The Yin and Yang of the Natural Product Triptolide and Its Interactions with XPB, an Essential Protein for Gene Expression and DNA Repair
Abstract
1. Introduction
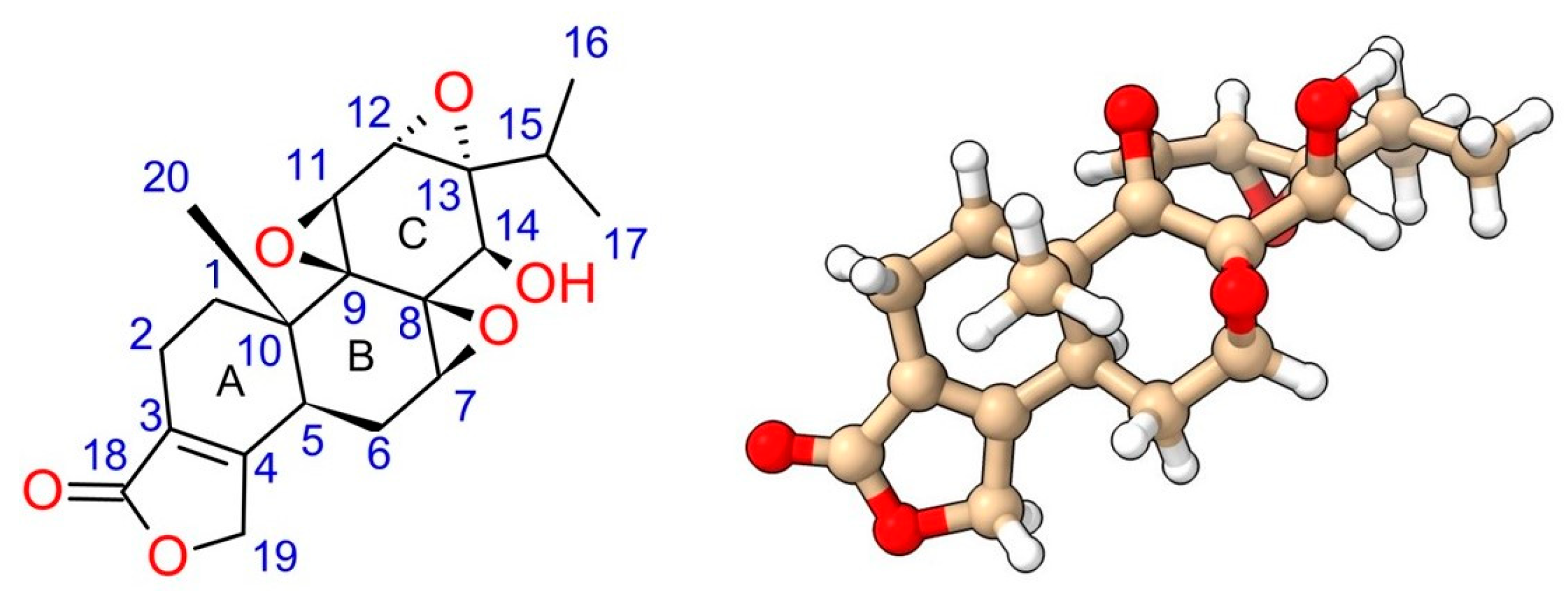
2. Chemical Properties of Triptolide and Existing Structure–Activity Relationship (SAR) Studies
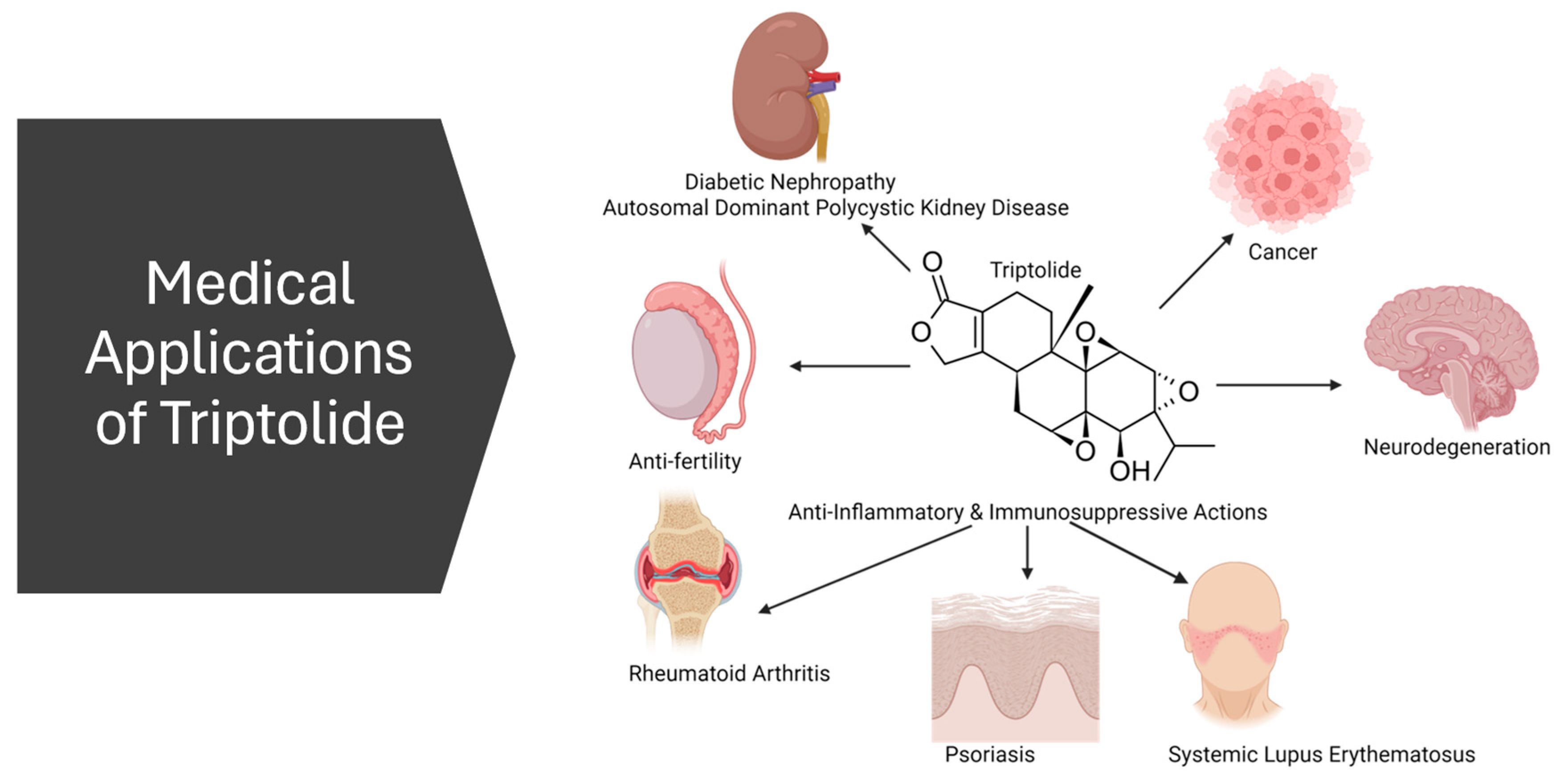
3. Potential Medical Applications
3.1. Anti-Inflammatory and Immunosuppression
3.2. Anticancer
3.3. Antifertility
3.4. Nephropathy
3.5. Neurodegeneration
4. Toxicity
4.1. Liver Toxicity
4.2. Cardiotoxicity
4.3. Nephrotoxicity
4.4. Conclusions on Toxicity
5. Pharmacokinetics
5.1. Absorption
5.2. Distribution
5.3. Metabolism
5.4. Excretion
5.5. Pharmacokinetic Studies in Human Subjects
5.6. Conclusions on Pharmacokinetics
6. Strategies to Improve Triptolide’s Pharmacological Profile
6.1. Structural Modification
6.1.1. Glutriptolides
6.1.2. Other C-14-βOH Conjugates
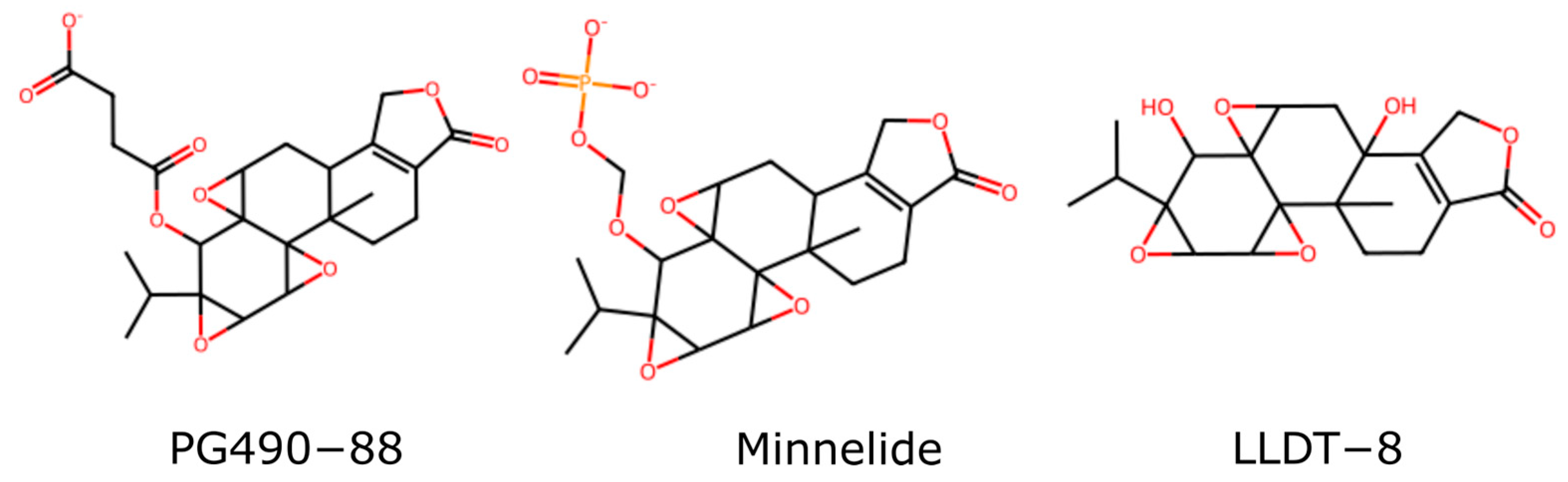
6.1.3. Additional Derivations of Triptolide
6.2. Delivery Systems
6.3. Microbiome
7. Protein Targets of Triptolide
7.1. Polycystin-2 (PC-2)
7.2. dCTP Pyro-Phosphatase 1 (DCTPP1)
7.3. Peroxiredoxin I (Prx I)
7.4. A-Disintegrin and Metalloprotease-10 (ADAM10)
7.5. TAK1 Binding Protein (TAB1)
7.6. The DNA–PK Complex Kinase Subunit (DNA–PKcs)
7.7. Xeroderma Pigmentosum B (XPB or ERCC3 Gene Product)
8. Important Downstream Effectors of Triptolide
8.1. p53
8.2. NF-κB
8.3. Nrf2
8.4. TNF-α
8.5. miRNA
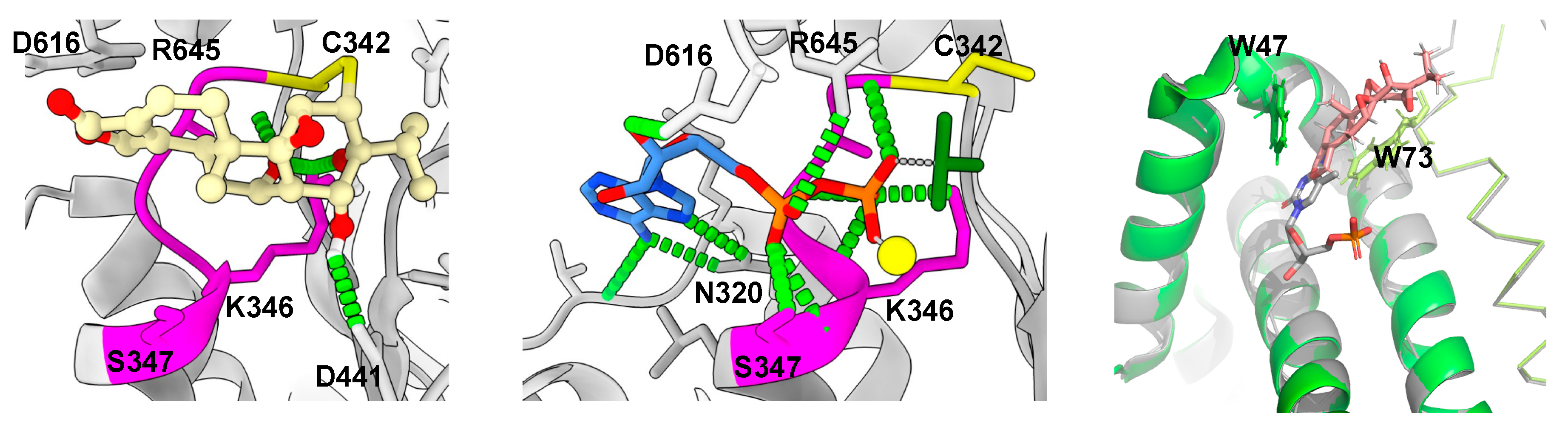
9. Structural Basis of Triptolide Interactions with XPB
