Prognostic Role of Specific KRAS Mutations Detected in Aspiration and Liquid Biopsies from Patients with Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Nucleic Acid Extraction and Detection of KRAS Mutations
2.3. Statistical Analysis
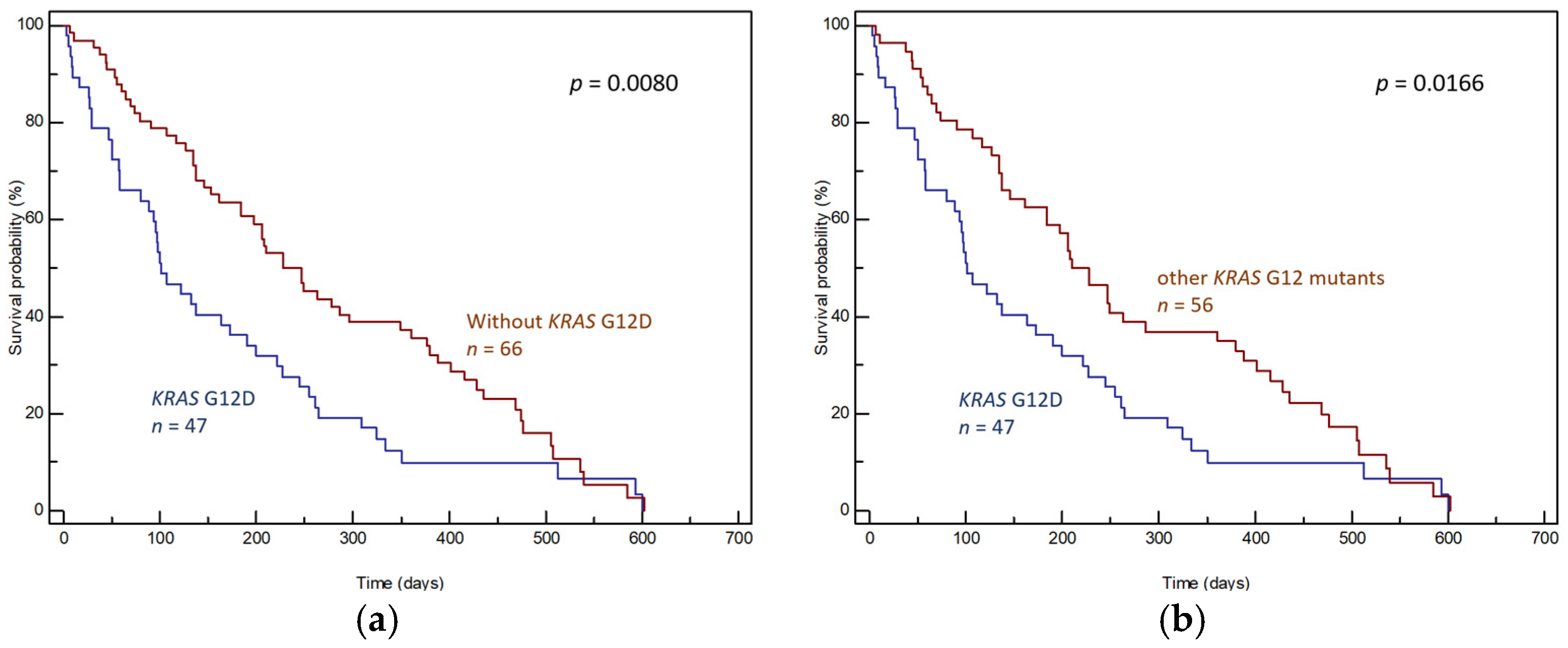
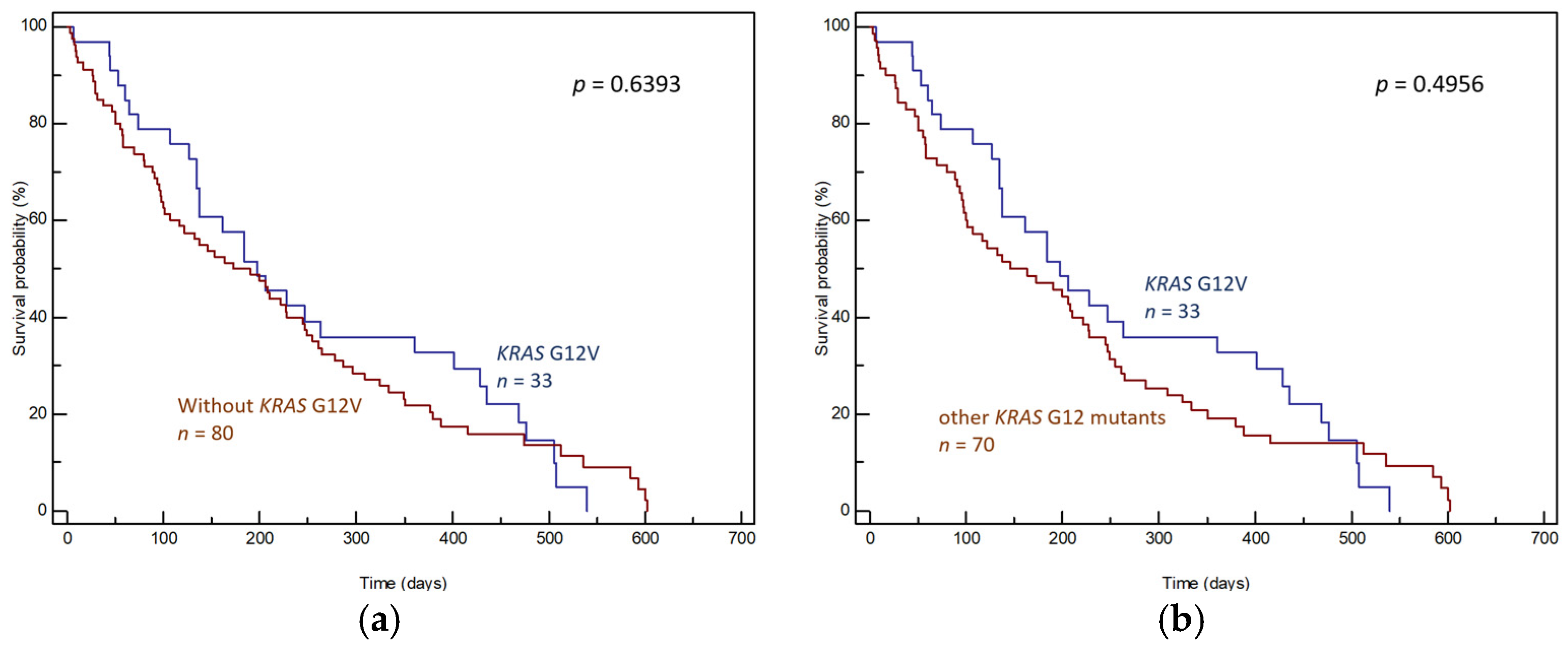
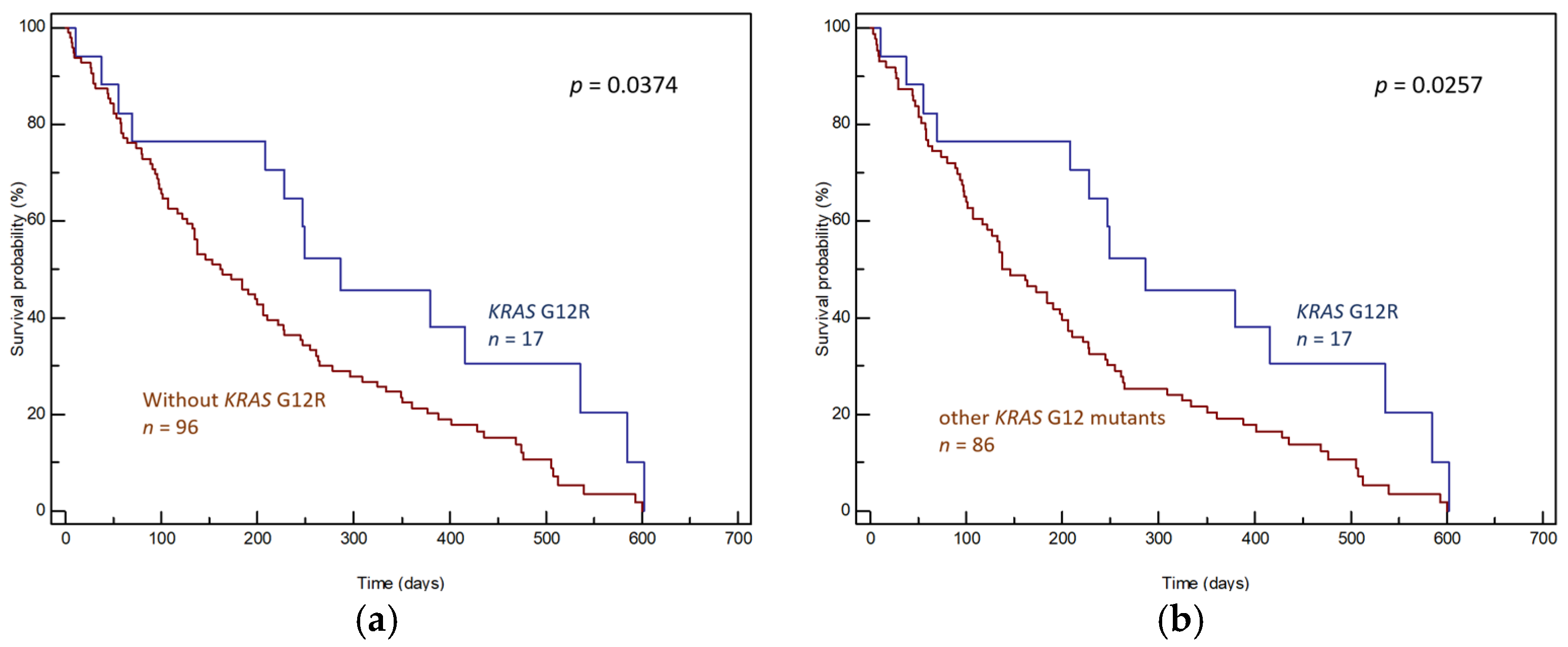
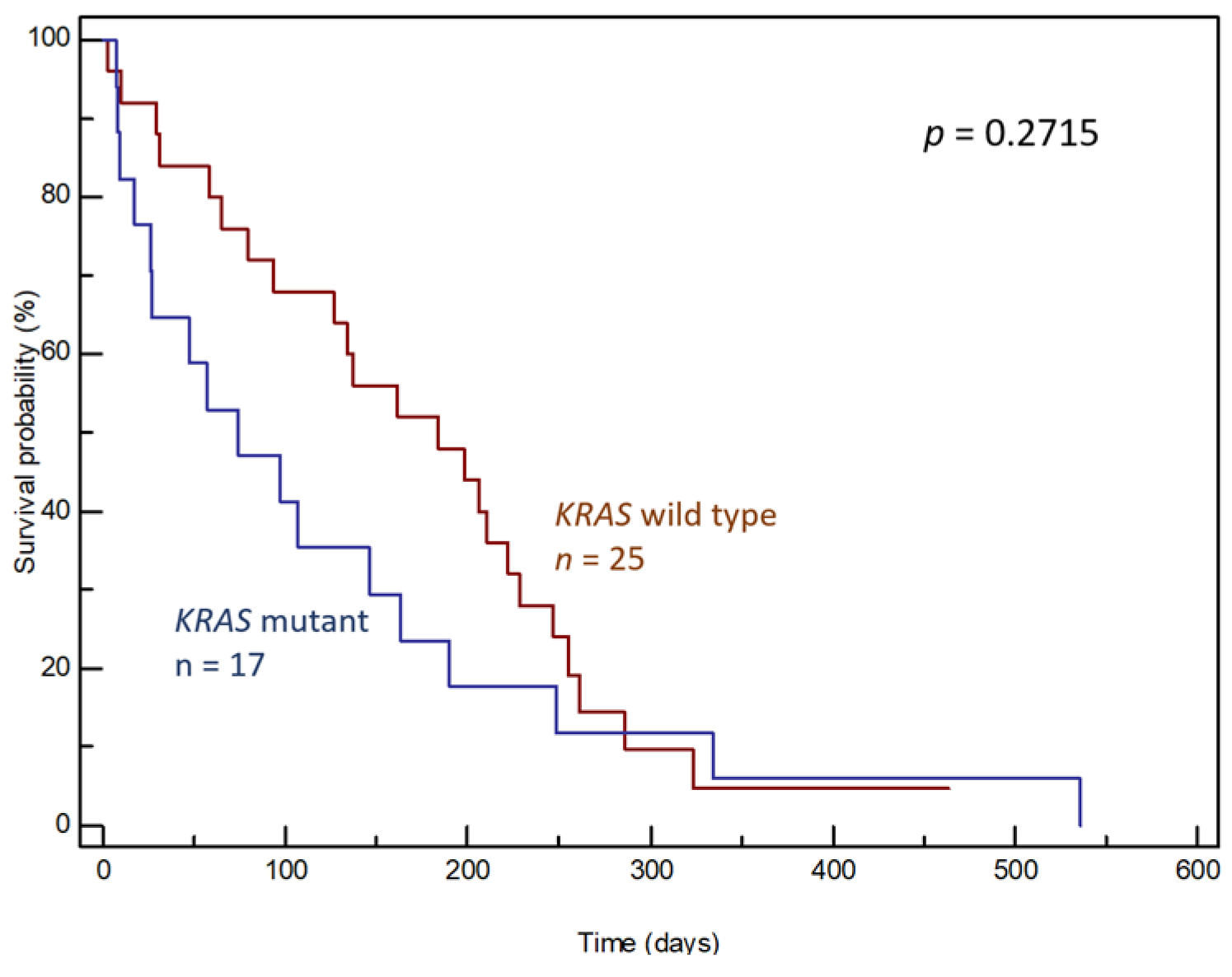
3. Results
3.1. Tumor Tissue Samples
3.2. ctDNA Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilic, I.; Ilic, M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J. Gastroenterol. 2022, 28, 4698–4715. [Google Scholar] [CrossRef] [PubMed]
- Whitley, A.; Kocián, P.; Nikov, A.; Krejčí, D.; Pehalová, L.; Blaha, M.; Dušek, L.; Gürlich, R. Early-onset pancreatic cancer: A national cancer registry study from the Czech Republic and review of the literature. J. Hepatobiliary Pancreat. Sci. 2023, 30, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Drebin, J.A.; Callery, M.P.; Fraker, D.; Kent, T.S.; Gates, J.; Vollmer, C.M., Jr. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB 2013, 15, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef]
- Goodsell, D.S. The molecular perspective: The ras oncogene. Oncologist 1999, 4, 263–264. [Google Scholar] [CrossRef]
