Assessing the Impact and Cost-Effectiveness of Exposome Interventions on Alzheimer’s Disease: A Review of Agent-Based Modeling and Other Data Science Methods for Causal Inference
Abstract
:1. Introduction
2. Challenges in Modeling the Exposome over the Life Course and Its Impacts on AD/ADRD
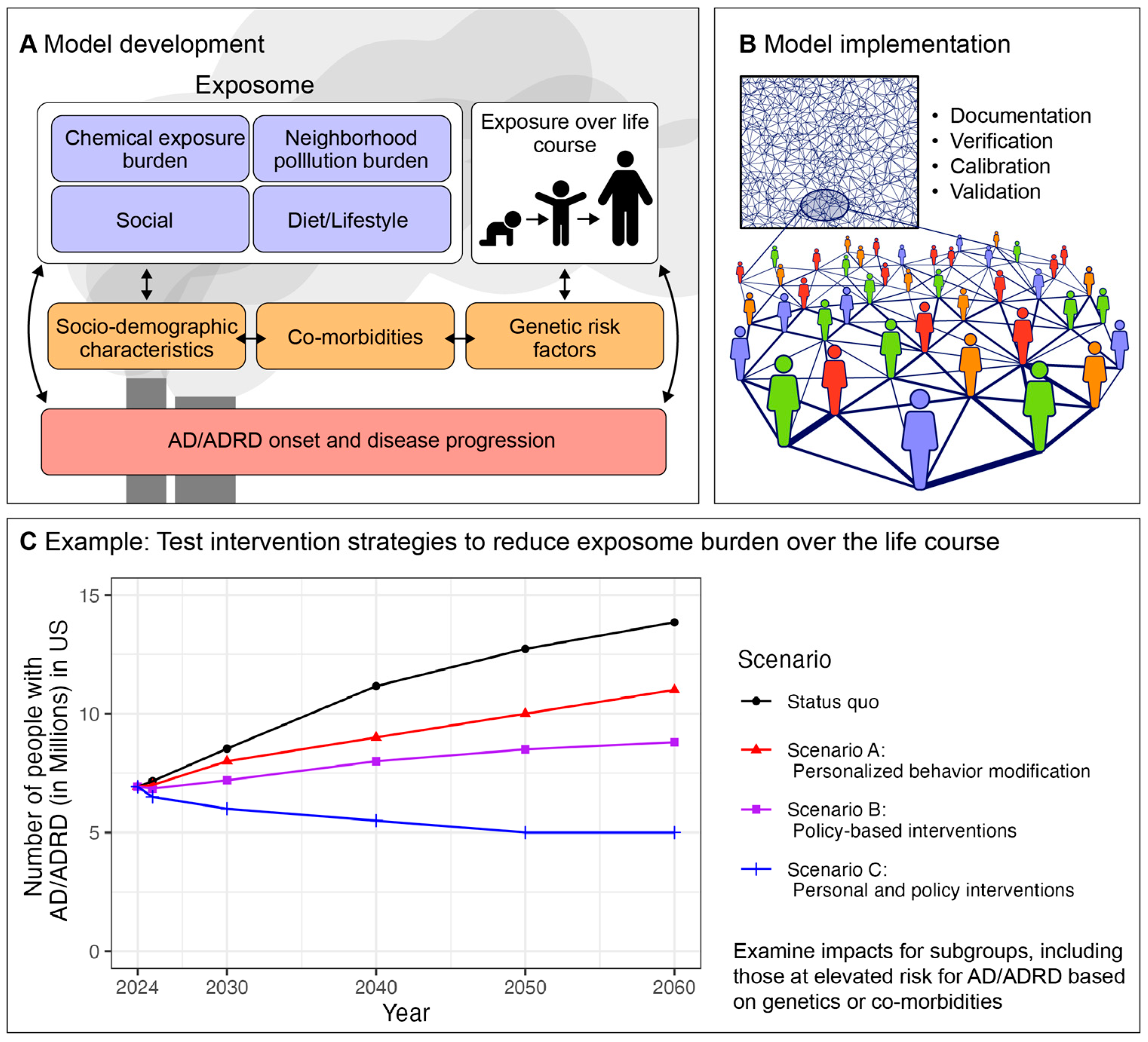
3. Agent-Based Modeling for Modeling the Impact of Exposome Interventions on Population-Level AD/ADRD Burden
4. Other Causal Inference Methods to Quantitatively Model the Impact of Exposome Interventions
5. Considerations of Economics and Cost-Effectiveness in Studying the Impact of Exposome Interventions
6. Future Directions and Big Data Considerations of Modeling the Exposome and Impacts of Interventions on Disease Burden: Innovative Use of Exposome Burden Scores and Artificial Intelligence
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [CrossRef]
- Wu, L.; Rosa-Neto, P.; Hsiung, G.Y.; Sadovnick, A.D.; Masellis, M.; Black, S.E.; Jia, J.; Gauthier, S. Early-onset familial Alzheimer’s disease (EOFAD). Can. J. Neurol. Sci. 2012, 39, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.N.; Solomon, M.B.; Privette Vinnedge, L.M. Novel molecular mechanisms in Alzheimer’s disease: The potential role of DEK in disease pathogenesis. Front. Aging Neurosci. 2022, 14, 1018180. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.N.; Clarnette, R.; Fisher, C.; Broe, G.A.; Brooks, W.S.; Montgomery, P.; Gandy, S.E. ApoE genotypes in Australia: Roles in early and late onset Alzheimer’s disease and Down’s syndrome. Neuroreport 1995, 6, 1513–1516. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Mayeux, R.; Stern, Y.; Ottman, R.; Tatemichi, T.K.; Tang, M.X.; Maestre, G.; Ngai, C.; Tycko, B.; Ginsberg, H. The apolipoprotein epsilon 4 allele in patients with Alzheimer’s disease. Ann. Neurol. 1993, 34, 752–754. [Google Scholar] [CrossRef]
- Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.; George-Hyslop, P.H.; Pericak-Vance, M.A.; Joo, S.H.; Rosi, B.L.; Gusella, J.F.; Crapper-MacLachlan, D.R.; Alberts, M.J.; et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43, 1467–1472. [Google Scholar] [CrossRef]
- Strittmatter, W.J.; Saunders, A.M.; Schmechel, D.; Pericak-Vance, M.; Enghild, J.; Salvesen, G.S.; Roses, A.D. Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 1977–1981. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Nandi, A.; Counts, N.; Bröker, J.; Malik, S.; Chen, S.; Han, R.; Klusty, J.; Seligman, B.; Tortorice, D.; Vigo, D.; et al. Cost of care for Alzheimer’s disease and related dementias in the United States: 2016 to 2060. npj Aging 2024, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- University of Southern California Sol Price School of Public Policy. The Most Expensive Medical Diseases and Procedures. Available online: https://healthadministrationdegree.usc.edu/blog/most-expensive-disease-to-treat-infographic (accessed on 7 August 2024).