10. Conclusions and Prospective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Zhou, Z.L.; Yang, Y.X.; Ding, J.; Li, Y.C.; Miao, Z.H. Triptolide: Structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat. Prod. Rep. 2012, 29, 457–475. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Dai, E. Therapeutic targets of thunder god vine (Tripterygium wilfordii hook) in rheumatoid arthritis (Review). Mol. Med. Rep. 2020, 21, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- He, Q.L.; Minn, I.; Wang, Q.; Xu, P.; Head, S.A.; Datan, E.; Yu, B.; Pomper, M.G.; Liu, J.O. Targeted Delivery and Sustained Antitumor Activity of Triptolide through Glucose Conjugation. Angew. Chem. Int. Ed. Engl. 2016, 55, 12035–12039. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.; Court, W.; Dailey, R.J.; Gilmore, C.; Bryan, R. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J. Am. Chem. Soc. 1972, 94, 7194–7195. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Xu, D.; Yue, S.; Yan, C.; Liu, W.; Fu, R.; Ma, W.; Tang, Y. Recent advances in the pharmacological applications and liver toxicity of triptolide. Chem. Biol. Interact. 2023, 382, 110651. [Google Scholar] [CrossRef]
- Zeng, L.S.; Yang, P.; Qin, Y.Y.; He, W.H.; Cao, L. Pharmacological activity and clinical progress of Triptolide and its derivatives LLDT-8, PG490-88Na, and Minnelide: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 10181–10203. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, Q.; Datan, E.; Lin, G.Q.; Minn, I.; Pomper, M.G.; Yu, B.; Romo, D.; He, Q.L.; Liu, J.O. Triptolide: Reflections on two decades of research and prospects for the future. Nat. Prod. Rep. 2021, 38, 843–860. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?intr=Minnelide (accessed on 11 September 2024).