- Downward, J. Control of ras activation. Cancer Surv. 1996, 27, 87–100. [Google Scholar]
- Boguski, M.S.; McCormick, F. Proteins regulating Ras and its relatives. Nature 1993, 366, 643–654. [Google Scholar] [CrossRef]
- Castellano, E.; Downward, J. RAS Interaction with PI3K, More Than Just Another Effector Pathway. Genes Cancer 2011, 2, 261–274. [Google Scholar] [CrossRef]
- Castagnola, P.; Giaretti, W. Mutant KRAS, chromosomal instability and prognosis in colorectal cancer. Biochim. Biophys. Acta 2005, 1756, 115–125. [Google Scholar] [CrossRef]
- Salek, C.; Benesova, L.; Zavoral, M.; Nosek, V.; Kasperova, L.; Ryska, M.; Strnad, R.; Traboulsi, E.; Minarik, M. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J. Gastroenterol. 2007, 13, 3714–3720. [Google Scholar] [CrossRef]
- Salek, C.; Minarikova, P.; Benesova, L.; Nosek, V.; Strnad, R.; Zavoral, M.; Minarik, M. Mutation status of K-ras, p53 and allelic losses at 9p and 18q are not prognostic markers in patients with pancreatic cancer. Anticancer Res. 2009, 29, 1803–1810. [Google Scholar] [PubMed]
