Bridging the Gap Between Tolerogenic Dendritic Cells In Vitro and In Vivo: Analysis of Siglec Genes and Pathways Associated with Immune Modulation and Evasion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dendritic Cell Culture for Database Generation
2.2. RNA Sequencing and Data Analysis
2.3. Flow Cytometric Analysis
2.4. Statistical Analysis
3. Results
3.1. High and Diverse Siglec Gene Expression in Immature Dendritic Cells Is Partially Retained by Mature Tolerogenic Dendritic Cells
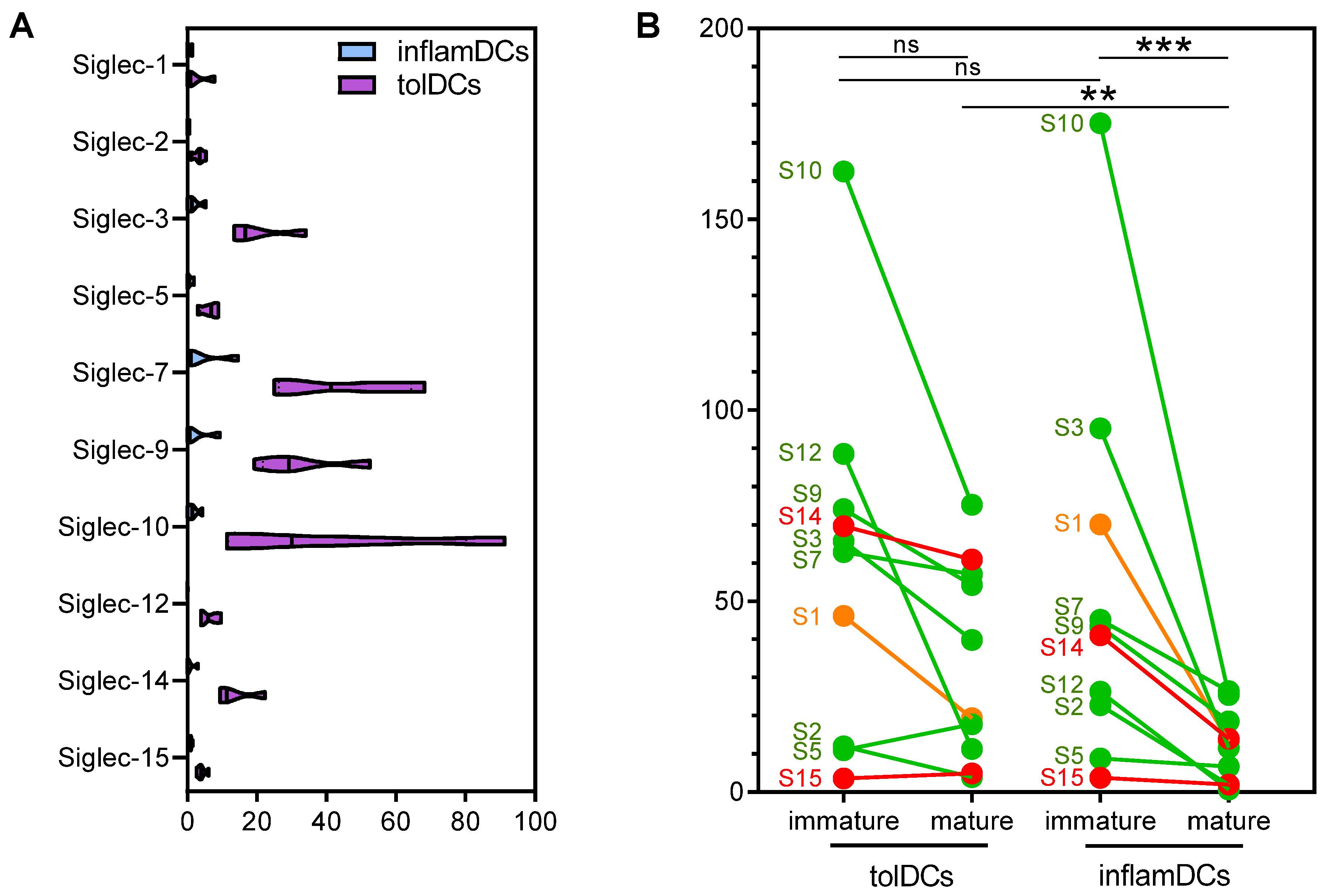
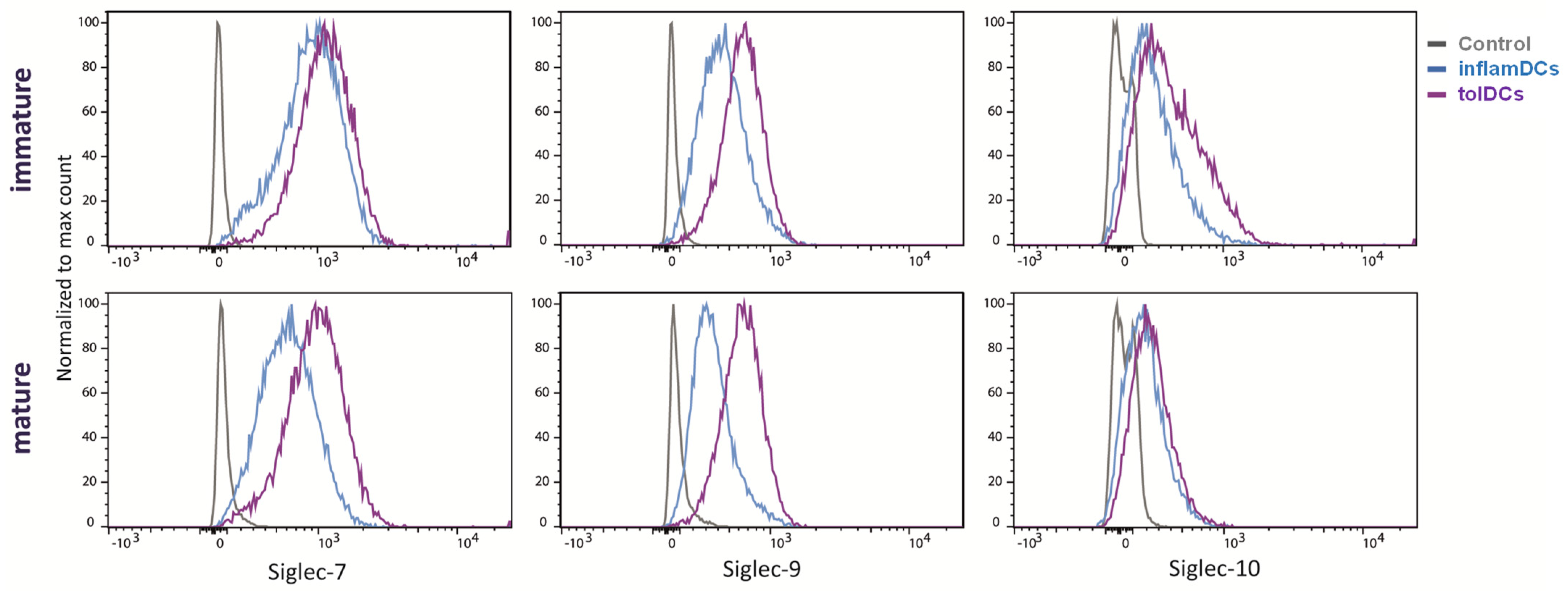
3.2. Siglec Protein Expression Largely Follows Siglec Gene Expression
3.3. Differentially Expressed Genes from the Siglec Interaction Networks Are Predominantly Associated with Inhibitory Siglecs and Enriched in the Immunoregulatory Interaction Network
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Théry, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Suwandi, J.S.; Nikolic, T.; Roep, B.O. Translating Mechanism of Regulatory Action of Tolerogenic Dendritic Cells to Monitoring Endpoints in Clinical Trials. Front. Immunol. 2017, 8, 1598. [Google Scholar] [CrossRef]
- Suwandi, J.S.; Toes, R.E.; Nikolic, T.; Roep, B.O. Inducing tissue specific tolerance in autoimmune disease with tolerogenic dendritic cells. Clin. Exp. Rheumatol. 2015, 33, S97–S103. [Google Scholar]
- Roep, B.O.; Wheeler, D.C.S.; Peakman, M. Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet Diabetes Endocrinol. 2019, 7, 65–74. [Google Scholar] [CrossRef]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the beta-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Coppieters, K.T.; Dotta, F.; Amirian, N.; Campbell, P.D.; Kay, T.W.; Atkinson, M.A.; Roep, B.O.; von Herrath, M.G. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 2012, 209, 51–60. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Nikolic, T.; Suwandi, J.S.; Wesselius, J.; Laban, S.; Joosten, A.M.; Sonneveld, P.; Mul, D.; Aanstoot, H.J.; Kaddis, J.S.; Zwaginga, J.J.; et al. Tolerogenic dendritic cells pulsed with islet antigen induce long-term reduction in T-cell autoreactivity in type 1 diabetes patients. Front. Immunol. 2022, 13, 1054968. [Google Scholar] [CrossRef]