- Vispe, S.; DeVries, L.; Creancier, L.; Besse, J.; Breand, S.; Hobson, D.J.; Svejstrup, J.Q.; Annereau, J.P.; Cussac, D.; Dumontet, C.; et al. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of short-lived mRNA. Mol. Cancer Ther. 2009, 8, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jin, Y.; Cheng, C.; Zhang, H.; Zou, W.; Zheng, Q.; Lu, Z.; Chen, Q.; Lai, Y.; Pan, J. Triptolide inhibits Bcr-Abl transcription and induces apoptosis in STI571-resistant chronic myelogenous leukemia cells harboring T315I mutation. Clin. Cancer Res. 2009, 15, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, H.; Ren, Y.; Chen, W.; Wang, Y.; Li, J.; Liu, H.; Xing, J.; Zhang, Y.; Li, N. Triptolide enhances carboplatin-induced apoptosis by inhibiting nucleotide excision repair (NER) activity in melanoma. Front. Pharmacol. 2023, 14, 1157433. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Xu, X. Triptolide potentiates lung cancer cells to cisplatin-induced apoptosis by selectively inhibiting the NER activity. Biomark. Res. 2015, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, Y.; Ge, S.; Liu, S.B. The roles of TPL in hematological malignancies. Hematology 2023, 28, 2231765. [Google Scholar] [CrossRef]
- Phillips, P.A.; Dudeja, V.; McCarroll, J.A.; Borja-Cacho, D.; Dawra, R.K.; Grizzle, W.E.; Vickers, S.M.; Saluja, A.K. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007, 67, 9407–9416. [Google Scholar] [CrossRef] [PubMed]
- Manzo, S.G.; Zhou, Z.L.; Wang, Y.Q.; Marinello, J.; He, J.X.; Li, Y.C.; Ding, J.; Capranico, G.; Miao, Z.H. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res. 2012, 72, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Titov, D.V.; Gilman, B.; He, Q.L.; Bhat, S.; Low, W.K.; Dang, Y.; Smeaton, M.; Demain, A.L.; Miller, P.S.; Kugel, J.F.; et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol. 2011, 7, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; DuPrez, K.T. XPB: An unconventional SF2 DNA helicase. Prog. Biophys. Mol. Biol. 2015, 117, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, D.H.; Schober, W.D.; Dietrich, M.F.; Pinilla, C.; Vassilev, L.T.; Reed, J.C.; Andreeff, M. Triptolide sensitizes AML cells to TRAIL-induced apoptosis via decrease of XIAP and p53-mediated increase of DR5. Blood 2008, 111, 3742–3750. [Google Scholar] [CrossRef]
- Nakazato, T.; Sagawa, M.; Kizaki, M. Triptolide induces apoptotic cell death of multiple myeloma cells via transcriptional repression of Mcl-1. Int. J. Oncol. 2014, 44, 1131–1138. [Google Scholar] [CrossRef]
- Modi, S.; Kir, D.; Giri, B.; Majumder, K.; Arora, N.; Dudeja, V.; Banerjee, S.; Saluja, A.K. Minnelide Overcomes Oxaliplatin Resistance by Downregulating the DNA Repair Pathway in Pancreatic Cancer. J. Gastrointest. Surg. 2016, 20, 13–23; discussion 23–14. [Google Scholar] [CrossRef]
- He, Q.L.; Titov, D.V.; Li, J.; Tan, M.; Ye, Z.; Zhao, Y.; Romo, D.; Liu, J.O. Covalent modification of a cysteine residue in the XPB subunit of the general transcription factor TFIIH through single epoxide cleavage of the transcription inhibitor triptolide. Angew. Chem. Int. Ed. Engl. 2015, 54, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Q.; Zhang, D.M.; Wang, H.B.; Liang, X.T. [Structure modification of triptolide, a diterpenoid from Tripterygium wilfordii]. Yao Xue Xue Bao 1992, 27, 830–836. [Google Scholar] [PubMed]
- Zhou, B.; Li, X.; Tang, H.; Miao, Z.; Feng, H.; Li, Y. Total synthesis of novel D-ring-modified triptolide analogues: Structure-cytotoxic activity relationship studies on the D-ring of triptolide. Org. Biomol. Chem. 2011, 9, 3176–3179. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shi, X.; Li, S.; Cui, J.; Lu, Z.; Jin, Y.; Lin, Y.; Pang, J.; Pan, J. Design, synthesis, and biological evaluation of novel water-soluble triptolide derivatives: Antineoplastic activity against imatinib-resistant CML cells bearing T315I mutant Bcr-Abl. Bioorg Med. Chem. 2010, 18, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Y.; Cui, L.; Yan, F.; Shen, S. Triptolide attenuates lipopolysaccharide-induced inflammatory responses in human endothelial cells: Involvement of NF-kappaB pathway. BMC Complement. Altern. Med. 2019, 19, 198. [Google Scholar] [CrossRef]
- Fang, W.Y.; Tseng, Y.T.; Lee, T.Y.; Fu, Y.C.; Chang, W.H.; Lo, W.W.; Lin, C.L.; Lo, Y.C. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-kappaB/TNF-α and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Kao, P. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R. D 2003, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Liu, X.; Wu, X.; Huang, L.; Gao, W. Triptolide: Pharmacological spectrum, biosynthesis, chemical synthesis and derivatives. Theranostics 2021, 11, 7199–7221. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Botchway, B.O.A.; Zhang, Y.; Liu, X. Triptolide activates the Nrf2 signaling pathway and inhibits the NF-kappaB signaling pathway to improve Alzheimer disease. Metab. Brain Dis. 2024, 39, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hui, L.; Xu, W.; Shen, H.; Chen, Q.; Long, L.; Zhu, X. Triptolide modulates the sensitivity of K562/A02 cells to adriamycin by regulating miR-21 expression. Pharm. Biol. 2012, 50, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Pigneux, A.; Mahon, F.X.; Uhalde, M.; Jeanneteau, M.; Lacombe, F.; Milpied, N.; Reiffers, J.; Belloc, F. Triptolide cooperates with chemotherapy to induce apoptosis in acute myeloid leukemia cells. Exp. Hematol. 2008, 36, 1648–1659. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Bermudez-Cruz, R.M. Natural Compounds That Target DNA Repair Pathways and Their Therapeutic Potential to Counteract Cancer Cells. Front. Oncol. 2020, 10, 598174. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Xiao, L.; Wang, D.; Ji, Y.C.; Yang, Y.P.; Ma, R.; Chen, X.H. Triptolide inhibits viability and induces apoptosis in liver cancer cells through activation of the tumor suppressor gene p53. Int. J. Oncol. 2017, 50, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Deng, S.; Deng, M.; Shi, Y.; Zhong, M.; Ding, L.; Jiang, Y.; Zhou, Y.; Carter, B.Z.; Xu, B. Therapeutic synergy of Triptolide and MDM2 inhibitor against acute myeloid leukemia through modulation of p53-dependent and -independent pathways. Exp. Hematol. Oncol. 2022, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; de Haydu, C.; Wilkinson, P.; Hooda, U.; Giri, B.; Oleas, J.M.; Rive, V.; Roy, S.; Dudeja, V.; Slomovitch, B.; et al. Minnelide, a prodrug, inhibits cervical cancer growth by blocking HPV-induced changes in p53 and pRb. Am. J. Cancer Res. 2021, 11, 2202. [Google Scholar]