- Benesova, L.; Halkova, T.; Bunganic, B.; Belsanova, B.; Zavoral, M.; Traboulsi, E.; Minarik, M. Comparison of Native Aspirates and Cytological Smears Obtained by EUS-Guided Biopsies for Effective DNA/RNA Marker Testing in Pancreatic Cancer. Pathol. Oncol. Res. 2020, 26, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.A.; Roslind, A.; Christensen, I.J.; Horn, T.; Høgdall, E.; Pedersen, L.N.; Kruhøffer, M.; Burcharth, F.; Wøjdemann, M.; Johansen, J.S. Frequencies and prognostic role of KRAS and BRAF mutations in patients with localized pancreatic and ampullary adenocarcinomas. Pancreas 2012, 41, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Oldani, A.; De Rosa, C.; Monni, M.; Terrone, A.; Miglio, U.; Garavoglia, M.; Boldorini, R. KRAS mutation analysis in ductal carcinoma of the pancreas; prognostic implications in elderly patients. BMC Surg. 2013, 13 (Suppl. S1), A31. [Google Scholar] [CrossRef]
- Bournet, B.; Muscari, F.; Buscail, C.; Assenat, E.; Barthet, M.; Hammel, P.; Selves, J.; Guimbaud, R.; Cordelier, P.; Buscail, L. KRAS G12D Mutation Subtype Is A Prognostic Factor for Advanced Pancreatic Adenocarcinoma. Clin. Transl. Gastroenterol. 2016, 7, e157. [Google Scholar] [CrossRef]
