e000317. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Wang, X.S.; Shi, Q.; Wang, J.; Wang, F.; Ren, S.; Jin, J.; Han, B.; Zhang, W.; et al. Compound Kushen Injection Reduces Severe Toxicity and Symptom Burden Associated with Curative Radiotherapy in Patients with Lung Cancer. J. Natl. Compr. Cancer Netw. 2023, 21, 821–830.e3. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, C.; Zhou, W.; Lu, S.; Tan, Y.; Wu, Z.; You, R.; Stalin, A.; Guo, F.; Zhang, J.; et al. Compound Kushen Injection inhibits epithelial-mesenchymal transition of gastric carcinoma by regulating VCAM1 induced by the TNF signaling pathway. Phytomedicine 2023, 118, 154984. [Google Scholar] [CrossRef]
- Yuan, Z.; Pan, Y.; Leng, T.; Chu, Y.; Zhang, H.; Ma, J.; Ma, X. Progress and Prospects of Research Ideas and Methods in the Network Pharmacology of Traditional Chinese Medicine. J. Pharm. Pharm. Sci. 2022, 25, 218–226. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, C.; Shi, J.; Huang, Z.; Lu, S.; Tan, Y.; You, R.; Hai, L.; Huang, J.; Guo, S.; et al. Elucidating the pharmacological effects of Compound Kushen injection on MYC-P15-CCND1 signaling pathway in nasopharyngeal carcinoma—An in vitro study. J. Ethnopharmacol. 2023, 315, 116702. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Bryant, S.H. PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009, 37, W623–W633. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Amberger, J.; Bocchini, C.; Hamosh, A. A New Face and New Challenges for Online Mendelian Inheritance in Man (OMIM®). Hum. Mutat. 2011, 32, 564–567. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Kryg, H.; et al. GeneCards Version 3: The human gene integrator. Database-J. Biol. Databases Curation 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.M.; Caluaco, B.J.; Madureira, J.M.C.; Cabongo, S.Q.; Gaieta, E.M.; Djata, F.; Colares, R.P.; Neto, M.M.; Fernandes, C.F.C.; Marinho, G.S.; et al. Screening of Potential Inhibitors Targeting the Main Protease Structure of SARS-CoV-2 via Molecular Docking, and Approach with Molecular Dynamics, RMSD, RMSF, H-Bond, SASA and MMGBSA. Mol. Biotechnol. 2024, 66, 1919–1933. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, H.; Liu, J.; Chen, L.; Zhang, Q.; Wang, Z. Identification and determination of the chemical constituents in a herbal preparation, compound kushen injection, by hplc and lc-dad-ms/ms. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 207–220. [Google Scholar] [CrossRef]
- Molavi, Z.; Razi, S.; Mirmotalebisohi, S.A.; Adibi, A.; Sameni, M.; Karami, F.; Niazi, V.; Niknam, Z.; Aliashrafi, M.; Taheri, M.; et al. Identification of FDA approved drugs against SARS-CoV-2 RNA dependent RNA polymerase (RdRp) and 3-chymotrypsin-like protease (3CLpro), drug repurposing approach. Biomed. Pharmacother. 2021, 138, 111544. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ma, W.; Gao, Y.; Zheng, W.; Zhang, B.; Peng, Y. Meta-analysis: Therapeutic effect of transcatheter arterial chemoembolization combined with compound kushen injection in hepatocellular carcinoma. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 178–188. [Google Scholar] [CrossRef]