- Huynh, P.N.; Hikim, A.P.S.; Wang, C.; Stefonovic, K.; Lue, Y.H.; Leung, A.; Atienza, V.; Baravarian, S.; Reutrakul, V.; Swerdloff, R.S. Long-Term Effects of Triptolide on Spermatogenesis, Epididymal Sperm Function, and Fertility in Male Rats. J. Androl. 2013, 21, 689–699. [Google Scholar] [CrossRef]
- Cao, W.; Liu, X.; Han, Y.; Song, X.; Lu, L.; Li, X.; Lin, L.; Sun, L.; Liu, A.; Zhao, H.; et al. (5R)-5-hydroxytriptolide for HIV immunological non-responders receiving ART: A randomized, double-blinded, placebo-controlled phase II study. Lancet Reg. Health West. Pac. 2023, 34, 100724. [Google Scholar] [CrossRef]
- Xiong, S.; Li, Y.; Xiang, Y.; Peng, N.; Shen, C.; Cai, Y.; Song, D.; Zhang, P.; Wang, X.; Zeng, X.; et al. Dysregulation of lncRNA and circRNA Expression in Mouse Testes after Exposure to Triptolide. Curr. Drug Metab. 2019, 20, 665–673. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.; Liu, M.; Gao, M.; Li, C.; Yan, H.; Li, C.; Sun, L.; Mo, L.; Wu, C.; et al. Critical Role of Hepatic Cyp450s in the Testis-Specific Toxicity of (5R)-5-Hydroxytriptolide in C57BL/6 Mice. Front. Pharmacol. 2017, 8, 832. [Google Scholar] [CrossRef]
- Hikim, A.P.S.; Lue, Y.H.; Wang, C.; Reutrakul, V.; Sangsuwan, R.; Swerdloff, R.S. Posttesticular Antifertility Action of Triptolide in the Male Rat: Evidence for Severe Impairment of Cauda Epididymal Sperm Ultrastructure. J. Androl. 2013, 21, 431–437. [Google Scholar] [CrossRef]
- Lue, Y.; Hikim, A.P.S.; Wang, C.; Leung, A.; Baravarian, S.; Reutrakul, V.; Sangsawan, R.; Chaichana, S.; Swerdloff, R.S. Triptolide: A Potential Male Contraceptive. J. Androl. 2013, 19, 479–486. [Google Scholar] [CrossRef]
- Ge, J.C.; Qian, Q.; Gao, Y.H.; Zhang, Y.F.; Li, Y.X.; Wang, X.; Fu, Y.; Ma, Y.M.; Wang, Q. Toxic effects of Tripterygium glycoside tablets on the reproductive system of male rats by metabolomics, cytotoxicity, and molecular docking. Phytomedicine 2023, 114, 154813. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, H.; Guo, G.; Wang, S.; He, L.; Chen, H.; Wu, J. Male germ cell apoptosis and epigenetic histone modification induced by Tripterygium wilfordii Hook F. PLoS ONE 2011, 6, e20751. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, H.; Cui, W.; Zhang, X.; Tang, Q.; Liu, N.; Lan, X.; Pan, C. Palliative effects of metformin on testicular damage induced by triptolide in male rats. Ecotoxicol. Environ. Saf. 2021, 222, 112536. [Google Scholar] [CrossRef] [PubMed]
- Leuenroth, S.J.; Bencivenga, N.; Igarashi, P.; Somlo, S.; Crews, C.M. Triptolide reduces cystogenesis in a model of ADPKD. J. Am. Soc. Nephrol. 2008, 19, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Leuenroth, S.J.; Crews, C.M. Studies on calcium dependence reveal multiple modes of action for triptolide. Chem. Biol. 2005, 12, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Tian, X.; Zhang, X.; Huang, S.; Ma, Y.; Wu, D.; Moeckel, G.; Somlo, S.; Wu, G. Human polycystin-2 transgene dose-dependently rescues ADPKD phenotypes in Pkd2 mutant mice. Am. J. Pathol. 2015, 185, 2843–2860. [Google Scholar] [CrossRef]
- Boletta, A. Emerging evidence of a link between the polycystins and the mTOR pathways. Pathogenetics 2009, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Xue, B.; Zhang, L.; Gong, Y.; Li, K.; Wang, H.; Jing, L.; Xie, J.; Wang, X. Triptolide inhibits amyloid-beta1-42-induced TNF-α and IL-1β production in cultured rat microglia. J. Neuroimmunol. 2008, 205, 32–36. [Google Scholar] [CrossRef]
- Ning, C.; Mo, L.; Chen, X.; Tu, W.; Wu, J.; Hou, S.; Xu, J. Triptolide derivatives as potential multifunctional anti-Alzheimer agents: Synthesis and structure-activity relationship studies. Bioorg Med. Chem. Lett. 2018, 28, 689–693. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Wang, Q.; Zhang, D.M.; Wang, J.Y.; Xiao, B.; Zheng, Y.; Wang, X.M. Triptolide Rescues Spatial Memory Deficits and Amyloid-β Aggregation Accompanied by Inhibition of Inflammatory Responses and MAPKs Activity in APP/PS1 Transgenic Mice. Curr. Alzheimer Res. 2016, 13, 288–296. [Google Scholar] [CrossRef]
- Shao, F.; Wang, G.; Xie, H.; Zhu, X.; Sun, J. Pharmacokinetic Study of Triptolide, a constituent of Immunosuppressive Chinese Herb Medicine, in Rats. Biol. Pharm. Bull. 2007, 30, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, F.; Guan, C.; Wang, W.; Sun, X.; Fu, X.; Huang, M.; Jin, J.; Huang, Z. Activation of Nrf2 protects against triptolide-induced hepatotoxicity. PLoS ONE 2014, 9, e100685. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, C.; Han, D.; Zhou, W.; Cui, Y.; Tang, X.; Xiao, C.; Wang, Y.; Gao, Y. SLC7A11/GPX4 Inactivation-Mediated Ferroptosis Contributes to the Pathogenesis of Triptolide-Induced Cardiotoxicity. Oxid. Med. Cell Longev. 2022, 2022, 3192607. [Google Scholar] [CrossRef]