- Haas, M.; Ormanns, S.; Baechmann, S.; Remold, A.; Kruger, S.; Westphalen, C.B.; Siveke, J.T.; Wenzel, P.; Schlitter, A.M.; Esposito, I.; et al. Extended RAS analysis and correlation with overall survival in advanced pancreatic cancer. Br. J. Cancer 2017, 116, 1462–1469. [Google Scholar] [CrossRef]
- Lee, M.R.; Woo, S.M.; Kim, M.K.; Han, S.S.; Park, S.J.; Lee, W.J.; Lee, D.E.; Choi, S.I.; Choi, W.; Yoon, K.A.; et al. Application of plasma circulating KRAS mutations as a predictive biomarker for targeted treatment of pancreatic cancer. Cancer Sci. 2024, 115, 1283–1295. [Google Scholar] [CrossRef]
- Tao, L.Y.; Zhang, L.F.; Xiu, D.R.; Yuan, C.H.; Ma, Z.L.; Jiang, B. Prognostic significance of K-ras mutations in pancreatic cancer: A meta-analysis. World J. Surg. Oncol. 2016, 14, 146. [Google Scholar] [CrossRef]
- Ihle, N.T.; Byers, L.A.; Kim, E.S.; Saintigny, P.; Lee, J.J.; Blumenschein, G.R.; Tsao, A.; Liu, S.; Larsen, J.E.; Wang, J.; et al. Effect of KRAS oncogene substitutions on protein behavior: Implications for signaling and clinical outcome. J. Natl. Cancer Inst. 2012, 104, 228–239. [Google Scholar] [CrossRef]
- Eser, S.; Reiff, N.; Messer, M.; Seidler, B.; Gottschalk, K.; Dobler, M.; Hieber, M.; Arbeiter, A.; Klein, S.; Kong, B.; et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell 2013, 23, 406–420. [Google Scholar] [CrossRef]
