The Molecular Basis of Multiple Morphological Abnormalities of Sperm Flagella and Its Impact on Clinical Practice
Abstract
:1. Introduction
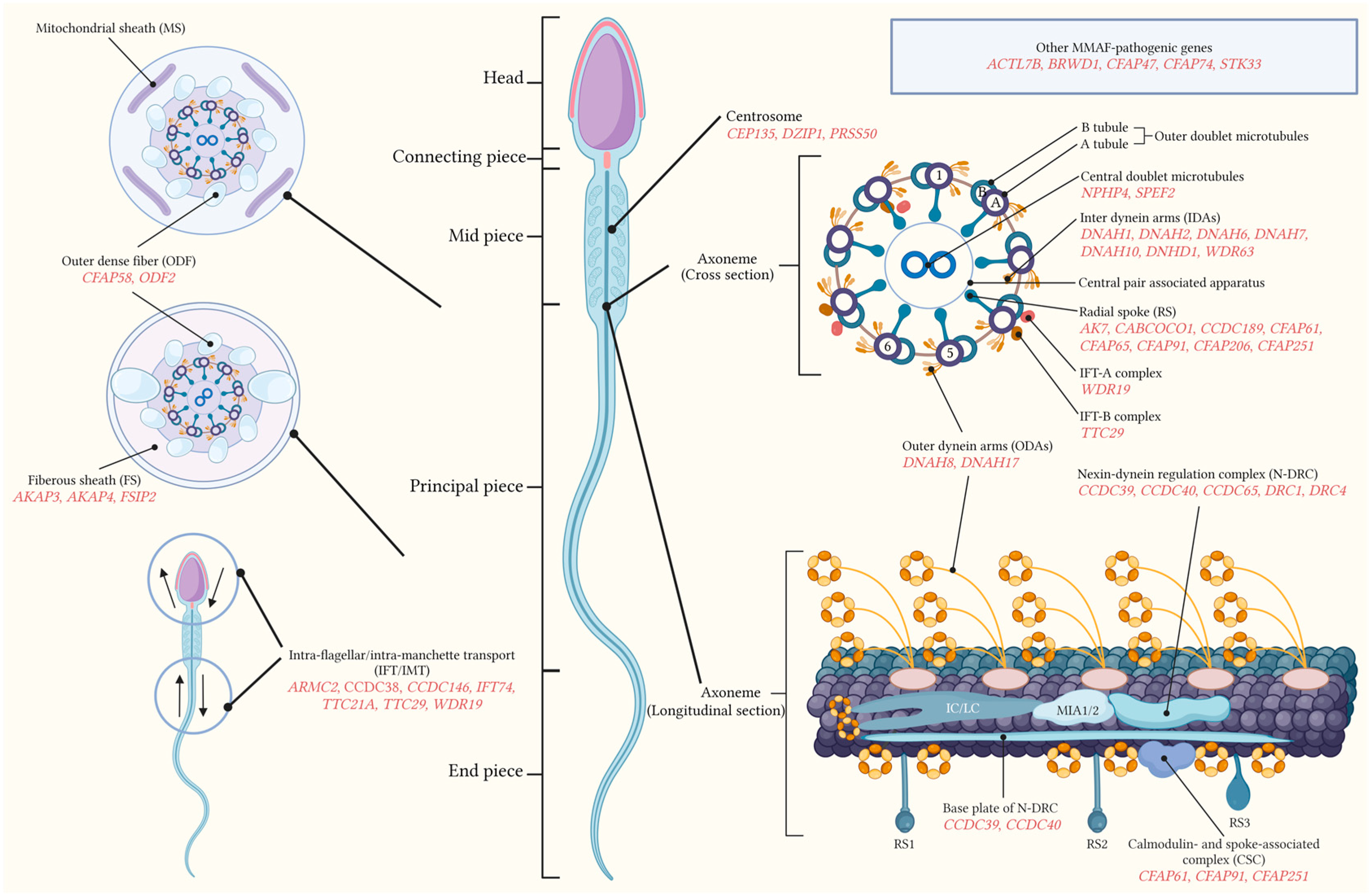
2. Multiple Morphological Abnormalities of the Sperm Flagella (MMAF)
2.1. Normal Sperm Flagella Structure
2.2. Concept of MMAF
2.3. Differential Diagnosis of MMAF
3. Molecular Genetics of MMAF
3.1. Axonemal-Associated Pathogenic Genes
3.1.1. Gene Mutations Related to the Outer Dynein Arm (ODA)
3.1.2. Gene Mutations Related to the Inner Dynein Arm (IDA)
3.1.3. Gene Mutations Related to the Nexin-Dynein Regulation Complex (N-DRC)
3.1.4. Gene Mutations Related to the Radial Spoke (RS)
3.1.5. Gene Mutations Related to the Central Doublet Microtubules
3.2. Peri-Axoneme-Associated Pathogenic Genes
3.3. Centrosome-Associated Pathogenic Genes
3.4. Pathogenic Genes Related to the Flagellar Assembly
3.5. Other Pathogenic Genes
4. Clinical Diagnosis and Treatment of MMAF Based on the Molecular Mechanisms
4.1. Diagnosis of MMAF
4.2. Treatment of MMAF
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Tu, C.F.; Tan, Y.Q. Insight on multiple morphological abnormalities of sperm flagella in male infertility: What is new? Asian J. Androl. 2020, 22, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ben Khelifa, M.; Coutton, C.; Zouari, R.; Karaouzène, T.; Rendu, J.; Bidart, M.; Yassine, S.; Pierre, V.; Delaroche, J.; Hennebicq, S.; et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014, 94, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sha, Y.; Wang, X.; Ding, L.; Liu, W.; Ji, Z.; Mei, L.; Huang, X.; Lin, S.; Kong, S.; et al. DNAH2 is a novel candidate gene associated with multiple morphological abnormalities of the sperm flagella. Clin. Genet. 2019, 95, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-M.; Zhu, Y.-T.; Gu, M.; Guo, S.-C.; Yu, H.; Li, K.-K.; Tang, D.-D.; Xu, Y.-P.; Lv, M.-R. Novel variants in DNAH6 cause male infertility associated with multiple morphological abnormalities of the sperm flagella (MMAF) and ICSI outcomes. Asian J. Androl. 2024, 26, 91–98. [Google Scholar] [CrossRef]
- Zhang, B.; Khan, I.; Liu, C.; Ma, A.; Khan, A.; Zhang, Y.; Zhang, H.; Kakakhel, M.B.S.; Zhou, J.; Zhang, W.; et al. Novel loss-of-function variants in DNAH17 cause multiple morphological abnormalities of the sperm flagella in humans and mice. Clin. Genet. 2021, 99, 176–186. [Google Scholar] [CrossRef]
- Lv, M.; Liu, W.; Chi, W.; Ni, X.; Wang, J.; Cheng, H.; Li, W.-Y.; Yang, S.; Wu, H.; Zhang, J.; et al. Homozygous mutations in DZIP1 can induce asthenoteratospermia with severe MMAF. J. Med. Genet. 2020, 57, 445–453. [Google Scholar] [CrossRef]
- Sha, Y.-W.; Xu, X.; Mei, L.-B.; Li, P.; Su, Z.-Y.; He, X.-Q.; Li, L. A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF). Gene 2017, 633, 48–53. [Google Scholar] [CrossRef]
- Liu, G.; Yin, X.; Xing, X.; Yin, S.; Shen, Y.; Zhang, H.; Lin, G.; Lu, G.; Li, W. Novel mutation in TTC21A triggers partial nonsense-mediated mRNA decay and causes male infertility with MMAF. Clin. Genet. 2022, 102, 459–460. [Google Scholar] [CrossRef]
- Cong, J.; Wang, X.; Amiri-Yekta, A.; Wang, L.; Kherraf, Z.-E.; Liu, C.; Cazin, C.; Tang, S.; Hosseini, S.H.; Tian, S.; et al. Homozygous mutations in CCDC34 cause male infertility with oligoasthenoteratozoospermia in humans and mice. J. Med. Genet. 2022, 59, 710–718. [Google Scholar] [CrossRef]
- Wang, M.; Kang, J.; Shen, Z.; Hu, Y.; Chen, M.; Cui, X.; Liu, H.; Gao, F. CCDC189 affects sperm flagellum formation by interacting with CABCOCO1. Natl. Sci. Rev. 2023, 10, nwad181. [Google Scholar] [CrossRef] [PubMed]
- Baldini, D.; Ferri, D.; Baldini, G.M.; Lot, D.; Catino, A.; Vizziello, D.; Vizziello, G. Sperm Selection for ICSI: Do We Have a Winner? Cells 2021, 10, 3566. [Google Scholar] [CrossRef] [PubMed]
- Marmor, D.; Grob-Menendez, F. Male infertility due to asthenozoospermia and flagellar anomaly: Detection in routine semen analysis. Int. J. Androl. 1991, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.; Christie, S.; Edmond, P. Ultrastructural tail defects in the spermatozoa from two men attending a subfertility clinic. J. Reprod. Fertil. 1973, 32, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, K.; Matsuda, T.; Horii, Y.; Yoshida, O. Three cases with different types of short-tailed spermatozoa. Urol. Int. 1993, 50, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, C.; Tharanne, M.J.; Lebos, C.; Lecomte, P.; Lansac, J. Tail stump spermatozoa: Morphogenesis of the defect. An ultrastructural study of sperm and testicular biopsy. Andrologia 1990, 22, 417–425. [Google Scholar] [CrossRef]
- Schwabe, G.C.; Hoffmann, K.; Loges, N.T.; Birker, D.; Rossier, C.; de Santi, M.M.; Olbrich, H.; Fliegauf, M.; Failly, M.; Liebers, U.; et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum. Mutat. 2008, 29, 289–298. [Google Scholar] [CrossRef]
- Escalier, D.; Touré, A. Morphological defects of sperm flagellum implicated in human male infertility. Med. Sci. 2012, 28, 503–511. [Google Scholar] [CrossRef]
- Curi, S.M.; Ariagno, J.I.; Chenlo, P.H.; Mendeluk, G.R.; Pugliese, M.N.; Sardi Segovia, L.M.; Repetto, H.E.H.; Blanco, A.M. Asthenozoospermia: Analysis of a large population. Arch. Androl. 2003, 49, 343–349. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021; p. xii. 276p. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010; p. xiv. 271p. [Google Scholar]
- Nsota Mbango, J.-F.; Coutton, C.; Arnoult, C.; Ray, P.F.; Touré, A. Genetic causes of male infertility: Snapshot on morphological abnormalities of the sperm flagellum. Basic Clin. Androl. 2019, 29, 2. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Shen, L.; Zheng, A.; Meng, Q.; Li, H.; Yang, S. Clinical detection, diagnosis and treatment of morphological abnormalities of sperm flagella: A review of literature. Front. Genet. 2022, 13, 1034951. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Lin, Y.-H.; Chen, H.-I.; Wang, Y.-Y.; Chiou, Y.-W.; Lin, H.-H.; Pan, H.-A.; Wu, C.-M.; Su, S.-M.; Hsu, C.-C.; et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum. Mutat. 2012, 33, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, M.; Thomas, L.; Bequignon, E.; Schmitt, A.; Stouvenel, L.; Montantin, G.; Tissier, S.; Duquesnoy, P.; Copin, B.; Chantot, S.; et al. Mutations in DNAH17, Encoding a Sperm-Specific Axonemal Outer Dynein Arm Heavy Chain, Cause Isolated Male Infertility Due to Asthenozoospermia. Am. J. Hum. Genet. 2019, 105, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; Lettieri, G.; Marinaro, C.; Longo, V.; Capone, S.; Forleo, A.; Pappalardo, S.; Montano, L.; Piscopo, M. Molecular Alterations and Severe Abnormalities in Spermatozoa of Young Men Living in the “Valley of Sacco River” (Latium, Italy): A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 11023. [Google Scholar] [CrossRef]
- Beurois, J.; Martinez, G.; Cazin, C.; Kherraf, Z.-E.; Amiri-Yekta, A.; Thierry-Mieg, N.; Bidart, M.; Petre, G.; Satre, V.; Brouillet, S.; et al. CFAP70 mutations lead to male infertility due to severe astheno-teratozoospermia. A case report. Hum. Reprod. 2019, 34, 2071–2079. [Google Scholar] [CrossRef]
- Jin, H.-J.; Wang, J.-L.; Geng, X.-Y.; Wang, C.-Y.; Wang, B.-B.; Chen, S.-R. CFAP70 is a solid and valuable target for the genetic diagnosis of oligo-astheno-teratozoospermia in infertile men. EBioMedicine 2023, 93, 104675. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Wu, B.; Shi, H.; Wang, L. Experimental and molecular support for Cfap70 as a causative gene of ‘multiple morphological abnormalities of the flagella’ with male infertility. Biol. Reprod. 2023, 109, 450–460. [Google Scholar] [CrossRef]