- Wei, Y.M.; Luan, Z.H.; Liu, B.W.; Wang, Y.H.; Chang, Y.X.; Xue, H.Q.; Ren, J.H. Autophagy in Triptolide-Mediated Cytotoxicity in Hepatic Cells. Int. J. Toxicol. 2019, 38, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Jiang, Z.; Duan, W.; Huang, J.; Zhang, L.; Hu, L.; He, L.; Li, F.; Xiao, Y.; Shu, B.; et al. Involvement of Mitochondrial Pathway in Triptolide-Induced Cytotoxicity in Human Normal Liver L-02 Cells. Biol. Pharm. Bull. 2008, 31, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Q.; Wang, Y.; Zhang, H.; Liu, X.; Zhou, H.; Yang, T. The molecular pathogenesis of triptolide-induced hepatotoxicity. Front. Pharmacol. 2022, 13, 979307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xi, C.; Wang, W.; Fu, X.; Jinqiang, L.; Qiu, Y.; Jin, J.; Xu, J.; Huang, Z. Triptolide-induced oxidative stress involved with Nrf2 contribute to cardiomyocyte apoptosis through mitochondrial dependent pathways. Toxicol. Lett. 2014, 230, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Qin, Z.; Jiang, S.; Zhang, L.; Zhang, G.; Huang, M.; Huang, Z.; Jin, J. MitoQ alleviates triptolide-induced cardiotoxicity via activation of p62/Nrf2 axis in H9c2 cells. Toxicol. Vitr. 2023, 86, 105487. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, W.; Wang, L.; Pan, J.; Cheng, Y.; Shen, F.; Huang, Z. Triptolide induces p53-dependent cardiotoxicity through mitochondrial membrane permeabilization in cardiomyocytes. Toxicol. Appl. Pharmacol. 2018, 355, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wan, F.; Lian, H.; Lu, Z.; Li, X.; Cao, D.; Jiang, Y.; Li, J. Friend or foe? The dual role of triptolide in the liver, kidney, and heart. Biomed. Pharmacother. 2023, 161, 114470. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Peng, S.; Wu, Z.; Zhou, Q.; Zhou, J. Toxicity of triptolide and the molecular mechanisms involved. Biomed. Pharmacother. 2017, 90, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, J.; Li, M.; Guan, C.; Wang, W.; Zhu, S.; Qiu, Y.; Huang, M.; Huang, Z. Role of Nrf2 in protection against triptolide-induced toxicity in rat kidney cells. Toxicol. Lett. 2012, 213, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.M.; Shen, G.L.; Xiao, W.B.; Tan, Y.; Lu, C.; Li, H. Assessment of the roles of P-glycoprotein and cytochrome P450 in triptolide-induced liver toxicity in sandwich-cultured rat hepatocyte model. Drug Metab. Dispos. 2013, 41, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, W.; Su, X.; Wu, S.; Lin, Y.; Li, J.; Wang, Y.; Chen, J.; Zhou, Y.; Qiu, P.; et al. Triptolide inhibits proliferation and invasion of malignant glioma cells. J. Neurooncol. 2012, 109, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Li, Y.; Yang, Y.; Yu, S.; Chen, Y. Triptolide Improves Cognitive Dysfunction in Rats with Vascular Dementia by Activating the SIRT1/PGC-1alpha Signaling Pathway. Neurochem. Res. 2019, 44, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, M.; Zhang, P.; Mu, B.; Bai, Z.; Li, L.; Yu, J. Triptolide promotes nerve repair after cerebral ischemia reperfusion injury by regulating the NogoA/NgR/ROCK pathway. Folia Neuropathol. 2024, 61, 51862. [Google Scholar] [CrossRef] [PubMed]
- Viegas, J.S.R.; Praca, F.G.; Kravicz, M.; Bentley, M. Therapeutic applications and delivery systems for triptolide. Drug Deliv. Transl. Res. 2020, 10, 1584–1600. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Guo, Y.; Xu, D.; Xiao, H.; Dai, Y.; Liu, K.; Chen, L.; Wang, H. Developmental toxicity and programming alterations of multiple organs in offspring induced by medication during pregnancy. Acta Pharm. Sin. B 2023, 13, 460–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Che, K.; Liu, Y. Pharmacokinetics, distribution and efficacy of triptolide PLGA microspheres after intra-articular injection in a rat rheumatoid arthritis model. Xenobiotica 2021, 51, 703–715. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.; Wu, Y. Absorption and Metabolism Characteristics of Triptolide as Determined by a Sensitive and Reliable LC-MS/MS Method. Molecules 2015, 20, 8928–8940. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, X.; Chen, X.Y.; Zhong, D.F. Excretion of [3H]triptolide and its metabolites in rats after oral administration. Acta Pharmacol. Sin. 2014, 35, 549–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borazanci, E.; Saluja, A.; Gockerman, J.; Velagapudi, M.; Korn, R.; Von Hoff, D.; Greeno, E. First-in-Human Phase I Study of Minnelide in Patients With Advanced Gastrointestinal Cancers: Safety, Pharmacokinetics, Pharmacodynamics, and Antitumor Activity. Oncologist 2024, 29, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yalikong, A.; Li, X.Q.; Zhou, P.H.; Qi, Z.P.; Li, B.; Cai, S.L.; Zhong, Y.S. A Triptolide Loaded HER2-Targeted Nano-Drug Delivery System Significantly Suppressed the Proliferation of HER2-Positive and BRAF Mutant Colon Cancer. Int. J. Nanomed. 2021, 16, 2323–2335. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, M.; Zhao, D.; Ru, D.; Duan, Y.; Ding, C.; Li, H. The Effect of Triptolide-Loaded Exosomes on the Proliferation and Apoptosis of Human Ovarian Cancer SKOV3 Cells. Biomed. Res. Int. 2019, 2019, 2595801. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zeng, T.; Li, J.; Huang, C.; Yu, M.; Wang, X.; Tan, L.; Zhang, M.; Li, A.; Hu, J. Kidney-targeted triptolide-encapsulated mesoscale nanoparticles for high-efficiency treatment of kidney injury. Biomater. Sci. 2019, 7, 5312–5323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, Q.; Chen, Y.; Xie, M.; Li, J.; Yao, S.; Li, M.; Lou, Z.; Cai, Y.; Sun, X. Mechanochemical preparation of triptolide-loaded self-micelle solid dispersion with enhanced oral bioavailability and improved anti-tumor activity. Drug Deliv. 2022, 29, 1398–1408. [Google Scholar] [CrossRef]
- Ren, Q.; Li, M.; Deng, Y.; Lu, A.; Lu, J. Triptolide delivery: Nanotechnology-based carrier systems to enhance efficacy and limit toxicity. Pharmacol. Res. 2021, 165, 105377. [Google Scholar] [CrossRef]
- Banerjee, S.; Thayanithy, V.; Sangwan, V.; Mackenzie, T.N.; Saluja, A.K.; Subramanian, S. Minnelide reduces tumor burden in preclinical models of osteosarcoma. Cancer Lett. 2013, 335, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Datan, E.; Minn, I.; Xu, P.; He, Q.L.; Ahn, H.H.; Yu, B.; Pomper, M.G.; Liu, J.O. A Glucose-Triptolide Conjugate Selectively Targets Cancer Cells under Hypoxia. iScience 2020, 23, 101536. [Google Scholar] [CrossRef]
- Nomura, A.; McGinn, O.; Dudeja, V.; Sangwan, V.; Saluja, A.K.; Banerjee, S. Minnelide effectively eliminates CD133(+) side population in pancreatic cancer. Mol. Cancer 2015, 14, 200. [Google Scholar] [CrossRef]
- Chen, B.J.; Liu, C.; Cui, X.; Fidler, J.M.; Chao, N.J. Prevention of Graft-Versus-Host Disease by a Novel Immunosuppressant, PG490–88, Through Inhibition of Alloreactive T Cell Expansion. Transplantation 2000, 70, 1442–1447. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.L.; Miao, Z.H.; Lin, L.P.; Feng, H.J.; Tong, L.J.; Ding, J.; Li, Y.C. Design and synthesis of novel C14-hydroxyl substituted triptolide derivatives as potential selective antitumor agents. J. Med. Chem. 2009, 52, 5115–5123. [Google Scholar] [CrossRef]