- Dai, M.; Jahanzaib, R.; Liao, Y.; Yao, F.; Li, J.; Teng, X.; Chen, K.; Cheng, W. Prognostic value of KRAS subtype in patients with PDAC undergoing radical resection. Front. Oncol. 2022, 12, 1074538. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; Yousef, M.; Chowdhury, S.; Abdilleh, K.; Knafl, M.; Edelkamp, P.; Alfaro-Munoz, K.; Chacko, R.; Peterson, J.; Smaglo, B.G.; et al. Impact of KRAS mutations and co-mutations on clinical outcomes in pancreatic ductal adenocarcinoma. NPJ Precis. Oncol. 2024, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Benesova, L.; Belsanova, B.; Suchanek, S.; Kopeckova, M.; Minarikova, P.; Lipska, L.; Levy, M.; Visokai, V.; Zavoral, M.; Minarik, M. Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal. Biochem. 2013, 433, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Yildirim, H.C.; Aktepe, O.H.; Dizdar, O.; Yalcin, S. A systematic review and meta-analysis of the association between circulating tumor DNA (ctDNA) and prognosis in pancreatic cancer. Crit. Rev. Oncol. Hematol. 2021, 168, 103528. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- COSMIC (Catalogue of Somatic Mutations in Cancer). Available online: http://cancer.sanger.ac.uk/cosmic/browse/tissue (accessed on 19 August 2024).