- Dil, S.; Khan, A.; Unar, A.; Yang, M.-L.; Ali, I.; Zeb, A.; Zhang, H.; Zhou, J.-T.; Zubair, M.; Khan, K.; et al. A novel homozygous frameshift variant in DNAH8 causes multiple morphological abnormalities of the sperm flagella in a consanguineous Pakistani family. Asian J. Androl. 2023, 25, 350–355. [Google Scholar] [CrossRef]
- Liu, C.; Miyata, H.; Gao, Y.; Sha, Y.; Tang, S.; Xu, Z.; Whitfield, M.; Patrat, C.; Wu, H.; Dulioust, E.; et al. Bi-allelic DNAH8 Variants Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Primary Male Infertility. Am. J. Hum. Genet. 2020, 107, 330–341. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, C.; Zhang, X.; Liu, X.; Li, J.; Qiao, X.; Liu, H.; Shen, Y. Loss-of-function mutation in DNAH8 induces asthenoteratospermia associated with multiple morphological abnormalities of the sperm flagella. Clin. Genet. 2020, 98, 396–401. [Google Scholar] [CrossRef]
- Sha, Y.; Wei, X.; Ding, L.; Mei, L.; Huang, X.; Lin, S.; Su, Z.; Kong, L.; Zhang, Y.; Ji, Z. DNAH17 is associated with asthenozoospermia and multiple morphological abnormalities of sperm flagella. Ann. Hum. Genet. 2020, 84, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, C.; Ni, F.; Yang, F.; Wei, H.; Li, T.; Wang, J.; Wang, B. Novel compound heterozygous variants of DNAH17 in a Chinese infertile man with multiple morphological abnormalities of sperm flagella. Andrologia 2022, 54, e14553. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liu, C.; Gao, Y.; Marley, J.L.; Li, W.; Ni, X.; Liu, W.; Chen, Y.; Wang, J.; Wang, C.; et al. Novel compound heterozygous variants in dynein axonemal heavy chain 17 cause asthenoteratospermia with sperm flagellar defects. J. Genet. Genom. 2020, 47, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Yang, T.; Shen, Q.; Liu, Y.; Wang, C.; Li, G.; Gao, Y.; Cao, Y.; He, X. Novel mutations in DNAH17 cause sperm flagellum defects and their influence on ICSI outcome. J. Assist. Reprod. Genet. 2023, 40, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Sun, Y.; Jiang, C.; Chen, D.; Yang, Y.; Shen, Y. A novel mutation in DNAH17 is present in a patient with multiple morphological abnormalities of the flagella. Reprod. Biomed. Online 2021, 43, 532–541. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.; Li, W.; Yang, X.; Li, Z.; Liu, W.; Li, C.; Zhu, Z.; Wang, L.; Wang, J.; et al. Biallelic Mutations in CFAP43 and CFAP44 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella. Am. J. Hum. Genet. 2017, 100, 854–864. [Google Scholar] [CrossRef]
- Coutton, C.; Vargas, A.S.; Amiri-Yekta, A.; Kherraf, Z.-E.; Ben Mustapha, S.F.; Le Tanno, P.; Wambergue-Legrand, C.; Karaouzène, T.; Martinez, G.; Crouzy, S.; et al. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 2018, 9, 686. [Google Scholar] [CrossRef]
- Wu, H.; Li, W.; He, X.; Liu, C.; Fang, Y.; Zhu, F.; Jiang, H.; Liu, W.; Song, B.; Wang, X.; et al. NovelCFAP43 andCFAP44 mutations cause male infertility with multiple morphological abnormalities of the sperm flagella (MMAF). Reprod. Biomed. Online 2019, 38, 769–778. [Google Scholar] [CrossRef]
- Sha, Y.-W.; Wang, X.; Xu, X.; Su, Z.-Y.; Cui, Y.; Mei, L.-B.; Huang, X.-J.; Chen, J.; He, X.-M.; Ji, Z.-Y.; et al. Novel Mutations in CFAP44 and CFAP43 Cause Multiple Morphological Abnormalities of the Sperm Flagella (MMAF). Reprod. Sci. 2019, 26, 26–34. [Google Scholar] [CrossRef]
- Ma, J.; Long, S.-H.; Yu, H.-B.; Xiang, Y.-Z.; Tang, X.-R.; Li, J.-X.; Liu, W.-W.; Han, W.; Jin, R.; Huang, G.-N.; et al. Patients with MMAF induced by novel biallelic CFAP43 mutations have good fertility outcomes after intracytoplasmic sperm injection. Asian J. Androl. 2023, 25, 564–571. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Zhou, L.; Li, H.; Zheng, B.; Yang, S. CFAP43-mediated intra-manchette transport is required for sperm head shaping and flagella formation. Zygote 2021, 29, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.-J.; Xu, S.-Y.; Dong, L.; Zhang, P.-H.; Zhuang, B.-L.; Huang, X.-P.; Li, G.-S.; You, Y.-D.; Chen, D.A.; Yu, X.-J.; et al. Novel DNAH1 Mutation Loci Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Literature Review. World J. Mens. Health 2022, 40, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; An, M.; Xu, Y.; Gao, Q.; Lu, M.; Li, Y.; Zhang, L.; Wang, H.; Xu, Z. Mutational landscape of DNAH1 in Chinese patients with multiple morphological abnormalities of the sperm flagella: Cohort study and literature review. J. Assist. Reprod. Genet. 2021, 38, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-Y.; Wei, T.-Y.; Feng, Z.-K.; Li, S.-J.; Zhao, R.; Yi, X.-L.; Hu, T.-L.; Zhao, H.; Li, C.-X.; Liu, Z.-G. Novel Biallelic DNAH1 Variations Cause Multiple Morphological Abnormalities of the Sperm Flagella. DNA Cell Biol. 2021, 40, 833–840. [Google Scholar] [CrossRef]
- Long, S.; Fu, L.; Ma, J.; Yu, H.; Tang, X.; Hu, T.; Han, W.; Liu, W.; Liao, H.; Fu, T.; et al. Novel biallelic variants in DNAH1 cause multiple morphological abnormalities of sperm flagella with favorable outcomes of fertility after ICSI in Han Chinese males. Andrology 2024, 12, 349–364. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, X.; Zhang, H.; Guo, J.; Zhang, C.; Li, J.; Yang, Y. Novel bi-allelic mutations in DNAH1 cause multiple morphological abnormalities of the sperm flagella resulting in male infertility. Transl. Androl. Urol. 2021, 10, 1656–1664. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.-Y.; Zhou, J.-P.; Tan, H.-P.; Hu, J.; Jin, L.; Zhu, L.-X. Novel compound heterozygous mutations in DNAH1 cause primary infertility in Han Chinese males with multiple morphological abnormalities of the sperm flagella. Asian J. Androl. 2023, 25, 512–519. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, S.; Sha, Y.; Zha, X.; Cheng, H.; Wang, A.; Liu, C.; Lv, M.; Ni, X.; Li, Q.; et al. Novel bi-allelic variants in DNAH2 cause severe asthenoteratozoospermia with multiple morphological abnormalities of the flagella. Reprod. Biomed. Online 2021, 42, 963–972. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Nawaz, S.; Choi, J.; Wang, H.; Hussain, S.; Nawaz, M.; Lopez-Giraldez, F.; Jeong, K.; Dong, W.; Oh, J.-N.; et al. Genetic Defects in DNAH2 Underlie Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella in Humans and Mice. Front. Cell Dev. Biol. 2021, 9, 662903. [Google Scholar] [CrossRef]
- Tu, C.; Nie, H.; Meng, L.; Yuan, S.; He, W.; Luo, A.; Li, H.; Li, W.; Du, J.; Lu, G.; et al. Identification of DNAH6 mutations in infertile men with multiple morphological abnormalities of the sperm flagella. Sci. Rep. 2019, 9, 15864. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, L.; Shen, Q.; Fu, F.; Xu, C.; Geng, H.; Lv, M.; Li, K.; Tang, D.; Song, B.; et al. Loss of function mutation in DNAH7 induces male infertility associated with abnormalities of the sperm flagella and mitochondria in human. Clin. Genet. 2022, 102, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Cong, J.; Zhang, Q.; He, X.; Zheng, R.; Yang, X.; Gao, Y.; Wu, H.; Lv, M.; Gu, Y.; et al. Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice. Am. J. Hum. Genet. 2021, 108, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, G.; Lv, M.; Wang, J.; Gao, Y.; Tang, F.; Xu, C.; Yang, W.; Yu, H.; Shao, Z.; et al. Bi-allelic variants in DNAH10 cause asthenoteratozoospermia and male infertility. J. Assist. Reprod. Genet. 2022, 39, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Barbotin, A.-L.; Cazin, C.; Wehbe, Z.; Boursier, A.; Amiri-Yekta, A.; Daneshipour, A.; Hosseini, S.-H.; Rives, N.; Feraille, A.; et al. New Mutations in DNHD1 Cause Multiple Morphological Abnormalities of the Sperm Flagella. Int. J. Mol. Sci. 2023, 24, 2559. [Google Scholar] [CrossRef]
- Lu, S.; Gu, Y.; Wu, Y.; Yang, S.; Li, C.; Meng, L.; Yuan, W.; Jiang, T.; Zhang, X.; Li, Y.; et al. Bi-allelic variants in human WDR63 cause male infertility via abnormal inner dynein arms assembly. Cell Discov. 2021, 7, 110. [Google Scholar] [CrossRef]