- Kitzen, J.J.; de Jonge, M.J.; Lamers, C.H.; Eskens, F.A.; van der Biessen, D.; van Doorn, L.; Ter Steeg, J.; Brandely, M.; Puozzo, C.; Verweij, J. Phase I dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumours. Eur. J. Cancer 2009, 45, 1764–1772. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wang, X.; Zheng, W.; Zhang, J.; Shi, F.; Liu, J. Redox-responsive self-assembly PEG nanoparticle enhanced triptolide for efficient antitumor treatment. Sci. Rep. 2018, 8, 12968. [Google Scholar] [CrossRef]
- Gao, C.; Song, X.D.; Chen, F.H.; Wei, G.L.; Guo, C.Y. The protective effect of natural medicines in rheumatoid arthritis via inhibit angiogenesis. Front. Pharmacol. 2024, 15, 1380098. [Google Scholar] [CrossRef]
- Zhou, R.; Tang, W.; He, P.L.; Yang, Y.F.; Li, Y.C.; Zuo, J.P. (5R)-5-hydroxytriptolide inhibits the immune response of human peripheral blood mononuclear cells. Int. Immunopharmacol. 2009, 9, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yang, Y.; Zhang, F.; Li, Y.C.; Zhou, R.; Wang, J.X.; Zhu, Y.N.; Li, X.Y.; Yang, Y.F.; Zuo, J.P. Prevention of graft-versus-host disease by a novel immunosuppressant, (5R)-5-hydroxytriptolide (LLDT-8), through expansion of regulatory T cells. Int. Immunopharmacol. 2005, 5, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ren, Y.; Ma, J.; Luo, X.; Li, J.; Wu, Y.; Gu, H.; Fu, C.; Cao, Z.; Zhang, J. Novel CD44-targeting and pH/redox-dual-stimuli-responsive core-shell nanoparticles loading triptolide combats breast cancer growth and lung metastasis. J. Nanobiotechnology 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hao, B.; Ju, D.; Liu, M.; Zhao, H.; Du, Z.; Xia, J. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis. Acta Pharm. Sin. B 2015, 5, 569–576. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Xu, H.; Yang, X. Anti-tumour and immuno-modulation effects of triptolide-loaded polymeric micelles. Eur. J. Pharm. Biopharm. 2008, 70, 741–748. [Google Scholar] [CrossRef]
- Jia, L.; Nie, X.Q.; Ji, H.M.; Yuan, Z.X.; Li, R.S. Multiple-Coated PLGA Nanoparticles Loading Triptolide Attenuate Injury of a Cellular Model of Alzheimer’s Disease. Biomed. Res. Int. 2021, 2021, 8825640. [Google Scholar] [CrossRef]
- Xu, L.; Pan, J.; Chen, Q.; Yu, Q.; Chen, H.; Xu, H.; Qiu, Y.; Yang, X. In vivo evaluation of the safety of triptolide-loaded hydrogel-thickened microemulsion. Food Chem. Toxicol. 2008, 46, 3792–3799. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.Q.; Jiang, X.J.; Wang, F.Y.; Shu, Z.X.; Gui, S.Y. Cubic and hexagonal liquid crystals as drug carriers for the transdermal delivery of triptolide. Drug Deliv. 2019, 26, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Shen, Y.; Liao, M.M.; Mao, X.; Mi, G.J.; You, C.; Guo, Q.Y.; Li, W.J.; Wang, X.Y.; Lin, N.; et al. Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: Enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine 2019, 15, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, M.; Chen, K.; Tian, Q.; Song, J.; Zhang, Z.; Xie, Z.; Yuan, Y.; Jia, Y.; Zhu, X.; et al. Novel Carboxylated Chitosan-Based Triptolide Conjugate for the Treatment of Rheumatoid Arthritis. Pharmaceutics 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mao, Y.; Li, K.; Shi, T.; Yao, H.; Yao, J.; Wang, S. Pharmacokinetics and tissue distribution study in mice of triptolide-loaded lipid emulsion and accumulation effect on pancreas. Drug Deliv. 2016, 23, 1344–1354. [Google Scholar] [CrossRef]
- Peng, R.; Ma, S.R.; Fu, J.; Han, P.; Pan, L.B.; Zhang, Z.W.; Yu, H.; Wang, Y. Transforming of Triptolide into Characteristic Metabolites by the Gut Microbiota. Molecules 2020, 25, 606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huang, J.F.; Cheng, Y.; Dai, M.Y.; Zhu, W.F.; Yang, X.W.; Gonzalez, F.J.; Li, F. Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome 2021, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Zhao, Q.; Dai, M.Y.; Xiao, X.R.; Zhang, T.; Zhu, W.F.; Li, F. Gut microbiota protects from triptolide-induced hepatotoxicity: Key role of propionate and its downstream signalling events. Pharmacol. Res. 2020, 155, 104752. [Google Scholar] [CrossRef]
- Feng, K.; Li, X.; Bai, Y.; Zhang, D.; Tian, L. Mechanisms of cancer cell death induction by triptolide: A comprehensive overview. Heliyon 2024, 10, e24335. [Google Scholar] [CrossRef]
- Yue, X.; Bai, C.; Xie, D.; Ma, T.; Zhou, P.K. DNA-PKcs: A Multi-Faceted Player in DNA Damage Response. Front. Genet. 2020, 11, 607428. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Hu, Z.; Tang, H.; Hu, Z.; Mao, Z.; Liu, B.; Xu, X.; Jiang, Y.; Wan, X. Triptolide impairs genome integrity by directly blocking the enzymatic activity of DNA-PKcs in human cells. Biomed. Pharmacother. 2020, 129, 110427. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, B.A.; Chen, E.Z.; Tang, S.; Belgum, H.S.; McCauley, J.A.; Evenson, K.A.; Etchison, R.G.; Jay-Dixon, J.; Patel, R.M.; Raza, A.; et al. Triptolide and its prodrug minnelide suppress Hsp70 and inhibit in vivo growth in a xenograft model of mesothelioma. Genes. Cancer 2015, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, B.O.; Wang, D.; Liu, W.; Zhang, H.; Liu, W.; Qiu, Z. Triptolide reduces the viability of osteosarcoma cells by reducing MKP-1 and Hsp70 expression. Exp. Ther. Med. 2016, 11, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Luo, J.; Li, P.; Zhou, Y.; Li, P.; Wang, F.; Mallio, C.A.; Rossi, G.; Jalal, A.H.; Filipovic, N.; et al. Triptolide promotes degradation of the unfolded gain-of-function Tp53(R175H/Y220C) mutant protein by initiating heat shock protein 70 transcription in non-small cell lung cancer. Transl. Lung Cancer Res. 2022, 11, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, M.K.; Gnudi, L. Diabetic Nephropathy: An Overview. In Diabetic Nephropathy: Methods and Protocols; Gnudi, L., Long, D.A., Eds.; Springer: New York, NY, USA, 2020; pp. 3–7. [Google Scholar]