- Manz, K.E.; Feerick, A.; Braun, J.M.; Feng, Y.-L.; Hall, A.; Koelmel, J.; Manzano, C.; Newton, S.R.; Pennell, K.D.; Place, B.J.; et al. Non-targeted analysis (NTA) and suspect screening analysis (SSA): A review of examining the chemical exposome. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 524–536. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders and Stroke. ONETOX: Neural Exposome and Toxicology Programs. Available online: https://www.ninds.nih.gov/current-research/research-funded-ninds/translational-research/onetox-neural-exposome-and-toxicology-programs/neural-exposome (accessed on 19 June 2024).
- Adkins-Jackson, P.B.; George, K.M.; Besser, L.M.; Hyun, J.; Lamar, M.; Hill-Jarrett, T.G.; Bubu, O.M.; Flatt, J.D.; Heyn, P.C.; Cicero, E.C.; et al. The structural and social determinants of Alzheimer’s disease related dementias. Alzheimers Dement 2023, 19, 3171–3185. [Google Scholar] [CrossRef]
- McMichael, A.J.; McGuinness, B.; Lee, J.; Minh, H.V.; Woodside, J.V.; McEvoy, C.T. Food insecurity and brain health in adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8728–8743. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Suzuki, N.; Fujiwara, T.; Hanazato, M.; Kondo, K. Neighborhood Food Environment and Dementia Incidence: The Japan Gerontological Evaluation Study Cohort Survey. Am. J. Prev. Med. 2019, 56, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Besser, L. Outdoor green space exposure and brain health measures related to Alzheimer’s disease: A rapid review. BMJ Open 2021, 11, e043456. [Google Scholar] [CrossRef]
- Zagnoli, F.; Filippini, T.; Jimenez, M.P.; Wise, L.A.; Hatch, E.E.; Vinceti, M. Is Greenness Associated with Dementia? A Systematic Review and Dose-Response Meta-analysis. Curr. Environ. Health Rep. 2022, 9, 574–590. [Google Scholar] [CrossRef]
- Buettner, L.L.; Langrish, S. Rural vs. urban caregivers of older adults with probable Alzheimer’s Disease: Perceptions regarding daily living and recreation needs. In Caregiving-Leisure and Aging; Routledge: London, UK, 2020; pp. 51–65. [Google Scholar]
- Stephen, R.; Hongisto, K.; Solomon, A.; Lönnroos, E. Physical Activity and Alzheimer’s Disease: A Systematic Review. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 733–739. [Google Scholar] [CrossRef]
- Toepper, M.; Falkenstein, M. Driving Fitness in Different Forms of Dementia: An Update. J. Am. Geriatr. Soc. 2019, 67, 2186–2192. [Google Scholar] [CrossRef]
- Babulal, G.M.; Williams, M.M.; Stout, S.H.; Roe, C.M. Driving Outcomes among Older Adults: A Systematic Review on Racial and Ethnic Differences over 20 Years. Geriatrics 2018, 3, 12. [Google Scholar] [CrossRef]
- Wang, K. Housing Instability and Socioeconomic Disparities in Health: Evidence from the U.S. Economic Recession. J. Racial Ethn. Health Disparities 2022, 9, 2451–2467. [Google Scholar] [CrossRef]
- Okoye, S.M.; Fabius, C.D.; Reider, L.; Wolff, J.L. Predictors of falls in older adults with and without dementia. Alzheimers Dement 2023, 19, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Coley, R.L.; Leventhal, T.; Lynch, A.D.; Kull, M. Relations between housing characteristics and the well-being of low-income children and adolescents. Dev. Psychol. 2013, 49, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Trani, J.F.; Moodley, J.; Maw, M.T.T.; Babulal, G.M. Association of Multidimensional Poverty With Dementia in Adults Aged 50 Years or Older in South Africa. JAMA Netw. Open 2022, 5, e224160. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Ogunniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Kaske, E.A.; Cramer, S.W.; Pena Pino, I.; Do, T.H.; Ladd, B.M.; Sturtevant, D.T.; Ahmadi, A.; Taha, B.; Freeman, D.; Wu, J.T.; et al. Injuries from Less-Lethal Weapons during the George Floyd Protests in Minneapolis. N. Engl. J. Med. 2021, 384, 774–775. [Google Scholar] [CrossRef]