- Chen, D.; Liang, Y.; Li, J.; Zhang, X.; Zheng, R.; Wang, X.; Zhang, H.; Shen, Y. A novel CCDC39 mutation causes multiple morphological abnormalities of the flagella in a primary ciliary dyskinesia patient. Reprod. Biomed. Online 2021, 43, 920–930. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, B.; Lei, C.; Yang, D.; Ding, S.; Lu, C.; Wang, L.; Guo, T.; Wang, R.; Luo, H. Novel Compound Heterozygous Variants in CCDC40 Associated with Primary Ciliary Dyskinesia and Multiple Morphological Abnormalities of the Sperm Flagella. Pharmgenom. Pers. Med. 2022, 15, 341–350. [Google Scholar] [CrossRef]
- Aprea, I.; Wilken, A.; Krallmann, C.; Nöthe-Menchen, T.; Olbrich, H.; Loges, N.T.; Dougherty, G.W.; Bracht, D.; Brenker, C.; Kliesch, S.; et al. Pathogenic gene variants in CCDC39, CCDC40, RSPH1, RSPH9, HYDIN, and SPEF2 cause defects of sperm flagella composition and male infertility. Front. Genet. 2023, 14, 1117821. [Google Scholar] [CrossRef]
- Jreijiri, F.; Cavarocchi, E.; Amiri-Yekta, A.; Cazin, C.; Hosseini, S.-H.; El Khouri, E.; Patrat, C.; Thierry-Mieg, N.; Ray, P.F.; Dulioust, E.; et al. CCDC65, encoding a component of the axonemal Nexin-Dynein regulatory complex, is required for sperm flagellum structure in humans. Clin. Genet. 2024, 105, 317–322. [Google Scholar] [CrossRef]
- Lei, C.; Yang, D.; Wang, R.; Ding, S.; Wang, L.; Guo, T.; Luo, H. DRC1 deficiency caused primary ciliary dyskinesia and MMAF in a Chinese patient. J. Hum. Genet. 2022, 67, 197–201. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.; Wu, H.; Zhang, X.; Yang, S.; Liu, C.; Liu, S.; Hua, R.; Zhou, S.; Zhao, S.; et al. Loss of DRC1 function leads to multiple morphological abnormalities of the sperm flagella and male infertility in human and mouse. Hum. Mol. Genet. 2021, 30, 1996–2011. [Google Scholar] [CrossRef] [PubMed]
- Kherraf, Z.-E.; Barbotin, A.-L.; Martinez, G.; Mazet, A.; Cazin, C.; Coutton, C.; Arnoult, C.; Thierry-Mieg, N.; Rives, N.; Rives-Feraille, A.; et al. A splice donor variant of GAS8 induces structural disorganization of the axoneme in sperm flagella and leads to nonsyndromic male infertility. Clin. Genet. 2024, 105, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Wang, Y.; Xu, W.; Zheng, N.; Deng, H.; Zhang, J.; Duan, Z.; Zha, X.; Zhang, W.; Song, G.; et al. A novel homozygous missense mutation in AK7 causes multiple morphological anomalies of the flagella and oligoasthenoteratozoospermia. J. Assist. Reprod. Genet. 2022, 39, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Tang, H.; Zhou, X.; He, J.; Liu, N.; Li, Y.; Xiang, W.; Yao, Z. A novel homozygous nonsense variant of AK7 is associated with multiple morphological abnormalities of the sperm flagella. Reprod. Biomed. Online 2024, 48, 103765. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Kherraf, Z.E.; Sun, S.; Zhang, X.; Cazin, C.; Coutton, C.; Zouari, R.; Zhao, S.; Hu, F.; et al. CFAP61 is required for sperm flagellum formation and male fertility in human and mouse. Development 2021, 148, dev199805. [Google Scholar] [CrossRef]
- Ma, A.; Zeb, A.; Ali, I.; Zhao, D.; Khan, A.; Zhang, B.; Zhou, J.; Khan, R.; Zhang, H.; Zhang, Y.; et al. Biallelic Variants in CFAP61 Cause Multiple Morphological Abnormalities of the Flagella and Male Infertility. Front. Cell Dev. Biol. 2021, 9, 803818. [Google Scholar] [CrossRef]
- Hu, T.; Meng, L.; Tan, C.; Luo, C.; He, W.-B.; Tu, C.; Zhang, H.; Du, J.; Nie, H.; Lu, G.-X.; et al. Biallelic CFAP61 variants cause male infertility in humans and mice with severe oligoasthenoteratozoospermia. J. Med. Genet. 2023, 60, 144–153. [Google Scholar] [CrossRef]
- Barbotin, A.-L.; Boursier, A.; Jourdain, A.-S.; Moerman, A.; Rabat, B.; Chehimi, M.; Thuillier, C.; Ghoumid, J.; Smol, T. Identification of a novel CFAP61 homozygous splicing variant associated with multiple morphological abnormalities of the flagella. J. Assist. Reprod. Genet. 2024, 41, 1499–1505. [Google Scholar] [CrossRef]
- Wang, W.; Tian, S.; Nie, H.; Tu, C.; Liu, C.; Li, Y.; Li, D.; Yang, X.; Meng, L.; Hu, T.; et al. CFAP65 is required in the acrosome biogenesis and mitochondrial sheath assembly during spermiogenesis. Hum. Mol. Genet. 2021, 30, 2240–2254. [Google Scholar] [CrossRef]
- Martinez, G.; Beurois, J.; Dacheux, D.; Cazin, C.; Bidart, M.; Kherraf, Z.-E.; Robinson, D.R.; Satre, V.; Le Gac, G.; Ka, C.; et al. Biallelic variants in MAATS1 encoding CFAP91, a calmodulin-associated and spoke-associated complex protein, cause severe astheno-teratozoospermia and male infertility. J. Med. Genet. 2020, 57, 708–716. [Google Scholar] [CrossRef]
- Shen, Q.; Martinez, G.; Liu, H.; Beurois, J.; Wu, H.; Amiri-Yekta, A.; Liang, D.; Kherraf, Z.-E.; Bidart, M.; Cazin, C.; et al. Bi-allelic truncating variants in CFAP206 cause male infertility in human and mouse. Hum. Genet. 2021, 140, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Tang, H.; Zheng, A.; Li, H.; Yang, S.; Xiang, J. Successful Results of Intracytoplasmic Sperm Injection of a Chinese Patient With Multiple Morphological Abnormalities of Sperm Flagella Caused by a Novel Splicing Mutation in CFAP251. Front. Genet. 2021, 12, 783790. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Unar, A.; Muhammad, Z.; Dil, S.; Zhang, B.; Sadaf, H.; Khan, M.; Ali, M.; Khan, R.; Shah, K.M.B.; et al. A novel NPHP4 homozygous missense variant identified in infertile brothers with multiple morphological abnormalities of the sperm flagella. J. Assist. Reprod. Genet. 2024, 41, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Nie, H.; Meng, L.; Wang, W.; Li, H.; Yuan, S.; Cheng, D.; He, W.; Liu, G.; Du, J.; et al. Novel mutations in SPEF2 causing different defects between flagella and cilia bridge: The phenotypic link between MMAF and PCD. Hum. Genet. 2020, 139, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Shamoto, N.; Narita, K.; Kubo, T.; Oda, T.; Takeda, S. CFAP70 Is a Novel Axoneme-Binding Protein That Localizes at the Base of the Outer Dynein Arm and Regulates Ciliary Motility. Cells 2018, 7, 124. [Google Scholar] [CrossRef]
- Ali, I.; Ali, H.; Unar, A.; Rahim, F.; Khan, K.; Dil, S.; Abbas, T.; Hussain, A.; Zeb, A.; Zubair, M.; et al. A novel homozygous missense TTC12 variant identified in an infertile Pakistani man with severe oligoasthenoteratozoospermia and primary ciliary dyskinesia. Mol. Genet. Genom. 2024, 299, 69. [Google Scholar] [CrossRef]
- Kamel, A.; Saberiyan, M.; Mirfakhraie, R.; Teimori, H. Reduced expression of CFAP44 and CFAP44-AS1 may affect sperm motility and morphology. Andrologia 2022, 54, e14447. [Google Scholar] [CrossRef]
- Lin, J.; Heuser, T.; Song, K.; Fu, X.; Nicastro, D. One of the nine doublet microtubules of eukaryotic flagella exhibits unique and partially conserved structures. PLoS ONE 2012, 7, e46494. [Google Scholar] [CrossRef]
- Urbanska, P.; Joachimiak, E.; Bazan, R.; Fu, G.; Poprzeczko, M.; Fabczak, H.; Nicastro, D.; Wloga, D. Ciliary proteins Fap43 and Fap44 interact with each other and are essential for proper cilia and flagella beating. Cell Mol. Life Sci. 2018, 75, 4479–4493. [Google Scholar] [CrossRef]