- Lu, T.; Kong, B.; Wang, Y.; Yu, J.; Pan, Y.; Chen, D.; Li, H.; Chen, X.; Yuan, Z.; Yang, Z.; et al. Compound Kushen injection combined with transarterial chemoembolization for hepatocellular carcinoma: An evidence map and overview of systematic reviews. J. Ethnopharmacol. 2024, 319 Pt 3, 117267. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Xu, J.; Zuo, H.; Tang, Y.; Huang, H.Y.; Chen, J.; Lin, Y.-C.-D.; Huang, H.D. Mechanism of Sophorae Flavescentis Radix (Kushen) in treating NSCLC: Insights from miRNA-mRNA network analysis. J. Ethnopharmacol. 2024, 319 Pt 3, 117232. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, G.; Wang, J.; Wang, S.; Tang, Q.; Sheng, H.; Wu, W.; Wang, S. Compound Kushen Injection Protects Skin from Radiation Injury via Regulating Bim. Front. Pharmacol. 2021, 12, 753068. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Yang, L.; Chen, L.; Miao, M.; Liu, Y.; Yin, Y.; Wei, M.; Liu, G.; An, Y.; et al. Compound Kushen injection attenuates angiotensin II-mediated heart failure by inhibiting the PI3K/Akt pathway. Int. J. Mol. Med. 2023, 51, 23. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Pei, T.; Guo, B.; Li, J.; Wang, H.; Ba, Q. Compound kushen injection in cancer treatments: Efficacy, active ingredients, and mechanisms. J. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100108. [Google Scholar] [CrossRef]
- Wang, W.; You, R.-L.; Qin, W.-J.; Hai, L.-N.; Fang, M.-J.; Huang, G.-H.; Kang, R.-X.; Li, M.-H.; Qiao, Y.-F.; Li, J.-W.; et al. Anti-tumor activities of active ingredients in Compound Kushen Injection. Acta Pharmacol. Sin. 2015, 36, 676–679. [Google Scholar] [CrossRef]
- Zhang, S.; Jia, X.; Dai, H.; Zhu, X.; Song, W.; Bian, S.; Wu, H.; Chen, S.; Tang, Y.; Chen, J.; et al. SERPINE2 promotes liver cancer metastasis by inhibiting c-Cbl-mediated EGFR ubiquitination and degradation. Cancer Commun. 2024, 44, 384–407. [Google Scholar] [CrossRef] [PubMed]
- Dustin, D.; Gu, G.; Fuqua, S.A.W. ESR1 mutations in breast cancer. Cancer 2019, 125, 3714–3728. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-X.; Du, G.-H.; Qin, X.-M.; Gao, L. Compound Kushen Injection intervenes metabolic reprogramming and epithelial-mesenchymal transition of HCC via regulating β-catenin/c-Myc signaling. Phytomedicine 2021, 93, 153781. [Google Scholar] [CrossRef] [PubMed]


| x- Center | y- Center | z- Center | x-Dimension | y-Dimension | z-Dimension | Shape | Spacing | |
|---|---|---|---|---|---|---|---|---|
| Isomatrine–EGFR | 36.02 | 178.10 | 164.24 | 126 | 130 | 126 | square | 0.992 |
| Isomatrine–ESR1 | −36.79 | −25.98 | −47.32 | 126 | 126 | 120 | square | 1.0 |
| Matrine–EGFR | 36.02 | 178.10 | 164.24 | 126 | 126 | 126 | square | 0.45 |
| Matrine–ESR1 | −36.79 | −25.98 | −47.32 | 126 | 124 | 126 | square | 0.47 |