- Nikolic, T.; Zwaginga, J.J.; Uitbeijerse, B.S.; Woittiez, N.J.; de Koning, E.J.; Aanstoot, H.J.; Roep, B.O. Safety and feasibility of intradermal injection with tolerogenic dendritic cells pulsed with proinsulin peptide-for type 1 diabetes. Lancet Diabetes Endocrinol. 2020, 8, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Beringer, D.X.; Kleijwegt, F.S.; Wiede, F.; van der Slik, A.R.; Loh, K.L.; Petersen, J.; Dudek, N.L.; Duinkerken, G.; Laban, S.; Joosten, A.; et al. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat. Immunol. 2015, 16, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Kleijwegt, F.S.; Roep, B.O. Infectious tolerance as candidate therapy for type 1 diabetes: Transfer of immunoregulatory properties from human regulatory T cells to other T cells and proinflammatory dendritic cells. Crit. Rev. Immunol. 2013, 33, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Kleijwegt, F.S.; Jansen, D.T.; Teeler, J.; Joosten, A.M.; Laban, S.; Nikolic, T.; Roep, B.O. Tolerogenic dendritic cells impede priming of naive CD8(+) T cells and deplete memory CD8(+) T cells. Eur. J. Immunol. 2013, 43, 85–92. [Google Scholar] [CrossRef]
- Ferreira, G.B.; Kleijwegt, F.S.; Waelkens, E.; Lage, K.; Nikolic, T.; Hansen, D.A.; Workman, C.T.; Roep, B.O.; Overbergh, L.; Mathieu, C. Differential protein pathways in 1,25-dihydroxyvitamin d(3) and dexamethasone modulated tolerogenic human dendritic cells. J. Proteome Res. 2012, 11, 941–971. [Google Scholar] [CrossRef]
- Kleijwegt, F.S.; Laban, S.; Duinkerken, G.; Joosten, A.M.; Koeleman, B.P.; Nikolic, T.; Roep, B.O. Transfer of regulatory properties from tolerogenic to proinflammatory dendritic cells via induced autoreactive regulatory T cells. J. Immunol. 2011, 187, 6357–6364. [Google Scholar] [CrossRef]
- Kleijwegt, F.S.; Laban, S.; Duinkerken, G.; Joosten, A.M.; Zaldumbide, A.; Nikolic, T.; Roep, B.O. Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J. Immunol. 2010, 185, 1412–1418. [Google Scholar] [CrossRef]
- Unger, W.W.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef]
- Suwandi, J.S.; Laban, S.; Vass, K.; Joosten, A.; van Unen, V.; Lelieveldt, B.P.F.; Höllt, T.; Zwaginga, J.J.; Nikolic, T.; Roep, B.O. Multidimensional analyses of proinsulin peptide-specific regulatory T cells induced by tolerogenic dendritic cells. J. Autoimmun. 2020, 107, 102361. [Google Scholar] [CrossRef]
- Mankan, A.K.; Czajka-Francuz, P.; Prendes, M.; Ramanan, S.; Koziej, M.; Vidal, L.; Saini, K.S. Intracellular DNA sensing by neutrophils and amplification of the innate immune response. Front. Immunol. 2023, 14, 1208137. [Google Scholar] [CrossRef]