- Dacheux, D.; Martinez, G.; Broster Reix, C.E.; Beurois, J.; Lores, P.; Tounkara, M.; Dupuy, J.-W.; Robinson, D.R.; Loeuillet, C.; Lambert, E.; et al. Novel axonemal protein ZMYND12 interacts with TTC29 and DNAH1, and is required for male fertility and flagellum function. Elife 2023, 12, RP87698. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zheng, J.; Wang, J.; Duan, S.; Zhang, W.; Yan, X.; Zhu, X. Vertebrate Dynein-f depends on Wdr78 for axonemal localization and is essential for ciliary beat. J. Mol. Cell Biol. 2019, 11, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Li, Y.; Li, K.; Xu, C.; Gao, Y.; Lv, M.; Guo, R.; Xu, Y.; Zhou, P.; et al. DNALI1 deficiency causes male infertility with severe asthenozoospermia in humans and mice by disrupting the assembly of the flagellar inner dynein arms and fibrous sheath. Cell Death Dis. 2023, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Ghanaeian, A.; Majhi, S.; McCafferty, C.L.; Nami, B.; Black, C.S.; Yang, S.K.; Legal, T.; Papoulas, O.; Janowska, M.; Valente-Paterno, M.; et al. Integrated modeling of the Nexin-dynein regulatory complex reveals its regulatory mechanism. Nat. Commun. 2023, 14, 5741. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xu, C.; Li, J.; Qiu, B.; Luo, J.; Hong, Q.; Tong, Y.; Fang, C.; Feng, Y.; Ma, R.; et al. Multi-scale structures of the mammalian radial spoke and divergence of axonemal complexes in ependymal cilia. Nat. Commun. 2024, 15, 362. [Google Scholar] [CrossRef] [PubMed]
- Auguste, Y.; Delague, V.; Desvignes, J.-P.; Longepied, G.; Gnisci, A.; Besnier, P.; Levy, N.; Beroud, C.; Megarbane, A.; Metzler-Guillemain, C.; et al. Loss of Calmodulin- and Radial-Spoke-Associated Complex Protein CFAP251 Leads to Immotile Spermatozoa Lacking Mitochondria and Infertility in Men. Am. J. Hum. Genet. 2018, 103, 413–420. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Lesich, K.A. Functional anatomy of the mammalian sperm flagellum. Cytoskeleton 2016, 73, 652–669. [Google Scholar] [CrossRef]
- Zhu, Z.-J.; Wang, Y.-Z.; Wang, X.-B.; Yao, C.-C.; Zhao, L.-Y.; Zhang, Z.-B.; Wu, Y.; Chen, W.; Li, Z. Novel mutation in ODF2 causes multiple morphological abnormalities of the sperm flagella in an infertile male. Asian J. Androl. 2022, 24, 463–472. [Google Scholar] [CrossRef]
- He, X.; Liu, C.; Yang, X.; Lv, M.; Ni, X.; Li, Q.; Cheng, H.; Liu, W.; Tian, S.; Wu, H.; et al. Bi-allelic Loss-of-function Variants in CFAP58 Cause Flagellar Axoneme and Mitochondrial Sheath Defects and Asthenoteratozoospermia in Humans and Mice. Am. J. Hum. Genet. 2020, 107, 514–526. [Google Scholar] [CrossRef]
- Sha, Y.; Sha, Y.; Liu, W.; Zhu, X.; Weng, M.; Zhang, X.; Wang, Y.; Zhou, H. Biallelic mutations of CFAP58 are associated with multiple morphological abnormalities of the sperm flagella. Clin. Genet. 2021, 99, 443–448. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Li, Y.; Sun, J.; Yang, Y.; Shen, Y. Novel mutations in FSIP2 lead to multiple morphological abnormalities of the sperm flagella and poor ICSI prognosis. Gene 2021, 781, 145536. [Google Scholar] [CrossRef]
- Fang, X.; Gamallat, Y.; Chen, Z.; Mai, H.; Zhou, P.; Sun, C.; Li, X.; Li, H.; Zheng, S.; Liao, C.; et al. Hypomorphic and hypermorphic mouse models of Fsip2 indicate its dosage-dependent roles in sperm tail and acrosome formation. Development 2021, 148, dev199216. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Xi, Q.; Zhu, L.; Jia, W.; Liu, Z.; Wang, C.; Zhou, X.; Zhang, D.; Xing, C.; Peng, X.; et al. Novel Compound Heterozygous Mutation in FSIP2 Causes Multiple Morphological Abnormalities of the Sperm Flagella (MMAF) and Male Infertility. Reprod. Sci. 2022, 29, 2697–2702. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, W.-Q.; Chen, Z.-Y.; Chen, Y.; Zhang, L.; Zheng, L.; Luo, T.; Chen, H.-Y. Successful outcomes of intracytoplasmic sperm injection-embryo transfer using ejaculated spermatozoa from two Chinese asthenoteratozoospermic brothers with a compound heterozygous FSIP2 mutation. Andrologia 2022, 54, e14351. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, Y.; Tang, S.; Wang, J.; Zhou, Y.; Tian, S.; Wu, H.; Cong, J.; He, X.; Jin, L.; et al. Homozygous variants in AKAP3 induce asthenoteratozoospermia and male infertility. J. Med. Genet. 2023, 60, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, D.; Tu, C.; Meng, L.; Tan, Y.; Ji, Z.; Cheng, J.; Lu, G.; Lin, G.; Zhang, H.; et al. Loss-of-function missense variant of AKAP4 induced male infertility through reduced interaction with QRICH2 during sperm flagella development. Hum. Mol. Genet. 2021, 31, 219–231. [Google Scholar] [CrossRef]
- Lee, K.H. Ectopic Expression of Cenexin1 S796A Mutant in ODF2(+/-) Knockout Background Causes a Sperm Tail Development Defect. Dev. Reprod. 2012, 16, 363–370. [Google Scholar] [CrossRef]
- Martinez, G.; Kherraf, Z.-E.; Zouari, R.; Fourati Ben Mustapha, S.; Saut, A.; Pernet-Gallay, K.; Bertrand, A.; Bidart, M.; Hograindleur, J.P.; Amiri-Yekta, A.; et al. Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella. Hum. Reprod. 2018, 33, 1973–1984. [Google Scholar] [CrossRef]
- Liu, W.; Wu, H.; Wang, L.; Yang, X.; Liu, C.; He, X.; Li, W.; Wang, J.; Chen, Y.; Wang, H.; et al. Homozygous loss-of-function mutations in FSIP2 cause male infertility with asthenoteratospermia. J. Genet. Genom. 2019, 46, 53–56. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, Y.; Li, Y.; Guo, J.; Wen, Y.; Jiang, C.; Yang, Y.; Shen, Y. FSIP2 plays a role in the acrosome development during spermiogenesis. J. Med. Genet. 2023, 60, 254–264. [Google Scholar] [CrossRef]
- Lv, M.; Tang, D.; Yu, H.; Geng, H.; Zhou, Y.; Shao, Z.; Li, K.; Gao, Y.; Guo, S.; Xu, C.; et al. Novel FSIP2 Variants Induce Super-Length Mitochondrial Sheath and Asthenoteratozoospermia in Humans. Int. J. Biol. Sci. 2023, 19, 393–411. [Google Scholar] [CrossRef]
- Xu, K.; Qi, H. Sperm-specific AKAP3 is a dual-specificity anchoring protein that interacts with both protein kinase a regulatory subunits via conserved N-terminal amphipathic peptides. Mol. Reprod. Dev. 2014, 81, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, S.; Goueli, S.A.; Davey, M.P.; Carr, D.W. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J. Biol. Chem. 1997, 272, 4747–4752. [Google Scholar] [CrossRef] [PubMed]
- Poursafari Talemi, E.; Hosseini, S.-H.; Gourabi, H.; Sabbaghian, M.; Mohseni Meybodi, A. Evaluation of The 1499T>C Variant in The AKAP3 Gene of Infertile Men with Multiple Morphological Abnormalities of The Sperm Flagella Phenotype: A Case-Control Study. Int. J. Fertil. Steril. 2024, 18, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M.; Musse, M.P.; Mandal, A.; Klotz, K.; Jayes, F.C.; Herr, J.C.; Gerton, G.L.; Moss, S.B.; Chemes, H.E. Molecular genetic analysis of two human sperm fibrous sheath proteins, AKAP4 and AKAP3, in men with dysplasia of the fibrous sheath. J. Androl. 2001, 22, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Limeta, A.; Loncarek, J. Human centrosome organization and function in interphase and mitosis. Semin. Cell Dev. Biol. 2021, 117, 30–41. [Google Scholar] [CrossRef]
- Scovell, J.M.; Bournat, J.C.; Szafran, A.T.; Solis, M.; Moore, J.; Rivera, A.; Chen, C.H.; Zhang, J.; Wilken, N.; Seth, A.; et al. PRSS50 is a testis protease responsible for proper sperm tail formation and function. Development 2021, 148, dev197558. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, Y.; Sheng, X.; Zhang, X.; Chen, Y.; Zhu, H.; Guo, Y.; Qi, Y.; Zhao, Y.; Zhou, Q.; et al. Absence of CEP78 causes photoreceptor and sperm flagella impairments in mice and a human individual. Elife 2023, 12, e76157. [Google Scholar] [CrossRef]
- Brunk, K.; Zhu, M.; Bärenz, F.; Kratz, A.-S.; Haselmann-Weiss, U.; Antony, C.; Hoffmann, I. Cep78 is a new centriolar protein involved in Plk4-induced centriole overduplication. J. Cell Sci. 2016, 129, 2713–2718. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, R.; Liang, C.; Liu, H.; Zhang, X.; Ma, Y.; Liu, M.; Zhang, W.; Yang, Y.; Liu, M.; et al. Loss-of-function mutations in CEP78 cause male infertility in humans and mice. Sci. Adv. 2022, 8, eabn0968. [Google Scholar] [CrossRef]
- Hao, L.; Scholey, J.M. Intraflagellar transport at a glance. J. Cell Sci. 2009, 122, 889–892. [Google Scholar] [CrossRef]