- Stefanoudakis, D.; Frountzas, M.; Schizas, D.; Michalopoulos, N.V.; Drakaki, A.; Toutouzas, K.G. Significance of TP53, CDKN2A, SMAD4 and KRAS in Pancreatic Cancer. Curr. Issues Mol. Biol. 2024, 46, 2827–2844. [Google Scholar] [CrossRef]
- Nusrat, F.; Khanna, A.; Jain, A.; Jiang, W.; Lavu, H.; Yeo, C.J.; Bowne, W.; Nevler, A. The Clinical Implications of KRAS Mutations and Variant Allele Frequencies in Pancreatic Ductal Adenocarcinoma. J. Clin. Med. 2024, 13, 2103. [Google Scholar] [CrossRef]
- Andreyev, H.J.; Norman, A.R.; Cunningham, D.; Oates, J.R.; Clarke, P.A. Kirsten ras mutations in patients with colorectal cancer: The multicenter “RASCAL” study. J. Natl. Cancer Inst. 1998, 90, 675–684. [Google Scholar] [CrossRef]
- Al-Mulla, F.; Milner-White, E.J.; Going, J.J.; Birnie, G.D. Structural differences between valine-12 and aspartate-12 Ras proteins may modify carcinoma aggression. J. Pathol. 1999, 187, 433–438. [Google Scholar] [CrossRef]
- De Roock, W.; Jonker, D.J.; Di Nicolantonio, F.; Sartore-Bianchi, A.; Tu, D.; Siena, S.; Lamba, S.; Arena, S.; Frattini, M.; Piessevaux, H.; et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA 2010, 304, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Marabese, M.; Rusconi, P.; Rulli, E.; Martelli, O.; Farina, G.; Scanni, A.; Broggini, M. Different types of K-Ras mutations could affect drug sensitivity and tumour behaviour in non-small-cell lung cancer. Ann. Oncol. 2011, 22, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Tejpar, S.; Celik, I.; Schlichting, M.; Sartorius, U.; Bokemeyer, C.; Van Cutsem, E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J. Clin. Oncol. 2012, 30, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef]
- McCormick, F. Progress in targeting RAS with small molecule drugs. Biochem. J. 2019, 476, 365–374. [Google Scholar] [CrossRef]
- Hofmann, M.H.; Gerlach, D.; Misale, S.; Petronczki, M.; Kraut, N. Expanding the Reach of Precision Oncology by Drugging All KRAS Mutants. Cancer Discov. 2022, 12, 924–937. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Yaeger, R.; Spira, A.I.; Pelster, M.S.; Sabari, J.K.; Hafez, N.; Barve, M.; Velastegui, K.; Yan, X.; Shetty, A.; et al. Adagrasib in Advanced Solid Tumors Harboring a KRAS(G12C) Mutation. J. Clin. Oncol. 2023, 41, 4097–4106. [Google Scholar] [CrossRef]
- Fiala, O.; Pesek, M.; Finek, J.; Benesova, L.; Belsanova, B.; Minarik, M. The dominant role of G12C over other KRAS mutation types in the negative prediction of efficacy of epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Cancer Genet. 2013, 206, 26–31. [Google Scholar] [CrossRef]
- Ghimessy, A.; Radeczky, P.; Laszlo, V.; Hegedus, B.; Renyi-Vamos, F.; Fillinger, J.; Klepetko, W.; Lang, C.; Dome, B.; Megyesfalvi, Z. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020, 39, 1159–1177. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.A.; Lawrence, S.A.; Richards, A.L.; Chou, J.F.; Wong, W.; Capanu, M.; Berger, M.F.; Donoghue, M.T.A.; Yu, K.H.; Varghese, A.M.; et al. Alterations in driver genes are predictive of survival in patients with resected pancreatic ductal adenocarcinoma. Cancer 2020, 126, 3939–3949. [Google Scholar] [CrossRef]