- Czajka-Francuz, P.; Prendes, M.J.; Mankan, A.; Quintana, Á.; Pabla, S.; Ramkissoon, S.; Jensen, T.J.; Peiró, S.; Severson, E.A.; Achyut, B.R.; et al. Mechanisms of immune modulation in the tumor microenvironment and implications for targeted therapy. Front. Oncol. 2023, 13, 1200646. [Google Scholar] [CrossRef] [PubMed]
- Lübbers, J.; Rodríguez, E.; van Kooyk, Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front. Immunol. 2018, 9, 2807. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Perdicchio, M.; Cornelissen, L.A.; Streng-Ouwehand, I.; Engels, S.; Verstege, M.I.; Boon, L.; Geerts, D.; van Kooyk, Y.; Unger, W.W. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget 2016, 7, 8771–8782. [Google Scholar] [CrossRef] [PubMed]
- Perdicchio, M.; Ilarregui, J.M.; Verstege, M.I.; Cornelissen, L.A.; Schetters, S.T.; Engels, S.; Ambrosini, M.; Kalay, H.; Veninga, H.; den Haan, J.M.; et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl. Acad. Sci. USA 2016, 113, 3329–3334. [Google Scholar] [CrossRef]
- Wang, J.; Manni, M.; Bärenwaldt, A.; Wieboldt, R.; Kirchhammer, N.; Ivanek, R.; Stanczak, M.; Zippelius, A.; König, D.; Rodrigues Manutano, N.; et al. Siglec Receptors Modulate Dendritic Cell Activation and Antigen Presentation to T Cells in Cancer. Front. Cell Dev. Biol. 2022, 10, 828916. [Google Scholar] [CrossRef]
- van Megen, K.M.; Chen, Z.; Joosten, A.M.; Laban, S.; Zwaginga, J.J.; Natarajan, R.; Nikolic, T.; Roep, B.O. 1,25-dihydroxyvitamin D3 induces stable and reproducible therapeutic tolerogenic dendritic cells with specific epigenetic modifications. Cytotherapy 2021, 23, 242–255. [Google Scholar] [CrossRef]
- Nikolic, T.; Woittiez, N.J.C.; van der Slik, A.; Laban, S.; Joosten, A.; Gysemans, C.; Mathieu, C.; Zwaginga, J.J.; Koeleman, B.; Roep, B.O. Differential transcriptome of tolerogenic versus inflammatory dendritic cells points to modulated T1D genetic risk and enriched immune regulation. Genes. Immun. 2017, 18, 176–183. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Kast, W.M.; Boog, C.J.; Roep, B.O.; Voordouw, A.C.; Melief, C.J. Failure or success in the restoration of virus-specific cytotoxic T lymphocyte response defects by dendritic cells. J. Immunol. 1988, 140, 3186–3193. [Google Scholar] [CrossRef]