- Lehti, M.S.; Sironen, A. Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 2016, 151, R43–R54. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Liang, Y.; Liu, M.; Yang, Y.; Liu, H.; Shen, Y. Novel biallelic mutations in TTC29 cause asthenoteratospermia and male infertility. Mol. Genet. Genom. Med. 2022, 10, e2078. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Wang, J.; Lv, M.; Liu, C.; Zhong, Y.; Tian, S.; Wu, H.; Cheng, H.; Gao, Y.; Tan, Q.; et al. A novel homozygous mutation in WDR19 induces disorganization of microtubules in sperm flagella and nonsyndromic asthenoteratospermia. J. Assist. Reprod. Genet. 2020, 37, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Lorès, P.; Kherraf, Z.-E.; Amiri-Yekta, A.; Whitfield, M.; Daneshipour, A.; Stouvenel, L.; Cazin, C.; Cavarocchi, E.; Coutton, C.; Llabador, M.-A.; et al. A missense mutation in IFT74, encoding for an essential component for intraflagellar transport of Tubulin, causes asthenozoospermia and male infertility without clinical signs of Bardet-Biedl syndrome. Hum. Genet. 2021, 140, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, B.; Liu, C.; Zhang, Z.; Wang, X.; Wang, L.; Xiao, S.; Chen, Y.; Wei, H.; Jiang, H.; et al. CCDC38 is required for sperm flagellum biogenesis and male fertility in mice. Development 2022, 149, dev200516. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, B.; Chen, Y.; Ma, S.; Wang, L.; Han, T.; Lin, X.; Yang, F.; Liu, C.; Zhao, J.; et al. CCDC146 is required for sperm flagellum biogenesis and male fertility in mice. Cell Mol. Life Sci. 2023, 81, 1. [Google Scholar] [CrossRef]
- Khan, I.; Dil, S.; Zhang, H.; Zhang, B.; Khan, T.; Zeb, A.; Zhou, J.; Nawaz, S.; Zubair, M.; Khan, K.; et al. A novel stop-gain mutation in ARMC2 is associated with multiple morphological abnormalities of the sperm flagella. Reprod. Biomed. Online 2021, 43, 913–919. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, C.; Xu, Y.; Wang, W.; Li, H.; Yang, S.; Zhao, J. Patient with multiple morphological abnormalities of sperm flagella caused by a novel ARMC2 mutation has a favorable pregnancy outcome from intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2022, 39, 1673–1681. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Q.; Su, L.; Meng, L.; Tan, C.; Wei, C.; Zhang, H.; Luo, T.; Zhang, Q.; Tan, Y.-Q.; et al. Identification of novel homozygous asthenoteratospermia-causing ARMC2 mutations associated with multiple morphological abnormalities of the sperm flagella. J. Assist. Reprod. Genet. 2024, 41, 1297–1306. [Google Scholar] [CrossRef]
- Chung, M.-I.; Kwon, T.; Tu, F.; Brooks, E.R.; Gupta, R.; Meyer, M.; Baker, J.C.; Marcotte, E.M.; Wallingford, J.B. Coordinated genomic control of ciliogenesis and cell movement by RFX2. Elife 2014, 3, e01439. [Google Scholar] [CrossRef]
- Lorès, P.; Dacheux, D.; Kherraf, Z.-E.; Nsota Mbango, J.-F.; Coutton, C.; Stouvenel, L.; Ialy-Radio, C.; Amiri-Yekta, A.; Whitfield, M.; Schmitt, A.; et al. Mutations in TTC29, Encoding an Evolutionarily Conserved Axonemal Protein, Result in Asthenozoospermia and Male Infertility. Am. J. Hum. Genet. 2019, 105, 1148–1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, X.; Liu, W.; Yang, S.; Wang, L.; Li, W.; Wu, H.; Tang, S.; Ni, X.; Wang, J.; et al. Bi-allelic Mutations in TTC29 Cause Male Subfertility with Asthenoteratospermia in Humans and Mice. Am. J. Hum. Genet. 2019, 105, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-R.; Li, Y.-C.; Luo, M.-L.; Guo, H.; Wang, T.-T.; Chen, J.-B.; Ma, Q.; Gu, Y.-L.; Jiang, Z.-M.; Gui, Y.-T. Identification and characteristics of the testes-specific gene, Ccdc38, in mice. Mol. Med. Rep. 2016, 14, 1290–1296. [Google Scholar] [CrossRef]
- Coutton, C.; Martinez, G.; Kherraf, Z.-E.; Amiri-Yekta, A.; Boguenet, M.; Saut, A.; He, X.; Zhang, F.; Cristou-Kent, M.; Escoffier, J.; et al. Bi-allelic Mutations in ARMC2 Lead to Severe Astheno-Teratozoospermia Due to Sperm Flagellum Malformations in Humans and Mice. Am. J. Hum. Genet. 2019, 104, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tu, C.; Wang, L.; Wu, H.; Houston, B.J.; Mastrorosa, F.K.; Zhang, W.; Shen, Y.; Wang, J.; Tian, S.; et al. Deleterious variants in X-linked CFAP47 induce asthenoteratozoospermia and primary male infertility. Am. J. Hum. Genet. 2021, 108, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dai, S.; Zhang, J.; Yang, Y.; Shen, Y.; Liu, H.; Yang, Y.; Jiang, C.; Tian, E. A novel mutation in CFAP47 causes male infertility due to multiple morphological abnormalities of the sperm flagella. Front. Endocrinol. 2023, 14, 1155639. [Google Scholar] [CrossRef]
- Liao, H.-Q.; Guo, Z.-Y.; Huang, L.-H.; Liu, G.; Lu, J.-F.; Zhang, Y.-F.; Xing, X.-W. WDR87 interacts with CFAP47 protein in the middle piece of spermatozoa flagella to participate in sperm tail assembly. Mol. Hum. Reprod. 2022, 29, gaac042. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.; Khan, A.; Zhao, D.; Ma, A.; Zhou, J.; Khan, I.; Khan, K.; Zhang, H.; Zhang, Y.; et al. Novel frameshift mutation in STK33 is associated with asthenozoospermia and multiple morphological abnormalities of the flagella. Hum. Mol. Genet. 2021, 30, 1977–1984. [Google Scholar] [CrossRef]
- Sha, Y.; Wei, X.; Ding, L.; Ji, Z.; Mei, L.; Huang, X.; Su, Z.; Wang, W.; Zhang, X.; Lin, S. Biallelic mutations of CFAP74 may cause human primary ciliary dyskinesia and MMAF phenotype. J. Hum. Genet. 2020, 65, 961–969. [Google Scholar] [CrossRef]
- Guo, T.; Tu, C.-F.; Yang, D.-H.; Ding, S.-Z.; Lei, C.; Wang, R.-C.; Liu, L.; Kang, X.; Shen, X.-Q.; Yang, Y.-F.; et al. Bi-allelic BRWD1 variants cause male infertility with asthenoteratozoospermia and likely primary ciliary dyskinesia. Hum. Genet. 2021, 140, 761–773. [Google Scholar] [CrossRef]
- Clement, T.M.; Geyer, C.B.; Willis, W.D.; Goulding, E.H.; Upadhyay, S.; Eddy, E.M. Actin-related protein ACTL7B ablation leads to OAT with multiple morphological abnormalities of the flagellum and male infertility in mice. Biol. Reprod. 2023, 108, 447–464. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, W.; Wu, H.; Lv, M.; Liu, W.; Liu, C.; Zhu, F.; Li, C.; Fang, Y.; Yang, C.; et al. Novel homozygous CFAP69 mutations in humans and mice cause severe asthenoteratospermia with multiple morphological abnormalities of the sperm flagella. J. Med. Genet. 2019, 56, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kissel, H.; Georgescu, M.-M.; Larisch, S.; Manova, K.; Hunnicutt, G.R.; Steller, H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell 2005, 8, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Yekta, A.; Coutton, C.; Kherraf, Z.-E.; Karaouzène, T.; Le Tanno, P.; Sanati, M.H.; Sabbaghian, M.; Almadani, N.; Sadighi Gilani, M.A.; Hosseini, S.H.; et al. Whole-exome sequencing of familial cases of multiple morphological abnormalities of the sperm flagella (MMAF) reveals new DNAH1 mutations. Hum. Reprod. 2016, 31, 2872–2880. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Huang, X.; Wu, B.; Dai, P.; Zhang, F.; Li, J.; Wang, L. Gene-knockout by iSTOP enables rapid reproductive disease modeling and phenotyping in germ cells of the founder generation. Sci. China Life Sci. 2024, 67, 1035–1050. [Google Scholar] [CrossRef]
- Ferreux, L.; Bourdon, M.; Chargui, A.; Schmitt, A.; Stouvenel, L.; Lorès, P.; Ray, P.; Lousqui, J.; Pocate-Cheriet, K.; Santulli, P.; et al. Genetic diagnosis, sperm phenotype and ICSI outcome in case of severe asthenozoospermia with multiple morphological abnormalities of the flagellum. Hum. Reprod. 2021, 36, 2848–2860. [Google Scholar] [CrossRef]
- Wambergue, C.; Zouari, R.; Fourati Ben Mustapha, S.; Martinez, G.; Devillard, F.; Hennebicq, S.; Satre, V.; Brouillet, S.; Halouani, L.; Marrakchi, O.; et al. Patients with multiple morphological abnormalities of the sperm flagella due to DNAH1 mutations have a good prognosis following intracytoplasmic sperm injection. Hum. Reprod. 2016, 31, 1164–1172. [Google Scholar] [CrossRef]