- Shen, H.; Lundy, J.; Strickland, A.H.; Harris, M.; Swan, M.; Desmond, C.; Jenkins, B.J.; Croagh, D. KRAS G12D Mutation Subtype in Pancreatic Ductal Adenocarcinoma: Does It Influence Prognosis or Stage of Disease at Presentation? Cells 2022, 11, 3175. [Google Scholar] [CrossRef]
- Diehl, A.C.; Hannan, L.M.; Zhen, D.B.; Coveler, A.L.; King, G.; Cohen, S.A.; Harris, W.P.; Shankaran, V.; Wong, K.M.; Green, S.; et al. KRAS Mutation Variants and Co-occurring PI3K Pathway Alterations Impact Survival for Patients with Pancreatic Ductal Adenocarcinomas. Oncologist 2022, 27, 1025–1033. [Google Scholar] [CrossRef]
- Ogura, T.; Yamao, K.; Hara, K.; Mizuno, N.; Hijioka, S.; Imaoka, H.; Sawaki, A.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Prognostic value of K-ras mutation status and subtypes in endoscopic ultrasound-guided fine-needle aspiration specimens from patients with unresectable pancreatic cancer. J. Gastroenterol. 2013, 48, 640–646. [Google Scholar] [CrossRef]
- Earl, J.; Garcia-Nieto, S.; Martinez-Avila, J.C.; Montans, J.; Sanjuanbenito, A.; Rodríguez-Garrote, M.; Lisa, E.; Mendía, E.; Lobo, E.; Malats, N.; et al. Circulating tumor cells (Ctc) and kras mutant circulating free DNA (cfdna)detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer 2015, 15, 797. [Google Scholar] [CrossRef]
- Kinugasa, H.; Nouso, K.; Miyahara, K.; Morimoto, Y.; Dohi, C.; Tsutsumi, K.; Kato, H.; Matsubara, T.; Okada, H.; Yamamoto, K. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer 2015, 121, 2271–2280. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, S.; Pandey, R.M.; Chauhan, S.S.; Saraya, A. High levels of cell-free circulating nucleic acids in pancreatic cancer are associated with vascular encasement.; metastasis and poor survival. Cancer Investig. 2015, 33, 78–85. [Google Scholar] [CrossRef]
- Hadano, N.; Murakami, Y.; Uemura, K.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Sueda, T.; Hiyama, E. Prognostic value of circulating tumour DNA in patients undergoing curative resection for pancreatic cancer. Br. J. Cancer 2016, 115, 59–65. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, C.; Jiang, J.; Luo, G.; Lu, Y.; Jin, K.; Guo, M.; Zhang, Z.; Xu, J.; Liu, L.; et al. Analysis of ctDNA to predict prognosis and monitor treatment responses in metastatic pancreatic cancer patients. Int. J. Cancer 2017, 140, 2344–2350. [Google Scholar] [CrossRef]