- Orozco, G.; Eerligh, P.; Sanchez, E.; Zhernakova, S.; Roep, B.O.; Gonzalez-Gay, M.A.; Lopez-Nevot, M.A.; Callejas, J.L.; Hidalgo, C.; Pascual-Salcedo, D.; et al. Analysis of a functional BTNL2 polymorphism in type 1 diabetes, rheumatoid arthritis, and systemic lupus erythematosus. Hum. Immunol. 2005, 66, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- van Lummel, M.; van Veelen, P.A.; de Ru, A.H.; Janssen, G.M.; Pool, J.; Laban, S.; Joosten, A.M.; Nikolic, T.; Drijfhout, J.W.; Mearin, M.L.; et al. Dendritic Cells Guide Islet Autoimmunity through a Restricted and Uniquely Processed Peptidome Presented by High-Risk HLA-DR. J. Immunol. 2016, 196, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Eerligh, P.; Barrera, P.; Weseloy, J.Z.; Huizinga, T.W.; Roep, B.O.; Wijmenga, C.; Koeleman, B.P. CTLA4 is differently associated with autoimmune diseases in the Dutch population. Hum. Genet. 2006, 119, 225. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Clayton, D.G.; Concannon, P.; Akolkar, B.; Cooper, J.D.; Erlich, H.A.; Julier, C.; Morahan, G.; Nerup, J.; Nierras, C.; et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009, 41, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Tegehall, A.; Ingvast, S.; Krogvold, L.; Dahl-Jorgensen, K.; Korsgren, O. Reduced expression of central innate defense molecules in pancreatic biopsies from subjects with Type 1 diabetes. Acta Diabetol. 2024, 61, 1117–1127. [Google Scholar] [CrossRef]
- Nikolic, T.; Roep, B.O. Regulatory multitasking of tolerogenic dendritic cells—Lessons taken from vitamin d3-treated tolerogenic dendritic cells. Front. Immunol. 2013, 4, 113. [Google Scholar] [CrossRef]
- Gibson, V.B.; Nikolic, T.; Pearce, V.Q.; Demengeot, J.; Roep, B.O.; Peakman, M. Proinsulin multi-peptide immunotherapy induces antigen-specific regulatory T cells and limits autoimmunity in a humanized model. Clin. Exp. Immunol. 2015, 182, 251–260. [Google Scholar] [CrossRef]
- van Halteren, A.G.; Tysma, O.M.; van Etten, E.; Mathieu, C.; Roep, B.O. 1alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J. Autoimmun. 2004, 23, 233–239. [Google Scholar] [CrossRef]
- Tree, T.I.; Lawson, J.; Edwards, H.; Skowera, A.; Arif, S.; Roep, B.O.; Peakman, M. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes 2010, 59, 1451–1460. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Battaglia, M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 2007, 7, 585–598. [Google Scholar] [CrossRef]