- Sha, Y.-W.; Wang, X.; Su, Z.-Y.; Mei, L.-B.; Ji, Z.-Y.; Bao, H.; Li, P. Patients with multiple morphological abnormalities of the sperm flagella harbouring CFAP44 or CFAP43 mutations have a good pregnancy outcome following intracytoplasmic sperm injection. Andrologia 2019, 51, e13151. [Google Scholar] [CrossRef]
- Wang, W.; Tu, C.; Nie, H.; Meng, L.; Li, Y.; Yuan, S.; Zhang, Q.; Du, J.; Wang, J.; Gong, F.; et al. Biallelic mutations in CFAP65 lead to severe asthenoteratospermia due to acrosome hypoplasia and flagellum malformations. J. Med. Genet. 2019, 56, 750–757. [Google Scholar] [CrossRef]
- Braham, A.; Ghedir, H.; Ben Khedher, M.B.; Ajina, M.; Saad, A.; Ibala-Romdhane, S. Nuclear sperm integrity and ICSI prognosis in Tunisian patients with MMAF syndrome (multiple morphological abnormalities of the sperm flagella). Hum. Fertil. 2023, 26, 1429–1438. [Google Scholar] [CrossRef]
- Porter, L.F.; Black, G.C.M. Personalized ophthalmology. Clin. Genet. 2014, 86, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Britten-Jones, A.C.; Jin, R.; Gocuk, S.A.; Cichello, E.; O’Hare, F.; Hickey, D.G.; Edwards, T.L.; Ayton, L.N. The safety and efficacy of gene therapy treatment for monogenic retinal and optic nerve diseases: A systematic review. Genet. Med. 2022, 24, 521–534. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Location | Gene Variation | Human/KO Mice Phenotype | Specific Phenotype | Mechanism | Reference |
|---|---|---|---|---|---|---|
| CFAP70 | Base of ODA | c.1723-1G>T | H 1 | Typical MMAF phenotypes 2; the absence of the central pair and ODAs | Disorganization of ODAs and the central pair | [27] |
| c.178T>A | ||||||
| c.2962C>T | H, M 3 | Typical MMAF phenotypes; severe deformation of the axoneme; low ratio of axoneme assembly | Blocking the interaction among CFAP70, QRICH2 and TTLL5; the failure of cytoplasmic preassembly of CFAP61 and CFAP91 | [28] | ||
| - | M | Typical MMAF phenotypes; disorganized microtubules and ODFs | Hindering the assembly of ODAs via DNAI1 and DNAI2; blocking the IFT via AKAP3 | [29] | ||
| DNAH8 | Dynein γ-heavy chain of ODA | c.6158_6159insT | H | Typical MMAF phenotypes; disorganized or missing ODAs and microtubules | Disruption of the interaction between DNAH8 and DNAH17 | [30] |
| c.11771C>T plus c.6689A>G | H, M | Typical MMAF phenotypes; the lack of microtubules; misarranged or supernumerary ODFs; disassembled or absent ODAs; disorganized microtubules and ODFs in KO mice | Disruption of the interaction between DNAH8 and DNAH17 | [31] | ||
| c.9427C>T plus c.12721G>A | ||||||
| c.6962_6968del | ||||||
| c.378_379del | H | Typical MMAF phenotypes; missing or irregular arrangement of ODFs; the absence of the central pair; disorganized outer doublet microtubules | The absence of ODAs due to the disruption of the interaction between DNAH8 and DNAH17 | [32] | ||
| DNAH17 | Dynein β-heavy chain of ODA | c.1293_1294del | H | Typical MMAF phenotypes; abnormal MS; cytoplasmic bags containing unassembled flagellar components; the absence of the central pair and outer microtubule doublets | Damaging the ODA structure | [25] |
| c.7994_8012del | ||||||
| c.5486G>A | ||||||
| c.10496C>T | ||||||
| c.10784T>C | ||||||
| c.10486_10497dup | ||||||
| c.4445C>T | H | Typical MMAF phenotypes; the absence of ODAs surrounded by a disorganized MS; defective FS | Damaging the ODA structure via the interaction with DNAI2 | [33] | ||
| c.6857C>T | ||||||
| c.5368C>T | H | Typical MMAF phenotypes; the absence of ODAs | Damaging the ODA structure | [34] | ||
| c.13183C>T | ||||||
| c.6308C>T | H, M | Typical MMAF phenotypes; disorganizations of the FS and ODFs; KO mice showed disorganized flagellar cross-sections, disrupted MS and FS, and missing ODAs and microtubules | - | [6] | ||
| c.11803C>T | ||||||
| c.5707C>T | ||||||
| c.12915+1C>T | H | Typical MMAF phenotypes; the absence of the central pair and outer doublet microtubules; disarranged ODFs | Impacting outer doublet microtubules 4–7 in sperm flagellar assembly | [35] | ||
| c.13202G>A | ||||||
| c.8512-2A>G | ||||||
| c.13294C>T | ||||||
| c.12865_12867delTCC and c.13105C>T | H | Typical MMAF phenotypes | - | [36] | ||
| c.612C>G and c.4237-6C>A | ||||||
| c.T2150C and c.G7136C | ||||||
| c.4810C>T | H | Typical MMAF phenotypes; thickened MS; disorganized ODFs; the absence of microtubules | Disruption of the interaction between DNAH8 and DNAH17 | [37] | ||
| CFAP43 and CFAP44 | Next to the outer doublet microtubules 5–6 bridge and connected to the IDA | c.2802T>A and c.4132C>T (CFAP43) | H, M | Typical MMAF phenotypes; hypertrophy and hyperplasia of FS; the absence of central microtubules; distorted cytoskeletal components; KO mice showed a lack of the central pair and they had disordered outer doublet microtubules and ODFs | Impacting the intra-flagellar transport | [38] |
| c.253C>T and c.3945_4431del (CFAP43) | ||||||
| c.386C>A and c.2802T>A (CFAP43) | ||||||
| c.2005_2006delAT (CFAP44) | ||||||
| c.3541−2A>C (CFAP43) | H, M | Typical MMAF phenotypes; defects in the ODFs, the FS, and the MS; partial or complete loss of the central doublet microtubules; disordered outer doublet microtubules; the worse motility in the Cfap43−/− mice; the absence of the head of RS interacting with the central pair; substantial structural disorganization with uneven distribution of the nine outer doublet microtubules and the absence of the central pair complex in the Cfap43−/− mice; defects in MS and FS in the Cfap44−/− mice; the mislocalization of the central pair and the loss of ODF 3 and 8 in the Cfap44−/− mice | Impairing the structure of the outer doublet microtubules 5–6; impacting the interaction between CFAP43 and CFAP44 | [39] | ||
| c.1240_1241delGT (CFAP43) | ||||||
| c.2658G>A (CFAP43) | ||||||
| c.2680C>T (CFAP43) | ||||||
| c.3882delA (CFAP43) | ||||||
| c.3352C>T (CFAP43) | ||||||
| c.1302dupT (CFAP43) | ||||||
| c.1040T>C (CFAP43) | ||||||
| c.2141+5G>A (CFAP43) | ||||||
| c.1890+1G>A (CFAP44) | ||||||
| c.3175C>T (CFAP44) | ||||||
| c.2818dupG (CFAP44) | ||||||
| c.1387G>T (CFAP44) | ||||||
| c.4767delT (CFAP44) | ||||||
| c.1140_1143del (CFAP43) | H | Typical MMAF phenotypes | - | [40] | ||
| c.739A>T (CFAP43) | ||||||
| c.1474G>C (CFAP43) | ||||||
| c.4600C>G (CFAP43) | ||||||
| c.4963C>T (CFAP44) | ||||||
| c.2935_2944del (CFAP44) | H | Typical MMAF phenotypes | - | [41] | ||
| c.T1769A (CFAP44) | ||||||
| c.G3262A and c.C1718A (CFAP44) | ||||||
| c.3661-2A>-(delA) (CFAP43) | ||||||
| c.585_735del (CFAP43) | H | Typical MMAF phenotypes; the loss of the central pair or severe disorganization of the FS, ODFs, and axonemal disassembly | - | [42] | ||
| c.944del (CFAP43) | ||||||
| c.4579dup (CFAP43) | ||||||
| c.3768+1G>A (CFAP43) | ||||||
| c.386C>A (CFAP43) | ||||||
| c.1418C>T (CFAP43) | ||||||
| c.2546T>C (CFAP43) | ||||||
| - | M | Typical MMAF phenotypes; the manchette did not elongate and disengaged from the head to the tail; the direction of abnormal manchette microtubules was perpendicular to the assembly direction of the flagellum | Impacting the intra-manchette transport (IMT) associated with CFAP43 | [43] | ||
| DNAH1 | Dynein heavy chain of IDA | c.6912C>A, c.7076G>T et al. | H | A high percentage of short flagella and a low percentage of bent flagella | The absence of DNAH1 removes the anchoring site of the RS3 | [44] |
| c.11726_11727del, c.4552C>T et al. | H | Typical MMAF phenotypes; the loss of sperm motility | - | [45] | ||
| c.1336G>C and c.2912G>A | H | Typical MMAF phenotypes; the absence of the IDA and RS; the displaced dense fibers and microtubules | Impacting the expression of DNALI1 | [46] | ||