- Ma, R.; Liu, L.; Liu, X.; Wang, Y.; Jiang, W.; Xu, L. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Exp. Ther. Med. 2013, 6, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Sayat, R.; Robertson, G.S.; Marignani, P.A. Triptolide: An inhibitor of a disintegrin and metalloproteinase 10 (ADAM10) in cancer cells. Cancer Biol. Ther. 2009, 8, 2054–2062. [Google Scholar] [CrossRef]
- Corson, T.W.; Cavga, H.; Aberle, N.; Crews, C.M. Triptolide directly inhibits dCTP pyrophosphatase. Chembiochem 2011, 12, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Q.-L.; Zhou, J.; Chikarmane, R.; Hauk, G.; Rachakonda, A.; Vaghasia, A.M.; Castagna, N.; Steinberg, R.C.; Pham, M.-T.; et al. Triptolide sensitizes cancer cells to nucleoside DNA methyltransferase inhibitors through inhibition of DCTPP1-mediated cell-intrinsic resistance. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Li, L.; Feng, X.; Ding, S.; Zheng, W.; Li, J.; Shen, P. TAB1: A target of triptolide in macrophages. Chem. Biol. 2014, 21, 246–256. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, Y.; Deng, Z.; Lee, O.Y.; Gao, P.; Chen, P.; Rose, R.J.; Zhao, H.; Zhang, Z.; Tao, X.P.; et al. Natural products triptolide, celastrol, and withaferin A inhibit the chaperone activity of peroxiredoxin I. Chem. Sci. 2015, 6, 4124–4130. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Duan, N.; Lan, Y.; Ma, P.; Zheng, H.; Zheng, W.; Li, J.; Hua, Z.-C. Computational prediction and experimental validation of low-affinity target of triptolide and its analogues. RSC Adv. 2015, 5, 34572–34579. [Google Scholar] [CrossRef]
- Jang, H.H.; Kim, S.Y.; Park, S.K.; Jeon, H.S.; Lee, Y.M.; Jung, J.H.; Lee, S.Y.; Chae, H.B.; Jung, Y.J.; Lee, K.O.; et al. Phosphorylation and concomitant structural changes in human 2-Cys peroxiredoxin isotype I differentially regulate its peroxidase and molecular chaperone functions. FEBS Lett. 2006, 580, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.; Castro, H.; Cruz, T.; Tse, E.; Koldewey, P.; Southworth, D.R.; Tomas, A.M.; Jakob, U. Mitochondrial peroxiredoxin functions as crucial chaperone reservoir in Leishmania infantum. Proc. Natl. Acad. Sci. USA 2015, 112, E616–E624. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Deng, C.; Lo, T.H.; Chan, K.Y.; Li, X.; Wong, C.M. Peroxiredoxin, Senescence, and Cancer. Cells 2022, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Piszczek, G.; Rhee, S.G.; Chock, P.B. Glutathionylation of peroxiredoxin I induces decamer to dimers dissociation with concomitant loss of chaperone activity. Biochemistry 2011, 50, 3204–3210. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.H.; Kidmose, R.T.; Jenner, L.B. Structure of TSA2 reveals novel features of the active-site loop of peroxiredoxins. Acta Crystallogr. D Struct. Biol. 2016, 72, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yu, H.; Chen, J.; Song, X.; Sun, L. ADAM10 promotes cell growth, migration, and invasion in osteosarcoma via regulating E-cadherin/β-catenin signaling pathway and is regulated by miR-122-5p. Cancer Cell Int. 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Atapattu, L.; Saha, N.; Chheang, C.; Eissman, M.F.; Xu, K.; Vail, M.E.; Hii, L.; Llerena, C.; Liu, Z.; Horvay, K.; et al. An activated form of ADAM10 is tumor selective and regulates cancer stem-like cells and tumor growth. J. Exp. Med. 2016, 213, 1741–1757. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.; Sidler, C.L.; Thali, R.F.; Winssinger, N.; Cheung, P.C.; Neumann, D. Autoactivation of transforming growth factor β-activated kinase 1 is a sequential bimolecular process. J. Biol. Chem. 2010, 285, 25753–25766. [Google Scholar] [CrossRef] [PubMed]
- Rimel, J.K.; Taatjes, D.J. The essential and multifunctional TFIIH complex. Protein Sci. 2018, 27, 1018–1037. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Ebright, R.H.; Reinberg, D. Mechanism of ATP-dependent promoter melting by transcription factor IIH. Science 2000, 288, 1418–1421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, F.; Huang, W.; Ousman, T.; Zhang, B.; Han, Y.; Clotaire, D.Z.; Wang, C.; Chang, H.; Luo, H.; Ren, X.; et al. Triptolide induces growth inhibition and apoptosis of human laryngocarcinoma cells by enhancing p53 activities and suppressing E6-mediated p53 degradation. PLoS ONE 2013, 8, e80784. [Google Scholar] [CrossRef] [PubMed]
- Dergai, O.; Hernandez, N. How to Recruit the Correct RNA Polymerase? Lessons from snRNA Genes. Trends Genet. 2019, 35, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.F.; Liu, L.; He, L.L.; Ye, J.; Lin, Z.J.; Yuan, D.L.; Deng, M.M.; Fang, Z.H.; Carter, B.Z.; Xu, B. Combining triptolide with ABT-199 is effective against acute myeloid leukemia through reciprocal regulation of Bcl-2 family proteins and activation of the intrinsic apoptotic pathway. Cell Death Dis. 2020, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, H.; Liu, T.; Tian, D.; Gu, L.; Zhou, M. Triptolide inhibits MDM2 and induces apoptosis in acute lymphoblastic leukemia cells through a p53-independent pathway. Mol. Cancer Ther. 2013, 12, 184–194. [Google Scholar] [CrossRef]
- Zheng, L.; Jia, J.; Dai, H.; Wan, L.; Liu, J.; Hu, L.; Zhou, M.; Qiu, M.; Chen, X.; Chang, L.; et al. Triptolide-Assisted Phosphorylation of p53 Suppresses Inflammation-Induced NF-κB Survival Pathways in Cancer Cells. Mol. Cell. Biol. 2017, 37, e00149-17. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, W.; Zhao, C.; Zhang, D.; Liang, P.; Wang, G.; Lu, L. Triptolide sensitizes human breast cancer cells to tumor necrosis factor-α-induced apoptosis by inhibiting activation of the nuclear factor-kappaB pathway. Mol. Med. Rep. 2016, 13, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Hu, Z.; Yang, Y.; Yin, Y.; Li, W.; Wu, L.; Fang, M. Anti-Inflammatory and Neuroprotective Effects of Triptolide via the NF-kappaB Signaling Pathway in a Rat MCAO Model. Anat. Rec. 2016, 299, 256–266. [Google Scholar] [CrossRef]