- Perets, R.; Greenberg, O.; Shentzer, T.; Semenisty, V.; Epelbaum, R.; Bick, T.; Sarji, S.; Ben-Izhak, O.; Sabo, E.; Hershkovitz, D. Mutant KRAS Circulating Tumor DNA Is an Accurate Tool for Pancreatic Cancer Monitoring. Oncologist 2018, 23, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Mosier, S.; Javed, A.A.; Teinor, J.A.; Gemenetzis, G.; Ding, D.; Haley, L.M.; Yu, J.; Burkhart, R.A.; Hasanain, A.; et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 4973–4984. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Suzuki, K.; Tamaki, S.; Abe, I.; Endo, Y.; Takayama, Y.; Ishikawa, H.; Kakizawa, N.; Saito, M.; Futsuhara, K.; et al. Longitudinal monitoring of KRAS-mutated circulating tumor DNA enables the prediction of prognosis and therapeutic responses in patients with pancreatic cancer. PLoS ONE 2019, 14, e0227366. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Shi, X.; Shen, J.; Gao, S.; Wang, H.; Shen, S.; Pan, Y.; Li, B.; Xu, X.; Shao, Z.; et al. Preoperative detection of KRAS G12D mutation in ctDNA is a powerful predictor for early recurrence of resectable PDAC patients. Br. J. Cancer 2020, 122, 857–867. [Google Scholar] [CrossRef] [PubMed]



| Monitored Characteristic | Number of Patients | |
|---|---|---|
| All cases | 118 (100%) | |
| Age | 67.9 ± 9.4 | |
| Gender | Male | 64 (54%) |
| Aged | 44–92 years, median 69 | |
| Female | 54 (46%) | |
| Aged | 44–87 years, median 66 | |
| Location of PDAC | Head | 81 (69%) |
| Body | 28 (24%) | |
| Tail | 9 (8%) | |
| Disease stage | II | 3 (3%) |
| III | 56 (47%) | |
| IV | 58 (49%) | |
| Unknown | 1 (1%) | |
| Treatment | Surgery + adjuvant chemotherapy | 16 (14%) |
| Surgery alone | 11 (9%) | |
| Chemotherapy alone | 44 (37%) | |
| Supportive care | 44 (37%) | |
| Unknown | 3 (3%) | |
| Result of KRAS Analysis | Tissue Sample (n = 118) | Plasma Sample (n = 45) |
|---|---|---|
| KRAS wild type | 12/118 (10%) | 25/45 (56%) |
| KRAS mutant | 106/118 (90%) | 20/45 (44%) |
| GGT/GAT (G12D) | 47/106 (44%) | 13/20 (65%) |
| GGT/GTT (G12V) | 33/106 (31%) | 2/20 (10%) |
| GGT/CGT (G12R) | 18/106 (17%) | 2/20 (10%) |
| GGT/TGT (G12C) | 5/106 (5%) | 0 |
| GGT/AGT (G12S) | 1/106 (1%) | 1/20 (5%) |
| GGT/GTT (G12V) + GGT/AGT (G12S) | 1/106 (1%) | 1/20 (5%) |
| GGT/GAT (G12D) + GGT/AGT (G12S) | 1/106 (1%) | 1/20 (5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hálková, T.; Bunganič, B.; Traboulsi, E.; Minárik, M.; Zavoral, M.; Benešová, L. Prognostic Role of Specific KRAS Mutations Detected in Aspiration and Liquid Biopsies from Patients with Pancreatic Cancer. Genes 2024, 15, 1302. https://doi.org/10.3390/genes15101302
Hálková T, Bunganič B, Traboulsi E, Minárik M, Zavoral M, Benešová L. Prognostic Role of Specific KRAS Mutations Detected in Aspiration and Liquid Biopsies from Patients with Pancreatic Cancer. Genes. 2024; 15(10):1302. https://doi.org/10.3390/genes15101302
Chicago/Turabian StyleHálková, Tereza, Bohuš Bunganič, Eva Traboulsi, Marek Minárik, Miroslav Zavoral, and Lucie Benešová. 2024. "Prognostic Role of Specific KRAS Mutations Detected in Aspiration and Liquid Biopsies from Patients with Pancreatic Cancer" Genes 15, no. 10: 1302. https://doi.org/10.3390/genes15101302