| c.1832T>C, c.2301-1G>T et al. | H | Typical MMAF phenotypes; the absence of the central pair; the absence of IDAs; the displacement of ODFs and/or the outer doublet microtubules; some unorganized axoneme clusters | Blocking the interaction between the C-terminus of DNAH1 in IDA3 and DNALI1; the absence of the RS3 anchoring site, resulting in the loss of the central pair | [47] | ||
| c.8170C>T and c.4670C>T | H | Typical MMAF phenotypes; missing central pair and disordered outer doublet microtubule arrangements | - | [48] | ||
| c.7435C>T | H | Typical MMAF phenotypes; the malformation or absence of the center pair; disorganized MS and FS | - | [49] | ||
| c.10757T>C | ||||||
| c.11726_11727delCT | ||||||
| c.12154delC | ||||||
| c.10627-3C>G | ||||||
| DNAH2 | Dynein heavy chain of IDA | c.9298C>T | H | Typical MMAF phenotypes; missing central pair and disordered outer doublet microtubule arrangements; missing or fragmented ODFs; partitional or hypertrophic MS | Decreasing the expression of DNALI1 | [4] |
| c.5770C>T | ||||||
| c.11500C>T | ||||||
| c.6960C>A | ||||||
| c.11503T>C | ||||||
| c.A2116C | H | Typical MMAF phenotypes; absence of the central pair; disrupted IDA | Impairing the ATPase activity of DNAH2; decreasing DNAH1 | [50] | ||
| c.C11635T | ||||||
| c.A5507G | ||||||
| c.G9291T | ||||||
| c.G4774A | ||||||
| c.G5771C | ||||||
| c.12720G>T | H, M | Typical MMAF phenotypes; KO mice showed abnormally arranged MS, misaligned ODFs and microtubule doublets, disorganized RS and IDA | Impacting the interaction between DNAH2 and DNAH1; blocking DNAH2′s role in annulus migration; impacting the IFT | [51] | ||
| DNAH6 | Dynein heavy chain of IDA | c.6582C>A | H | Typical MMAF phenotypes; the absence of the central pair; disorganization of the peripheral doublet microtubules and the ODFs; disorganized MS and FS | Reducing the ATPase activity and microtubule-binding capacity of DNAH6; destroying the flagellar axoneme assembly | [52] |
| c.11258G>A | ||||||
| c.5264C>T and c.8726A>G | H | Typical MMAF phenotypes; the absence of the central pair; affected RS | Blocking the interaction among DNAH6, DNAH1 (IDA component), SPAG6 (central pair component), RSPH1 (RS component), AKAP4 (FS component), and ACTL7A (acrosome-associated protein) | [5] | ||
| c.8852-1G>A and c.10127T>A | ||||||
| c.9250C>G | ||||||
| DNAH7 | Dynein heavy chain of IDA | c.2478dupA | H | Typical MMAF phenotypes; severe defects in the MS; severe IDA loss | Blocking the interaction among DNAH7, TBC1D21, and TOMM20 | [53] |
| DNAH10 | Dynein heavy chain of IDA | c.12838G>A | H, M | Typical MMAF phenotypes; the absence of IDAs; disorganization of axonemal or peri-axonemal structures; disorganized MS, ODFs, and microtubules in KO mice | Impacting the IMT and IFT | [54] |
| c.7601C>T | ||||||
| c.5663G>A | ||||||
| c.11887C>T | ||||||
| c.7260dup | ||||||
| c.12235del | ||||||
| c.2514delG | H | Typical MMAF phenotypes; IDA deficiency | Impacting the interaction among DNAH10, DNAH1 (IDA component), DNAI1 (ODA component), SPAG6 (central pair component), and AKAP4 (FS component) | [55] | ||
| c.10820T>C and c.12692C>T | ||||||
| DNHD1 | Dynein heavy-chain component | c.8782C>T | H | Typical MMAF phenotypes; the absence of the central pair complex; abnormal ODF and MS | - | [56] |
| c.5989G>A | ||||||
| c.2581C>T and c.6031C>T | ||||||
| WDR63 (DNAI3) | Dynein intermediate chain of IDA | c.163C>T | H, M | Typical MMAF phenotypes; disorganized “9 + 2” axoneme combined with aberrant IDAs, ODAs, ODFs, FSs, and N-DRCs in KO mice | Impacting the IDA assembly by blocking the interaction among WDR63, WDR78, DNAH2, and DNAH10 | [57] |
| c.1075C>T | ||||||
| CCDC39 and CCDC40 | Base plate component | c.983T>C (CCDC39) | H | Typical MMAF phenotypes; the absence of the central pair; the disorganized outer doublet microtubules | - | [58] |
| c.901C>T (CCDC40) | H | Immotility; typical MMAF phenotypes | - | [59] | ||
| c.2065_2068dup (CCDC40) | ||||||
| c.1072del and c.1007-1010del (CCDC39) | H | Typical MMAF phenotypes; unassembled axonemal and peri-axonemal components; severe tubular disorganization of the flagellar axoneme | The absence of CCDC39 | [60] | ||
| c.1675G>T (CCDC40) | ||||||
| c.248del and c.736_755dup (CCDC40) | ||||||
| CCDC65 | A component interconnecting microtubule doublets | c.1208del | H | Typical MMAF phenotypes; disorganization with abnormal doublet positioning; severe midpiece defects | Impairing the interaction among CCDC65, DNAI1 (located at the ODA), DNALI1 (located at the IDA), and SPAG6 (located at the central pair complex) | [61] |
| c.1126C>T | ||||||
| DRC1 | Core structural component of the N-DRC | c.1296G>A | H | Typical MMAF phenotypes | - | [62] |
| c.C1660T | H, M | Typical MMAF phenotypes; severe axonemal disorganization and unassembled microtubule doublets; KO mice showed the absence of sperm motility, separated ODFs, and the absence of N-DRCs, RSs, and dynein arms | Decreasing flagellum axoneme stability | [63] | ||
| c.C238T | ||||||
| DRC4 (GAS8) | Core structural component of the N-DRC | c.1011+2T>C | H | Typical MMAF phenotypes; immotile sperm; defects in MS and FS; the absence of some peripheral microtubule doublets | Destroying the N-DRC structure; impairing the interaction among DRC4, DRC1, and CCDC65 | [64] |
| AK7 | Associated with the protein kinase A located at the RS | c.1846G>A | H | Typical MMAF phenotypes; low motility of the sperm | - | [65] |
| c.1153A>T | H | Typical MMAF phenotypes; multiple axonemes in uniflagellate spermatozoa; mitochondrial vacuolization; the absence of a central pair | Impacts on the interaction between AK7 and DNAH protein family; destroying the regulatory function of AK7 on mitochondria | [66] | ||
| CCDC189 | Attached to the RS | - | M | Impaired MS and coiled FS; structural abnormalities of microtubules | Blocking the interaction between CCDC189 and the RS via RSPH1 and CABCOCO1; impacts on the IFT via blocking the interaction with IFT20 and IFT88 | [11] |
| CFAP61 | Main component of the CSC | c.143+5G>A | H, M | Impaired motility of sperm along with typical MMAF phenotypes; KO mice showed separated microtubules and ODFs and lost certain RS complex components | Impacting the role of CFAP61 in the late stages of RS assembly | [67] |
| c.451_452del | H | Typical MMAF phenotypes; disorganized axoneme structure along with the disappearance of microtubules, dynein arms, RS | Affecting the normal assembly of the central pair, RS, and IDA | [68] | ||
| c.847C>T | ||||||
| c.1654C>T and c.2911G>A | H | Typical MMAF phenotypes; absence of central pair microtubules and MS malformation | - | [69] | ||
| c.144-2A>G and c.1666G>A | ||||||
| c.1245+6T>C | H | Severe axoneme disorganization such as the absence of central or outer doublet microtubules, missing RS, and misshapen MS | Severe defects to CSC | [70] | ||
| CFAP65 (CCDC108) | Unknown | - | M | Disorganized axoneme and peri-axoneme structures, such as absence or disorganization of outer doublet microtubules and ODFs; the absence of the central pair complex; the unstable formation of MS | Interfering with the interaction between CFAP65 and the RS component RSPH1 | [71] |
| CFAP91 (MAATS1) | A component of the CSC | c.682+1G>A | H | Typical MMAF phenotypes; the absence of the central pair complex; an abnormal number of ODFs | Destabilizing the RS and CSC structure; impacting the interaction among CFAP91, CFAP251, and radial spoke protein 3 (RSP3) | [72] |
| c.124G>C | ||||||
| CFAP206 | Microtubule-docking adapter for the RS and IDA | c.1430dupA | H, M | Typical MMAF phenotypes; disorganized RS and CSC; distorted axoneme with a lack of peripheral doublets, the absence of the central pair, or abnormal distribution of ODFs in KO mice | Blocking the formation of RS and CSC | [73] |