- Park, S.W.; Kim, Y.I. Triptolide induces apoptosis of PMA-treated THP-1 cells through activation of caspases, inhibition of NF-kappaB and activation of MAPKs. Int. J. Oncol. 2013, 43, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Hong, O.Y.; Jang, H.Y.; Park, K.H.; Jeong, Y.J.; Kim, J.S.; Chae, H.S. Triptolide inhibits matrix metalloproteinase-9 expression and invasion of breast cancer cells through the inhibition of NF-kappaB and AP-1 signaling pathways. Oncol. Lett. 2021, 22, 562. [Google Scholar] [CrossRef]
- Yan, C.; Dodd, T.; He, Y.; Tainer, J.A.; Tsutakawa, S.E.; Ivanov, I. Transcription preinitiation complex structure and dynamics provide insight into genetic diseases. Nat. Struct. Mol. Biol. 2019, 26, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Schier, A.C.; Taatjes, D.J. Structure and mechanism of the RNA polymerase II transcription machinery. Genes Dev. 2020, 34, 465–488. [Google Scholar] [CrossRef]
- Hou, Z.; Yan, M.; Li, H.; Wang, W.; You, S.; Wang, M.; Du, T.; Gong, H.; Li, W.; Guo, L.; et al. Variable p53/Nrf2 crosstalk contributes to triptolide-induced hepatotoxic process. Toxicol. Lett. 2023, 379, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, D.; Wu, L.; Li, Y. Protective effects of triptolide on retinal ganglion cells in a rat model of chronic glaucoma. Drug Des. Devel Ther. 2015, 9, 6095–6107. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, Y.; Liu, C.; Guo, W.; Li, X.; Su, X.; Wan, H.; Sun, Y.; Lin, N. Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS ONE 2013, 8, e77513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Chang, Y.; Han, B.; Li, R.; Wei, Y. MicroRNAs as crucial mediators in the pharmacological activities of triptolide (Review). Exp. Ther. Med. 2021, 21, 499. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, T.N.; Mujumdar, N.; Banerjee, S.; Sangwan, V.; Sarver, A.; Vickers, S.; Subramanian, S.; Saluja, A.K. Triptolide induces the expression of miR-142-3p: A negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol. Cancer Ther. 2013, 12, 1266–1275. [Google Scholar] [CrossRef]
- He, Q.; Zhang, B.; Hu, F.; Long, J.; Shi, Q.; Pi, X.; Chen, H.; Li, J. Triptolide Inhibits the Proliferation of HaCaT Cells Induced by IL22 via Upregulating miR-181b-5p. Drug Des. Devel Ther. 2020, 14, 2927–2935. [Google Scholar] [CrossRef] [PubMed]

| Delivery System | Year | Type of Study | Route of Admin | Pros | Ref. |
|---|---|---|---|---|---|
| Liposome Hydrogel | 2015 | in vivo | transdermal | Slower time to peak, more extended stability of plasma concentration vs. oral administration, and improved bioavailability. | [90] |
| Polymeric Micelles | 2008 | in vitro and in vivo | intravenous | Prolonged blood circulation, reduced cumulative toxicity, deeper tumor penetration, and improved endocytosis. | [91] |
| PLGA Nanoparticles | 2021 | in vitro | intranasal | Novel nasal brain targeting preparation, PLGA is FDA approved, has a sustained release profile, better transcellular permeability, reduced cytotoxicity, and attenuated oxidative stress. | [92] |
| Stimuli-Responsive Nanoparticles | 2021 | in vitro and in vivo | intravenous | pH/redox-dual-stimuli-responsive drug release profile, selective tumor uptake and high tumor tissue accumulation, CD44 targeting. | [89] |
| Exosomes | 2019 | in vitro and in vivo | intraperitoneal | Antitumor effect in vivo is superior to free triptolide. | [75] |
| Microemulsions | 2008 | in vivo | transdermal | Only mild reversible skin irritation signs and a good safety profile were observed. | [93] |
| Nano-Drugs | 2021 | in vitro and in vivo | intravenous | Good cancer targeting, enhanced apoptosis, remarkable tumor growth inhibition in vivo, and reduced toxicity. | [74] |
| Solid Dispersions | 2022 | in vitro and in vivo | intragastric | Enhanced oral bioavailability and improved antitumor activity. | [77] |
| Target | Triptolide Binding Mode | Activities | Affected Functions | Validation | Ref. |
|---|---|---|---|---|---|
| XPB | Irreversible | ATPase | NER, transcription | Immunoprecipitation and immunoblot | [7,16,21,101] |
| DNA-PKcs | Irreversible | Kinase | DNA repair, cell cycle | Computational prediction, thermal shift assay, co-immunoprecipitation, activity assays | [7,101,102,103] |
| HSP70 | Reduced Expression | Chaperone | Protein folding, stress response | Immunoblot, qPCR, immunohistochemistry, assays | [14,104,105,106] |
| PC-2 | Reversible | Calcium channel | Calcium release | Chromatographic protein fractionation, MALDI-MS analysis, and immunoblot | [7,45,101,107,108] |
| ADAM10 | Reduced Expression | Proteinase | Protein metabolism | Affinity chromatography and mass spec | [7,101,109] |
| DCTPP1 | Reversible | Pyrophosphatase | Nucleotide metabolism | Photoaffinity pull-down assay | [7,101,110,111] |
| TAB1 | Reversible | TAK1 (kinase) activation | TAK1 kinase | Pull-down assays, chemical proteomics | [7,101,112] |
| PRXI | Irreversible | Chaperone | Protein folding | Competition binding assay, size exclusion chromatography, mass spectrometry, activity assays, small molecule probes, immunoblot | [7,101,113] |
| Erα | Reversible | Mitogenic | Proliferation | Computational prediction, surface plasmon resonance, isothermal titration calorimetry, reporter gene assays | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorrie, D.; Bravo, M.; Fan, L. The Yin and Yang of the Natural Product Triptolide and Its Interactions with XPB, an Essential Protein for Gene Expression and DNA Repair. Genes 2024, 15, 1287. https://doi.org/10.3390/genes15101287
Gorrie D, Bravo M, Fan L. The Yin and Yang of the Natural Product Triptolide and Its Interactions with XPB, an Essential Protein for Gene Expression and DNA Repair. Genes. 2024; 15(10):1287. https://doi.org/10.3390/genes15101287
Chicago/Turabian StyleGorrie, David, Marco Bravo, and Li Fan. 2024. "The Yin and Yang of the Natural Product Triptolide and Its Interactions with XPB, an Essential Protein for Gene Expression and DNA Repair" Genes 15, no. 10: 1287. https://doi.org/10.3390/genes15101287
APA StyleGorrie, D., Bravo, M., & Fan, L. (2024). The Yin and Yang of the Natural Product Triptolide and Its Interactions with XPB, an Essential Protein for Gene Expression and DNA Repair. Genes, 15(10), 1287. https://doi.org/10.3390/genes15101287