| Oxymatrine–EGFR | 36.02 | 178.10 | 164.24 | 126 | 126 | 126 | square | 0.93 |
| Oxymatrine–ESR1 | −36.79 | −25.98 | −47.32 | 126 | 142 | 126 | square | 1.0 |
| Ingredients | PubChem CID | |
|---|---|---|
| Piscidic Acid | 120693 | CKI 1 |
| Macrozamin | 9576780 | CKI 2 |
| Oxysophoridine | 71773433 | CKI 3 |
| Oxymatrine | 114850 | CKI 4 |
| Oxysophocarpine | 24721085 | CKI 5 |
| Sophoridine | 165549 | CKI 6 |
| Sophoranol | 12442899 | CKI 7 |
| Baptifoline | 621307 | CKI 8 |
| 9a-Hydroxymatrine | 15385684 | CKI 9 |
| N-methylcytisine | 670971 | CKI 10 |
| Lamprolobine | 87752 | CKI 11 |
| Isomatrine | 5271984 | CKI 12 |
| Matrine | 91466 | CKI 13 |
| Sophocarpine | 115269 | CKI 14 |
| Trifolirhizin | 442827 | CKI 15 |
| 9α-Hydroxysophocarpine | 132555388 | CKI 16 |
| Target | Degree | BC | Target | Degree | BC |
|---|---|---|---|---|---|
| HDAC1 | 20 | 295.966 | HSD17B7 | 4.0 | 0.0 |
| ESR1 | 16 | 543.3 | CHRNA1 | 4.0 | 2.0 |
| EGFR | 14 | 546.0 | BRD4 | 4.0 | 0.0 |
| JAK2 | 14 | 364.0 | ROCK2 | 2.0 | 0.0 |
| CHRNA4 | 14 | 40.5 | ROCK1 | 2.0 | 0.0 |
| CHRNB2 | 14 | 40.5 | PARP2 | 2.0 | 0.0 |
| CHRNA3 | 14 | 40.5 | ITGB3 | 2.0 | 0.0 |
| CHRNB4 | 14 | 40.5 | ITGA2B | 2.0 | 0.0 |
| HDAC2 | 12 | 25.8 | IMPDH2 | 2.0 | 0.0 |
| CHRNA7 | 12 | 0 | IMPDH1 | 2.0 | 0.0 |
| CHRNA9 | 12 | 0 | HDAC10 | 2.0 | 0.0 |
| CHRNA2 | 12 | 0 | HCN4 | 2.0 | 0.0 |
| KDM1A | 10 | 11.167 | HCN1 | 2.0 | 0.0 |
| AR | 10 | 24.8 | FEN1 | 2.0 | 0.0 |
| HDAC3 | 10 | 0.8 | PRKCA | 2.0 | 0.0 |
| PARP1 | 10 | 126.0 | HDAC6 | 2.0 | 0.0 |
| JAK1 | 6 | 0 | CYP2C9 | 2.0 | 0.0 |
| HSPA8 | 6 | 280.0 | CYP2C19 | 2.0 | 0.0 |
| ERG28 | 6.0 | 31.0 | CTSL | 2.0 | 0.0 |
| SIGMAR1 | 6.0 | 180.0 | CTSK | 2.0 | 0.0 |
| CYP51A1 | 6.0 | 31.0 | CHRNG | 2.0 | 0.0 |
| CIR1 | 6.0 | 0 | CHRND | 2.0 | 0.0 |
| HSPA5 | 232.0 | BAZ2A | 2.0 | 0.0 | |
| HSPA5 | 4.0 | 232.0 | ADRA1B | 2.0 | 0.0 |
| ESR2 | 4.0 | 0.0 | ADRA1A | 2.0 | 0.0 |
| Ingredient | Minimum Binding Energy (kcal/mol) | |
|---|---|---|
| EGFR | ESR1 | |
| Matrine | −5.5 | −5.5 |
| Oxymatrine | −9.5 | −7.4 |
| Isomatrine | −7.5 | −6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Liu, J.; Wei, Z.; Peng, C.; Zhao, Y.; Ding, Y.; Shi, J.; Zhao, J. Unveiling the Mechanism of Compound Ku-Shen Injection in Liver Cancer Treatment through an Ingredient–Target Network Analysis. Genes 2024, 15, 1278. https://doi.org/10.3390/genes15101278
Zou W, Liu J, Wei Z, Peng C, Zhao Y, Ding Y, Shi J, Zhao J. Unveiling the Mechanism of Compound Ku-Shen Injection in Liver Cancer Treatment through an Ingredient–Target Network Analysis. Genes. 2024; 15(10):1278. https://doi.org/10.3390/genes15101278
Chicago/Turabian StyleZou, Wenkui, Jiazhen Liu, Zexing Wei, Chunhua Peng, Ying Zhao, Yue Ding, Jifan Shi, and Juan Zhao. 2024. "Unveiling the Mechanism of Compound Ku-Shen Injection in Liver Cancer Treatment through an Ingredient–Target Network Analysis" Genes 15, no. 10: 1278. https://doi.org/10.3390/genes15101278