| CFAP251 (WDR66) | A component of the CSC | c.1192-3C>G | H | Higher rates of absent and coiled flagella; disorganization in axonemal and other peri-axonemal structures | - | [74] |
| NPHP4 | A structural protein in the cilia | c.1490C>G | H | Typical MMAF phenotypes; “9 + 0” arrangements of microtubules with an absent central pair | Impairing of the interaction between NPHP4 and the central pair | [75] |
| SPEF2 | A component of the central-pair-associated apparatus C1b | c.2507+5delG | H, M | Typical MMAF phenotypes; disorganized and scattered MS, ODFs, and FS; defects in both the central pairs and RS; unassembled axonemal components such as microtubule-like structure, fibrous elements, and mitochondria in KO mice | - | [76] |
| c.C4096T | ||||||
| c.2649dupA | ||||||
| c.3400delA | ||||||
| c.3922dupA |
| Gene Name | Location | Gene Variation | Human/KO Mice Phenotype | Specific Phenotype | Mechanism | Reference |
|---|---|---|---|---|---|---|
| ODF2 | A component of ODFs | c.202A>G | H 1 | Tail deformity; high proportion of outer dense fiber deficiencies | Impairing the ODF assembly | [89] |
| CFAP58 | The entire flagella and predominantly concentrate in the midpiece | c.2092C>T | H, M 2 | Typical MMAF phenotypes 3; short and coiled flagella in KO mice | Causing defective flagellogenesis | [90] |
| c.1429del and c.2092C>T | ||||||
| c.2052del | ||||||
| c.1696C>T | ||||||
| c.2274C>A | ||||||
| c.323C>T and c.1855C>T | H | Typical MMAF phenotypes | [91] | |||
| c.1883A>G and c.2020G>T | ||||||
| FSIP2 | A component of FS | c.16246_16247ins CCCAAATATCACC and c.17323C>T | H | Typical MMAF phenotypes | Disrupting sperm flagellar development | [92] |
| c.8368_8369insC | H, M | Typical MMAF phenotypes; dysplastic and disorganized MS; absent FS; MMAF phenotypes, absent FS, and exposed axonemes in knock-in mice | [93] | |||
| c.1494C > A and c.11020_11024del | H | Typical MMAF phenotypes; abnormal mitochondrial arrangement and disorganization; dysplastic FS | [94] | |||
| c.1750T>A and c.13600A>G | H | Typical MMAF phenotypes; abnormal heads | [95] | |||
| AKAP3 | A structural protein of FS | c.2286_2287del | H | Typical MMAF phenotypes; abnormal acrosomal morphology | Affecting FS assembly | [96] |
| c.44G>A | ||||||
| AKAP4 | A structural protein of FS | c.1285C>T | H | Typical MMAF phenotypes; missing or inhomogeneous FS | Affecting the interaction with QRICH2 and its normal expression | [97] |
| Gene Name | Location | Gene Variation | Human/KO Mice Phenotype | Specific Phenotype | Mechanism | Reference |
|---|---|---|---|---|---|---|
| CEP135 | Centriole | c.A1364T | H 1 | Typical MMAF phenotypes 2 | Massive formation of filamentous aggregates impairing centriole biogenesis | [8] |
| DZIP1 | Centrioles and pericentriolar matrixes | c.188G>A | H, M 3 | More than 90% have no flagellate; absent or very short axoneme; absent flagella, cytoplasm residual, and abnormal heads in KO mice | Centrosome damage caused by the absence of DZIP1 | [7] |
| c.690T>G | ||||||
| PRSS50 | Murine sperm midpiece | - | M | - | PRSS50-NF-κB-LRWD1 pathway affecting the level of LRWD1 and AKAP4 | [108] |
| CEP78 | Distal region of mature centrioles | c.1629-2A>G | H, M | MMAF phenotype; head abnormalities; multiple abnormalities of sperm head and flagella in KO mice; defective microtubule arrangements and elongated centrioles in KO mice | Affecting the interaction and stability with IFT20 and TTC21A, resulting in an influence on the regulation of centrosomes and ciliary length | [109] |
| Gene Name | Location | Gene Variation | Human/KO Mice Phenotype | Specific Phenotype | Mechanism | Reference |
|---|---|---|---|---|---|---|
| TTC21A (IFT139A) | An IFT complex component | c.3450+2delT | H 1 | Typical MMAF phenotypes 2; absent or misplaced central-pair microtubules and peripheral doublet microtubules | - | [9] |
| TTC29 | A potential member of the IFT-B complex | c.254+1G>A and c.1185C>G | H | Typical MMAF phenotype; abnormally shaped heads; defective sperm acrosomes | Affecting the development of flagella | [114] |
| WDR19 | A core component in IFT-A | c.3811A>G | H | Typical MMAF phenotype | Lead to the abnormal expression of other IFT partials | [115] |
| IFT74 | A core component in IFT | c.256G>A | H | Typical MMAF phenotype; abnormal mitochondrial sheath; cytoplasmic bags containing unassembled flagellar components; missing some peripheral doublets | Affecting IFT74 mRNA splicing and inducing mutant proteins with abnormal subcellular localization along the flagellum | [116] |
| CCDC38 | Manchette and sperm tail | - | M 3 | MMAF phenotype in KO mice | Affecting ODF2 transportation | [117] |
| CCDC146 | Sperm connecting piece and sperm tail | - | M | MMAF phenotype; abnormal sperm heads in KO mice | Affecting interaction with CCDC38 and CCDC42 in the IFT pathway | [118] |
| ARMC2 | Axonemal central pair complex | c.182C>G | H | Typical MMAF phenotype; complete absence of central pair complex; axonemal ultrastructure disorganization | - | [119] |
| c.1264C>T | H | Typical MMAF phenotype; missing central microtubule pairs | Affecting flagellar structures | [120] | ||
| c.314C>T | H | Typical MMAF phenotype; abnormal expression and localization of TOMM20 and AKAP4 | Affecting CPC assembly/stability and flagellar assembly | [121] | ||
| c.2227A>G |
| Gene Name | Location | Gene Variation | Human/KO Mice Phenotype | Specific Phenotype | Mechanism | Reference |
|---|---|---|---|---|---|---|
| CFAP47 | - | c.7154T>A | H 1, M 2 | Typical MMAF phenotype 3; bent flagella in KO mice | - | [127] |
| c.5224A>G | ||||||
| c.8668C>A | ||||||
| - | c.1414G>A | H | Typical MMAF phenotype; numerous malformed sperm heads, defective sperm annulus, and aplasia sperm mitochondrial sheaths | Influencing the expression of CFAP65, CFAP69, and SEPTIN4 to regulate spermatogenesis | [128] | |
| - | c.706G>A and c.1337C>T | H | Typical MMAF phenotype | Forming a complex with WDR87 and participating in the assembly of the midpiece of flagella via IMT-IFT transport | [129] | |
| STK33 | - | c.1235del | H | Typical MMAF phenotype | - | [130] |
| CFAP74 | - | c.983G>A and c.3532G>A | H | Lost or short tail defects and an incomplete mitochondrial sheath | Affect the ultrastructure of the sperm axoneme and MSs | [131] |
| c.652C>T and c.4331G>C | ||||||
| BRWD1 | - | c.C523T | H | Typical MMAF phenotype; missing ODAs and IDAs | - | [132] |
| c.A5573T | ||||||
| c.G166A and c.T1016C | ||||||
| ACTL7B | Developing acrosome; within the nucleus of early spermatids; flagellum connecting region | - | M | Conspicuous absence of the flagella connecting pieces; severe disruptions in flagellar midpiece structure in KO mice | Affecting the structural integrity of the sperm flagellar junction, axial filament, and mitochondrial sheath | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yu, S.; Zhang, W. The Molecular Basis of Multiple Morphological Abnormalities of Sperm Flagella and Its Impact on Clinical Practice. Genes 2024, 15, 1315. https://doi.org/10.3390/genes15101315
Zhou Y, Yu S, Zhang W. The Molecular Basis of Multiple Morphological Abnormalities of Sperm Flagella and Its Impact on Clinical Practice. Genes. 2024; 15(10):1315. https://doi.org/10.3390/genes15101315
Chicago/Turabian StyleZhou, Yujie, Songyan Yu, and Wenyong Zhang. 2024. "The Molecular Basis of Multiple Morphological Abnormalities of Sperm Flagella and Its Impact on Clinical Practice" Genes 15, no. 10: 1315. https://doi.org/10.3390/genes15101315
APA StyleZhou, Y., Yu, S., & Zhang, W. (2024). The Molecular Basis of Multiple Morphological Abnormalities of Sperm Flagella and Its Impact on Clinical Practice. Genes, 15(10), 1315. https://doi.org/10.3390/genes15101315