- Keumatio Doungtsop, B.C.; Nardini, E.; Kalay, H.; Versteeg, S.A.; Lubbers, J.; van Barneveld, G.; Li, E.R.J.; van Vliet, S.J.; van Ree, R.; de Jong, E.C.; et al. Sialic acid-modified der p 2 allergen exerts immunomodulatory effects on human PBMCs. J. Allergy Clin. Immunol. Glob. 2024, 3, 100193. [Google Scholar] [CrossRef] [PubMed]

| Pathway | Gene Count | Enrichment Strength | FDR | |
|---|---|---|---|---|
| Observed | Total | |||
| Immunoregulatory interactions between Lymphoid and non-Lymphoid cells | 15 | 130 | 1.7 | 4.5 × 10−18 |
| Immune System | 30 | 1979 | 0.8 | 3.1 × 10−16 |
| Adaptive Immune System | 21 | 758 | 1.1 | 5.3 × 10−15 |
| Neutrophil degranulation | 14 | 476 | 1.1 | 1.9 × 10−9 |
| Innate Immune System | 18 | 1041 | 0.9 | 3.6 × 10−9 |
| Other semaphorin interactions | 4 | 19 | 2.0 | 8.4 × 10−5 |
| DAP12 interactions | 4 | 39 | 1.6 | 9.7 × 10−4 |
| Cell surface interactions at the vascular wall | 5 | 139 | 1.2 | 5.4 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, D.T.S.L.; Nikolic, T.; den Hollander, N.H.M.; Zwaginga, J.J.; Roep, B.O. Bridging the Gap Between Tolerogenic Dendritic Cells In Vitro and In Vivo: Analysis of Siglec Genes and Pathways Associated with Immune Modulation and Evasion. Genes 2024, 15, 1427. https://doi.org/10.3390/genes15111427
Jansen DTSL, Nikolic T, den Hollander NHM, Zwaginga JJ, Roep BO. Bridging the Gap Between Tolerogenic Dendritic Cells In Vitro and In Vivo: Analysis of Siglec Genes and Pathways Associated with Immune Modulation and Evasion. Genes. 2024; 15(11):1427. https://doi.org/10.3390/genes15111427
Chicago/Turabian StyleJansen, Diahann T. S. L., Tatjana Nikolic, Nicoline H. M. den Hollander, Jaap Jan Zwaginga, and Bart O. Roep. 2024. "Bridging the Gap Between Tolerogenic Dendritic Cells In Vitro and In Vivo: Analysis of Siglec Genes and Pathways Associated with Immune Modulation and Evasion" Genes 15, no. 11: 1427. https://doi.org/10.3390/genes15111427
APA StyleJansen, D. T. S. L., Nikolic, T., den Hollander, N. H. M., Zwaginga, J. J., & Roep, B. O. (2024). Bridging the Gap Between Tolerogenic Dendritic Cells In Vitro and In Vivo: Analysis of Siglec Genes and Pathways Associated with Immune Modulation and Evasion. Genes, 15(11), 1427. https://doi.org/10.3390/genes15111427