- Cox, R.J.A.; Wallace, R.B. The Role of Incarceration as a Risk Factor for Cognitive Impairment. J. Gerontol. B Psychol. Sci. Soc. Sci. 2022, 77, e247–e262. [Google Scholar] [CrossRef]
- Powell, W.R.; Buckingham, W.R.; Larson, J.L.; Vilen, L.; Yu, M.; Salamat, M.S.; Bendlin, B.B.; Rissman, R.A.; Kind, A.J.H. Association of Neighborhood-Level Disadvantage With Alzheimer Disease Neuropathology. JAMA Netw. Open 2020, 3, e207559. [Google Scholar] [CrossRef]
- Kind, A.J.H.; Buckingham, W.R. Making Neighborhood-Disadvantage Metrics Accessible—The Neighborhood Atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef]
- Huang, L.Y.; Hu, H.Y.; Wang, Z.T.; Ma, Y.H.; Dong, Q.; Tan, L.; Yu, J.T. Association of Occupational Factors and Dementia or Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2020, 78, 217–227. [Google Scholar] [CrossRef]
- Parker, S.K.; Ward, M.K.; Fisher, G.G. Can high-quality jobs help workers learn new tricks? A multidisciplinary review of work design for cognition. Acad. Manag. Ann. 2021, 15, 406–454. [Google Scholar] [CrossRef]
- Yaffe, K.; Falvey, C.; Harris, T.B.; Newman, A.; Satterfield, S.; Koster, A.; Ayonayon, H.; Simonsick, E. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. Bmj 2013, 347, f7051. [Google Scholar] [CrossRef] [PubMed]
- Marden, J.R.; Tchetgen Tchetgen, E.J.; Kawachi, I.; Glymour, M.M. Contribution of Socioeconomic Status at 3 Life-Course Periods to Late-Life Memory Function and Decline: Early and Late Predictors of Dementia Risk. Am. J. Epidemiol. 2017, 186, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Sisco, S.; Gross, A.L.; Shih, R.A.; Sachs, B.C.; Glymour, M.M.; Bangen, K.J.; Benitez, A.; Skinner, J.; Schneider, B.C.; Manly, J.J. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J. Gerontol. B Psychol. Sci. Soc. Sci. 2015, 70, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Arce Rentería, M.; Vonk, J.M.J.; Felix, G.; Avila, J.F.; Zahodne, L.B.; Dalchand, E.; Frazer, K.M.; Martinez, M.N.; Shouel, H.L.; Manly, J.J. Illiteracy, dementia risk, and cognitive trajectories among older adults with low education. Neurology 2019, 93, e2247–e2256. [Google Scholar] [CrossRef]
- Kobayashi, L.C.; Berkman, L.F.; Wagner, R.G.; Kahn, K.; Tollman, S.; Subramanian, S.V. Education modifies the relationship between height and cognitive function in a cross-sectional population-based study of older adults in Rural South Africa. Eur. J. Epidemiol. 2019, 34, 131–139. [Google Scholar] [CrossRef]
- Eng, C.W.; Glymour, M.M.; Gilsanz, P.; Mungas, D.M.; Mayeda, E.R.; Meyer, O.L.; Whitmer, R.A. Do the Benefits of Educational Attainment for Late-life Cognition Differ by Racial/Ethnic Group?: Evidence for Heterogenous Treatment Effects in the Kaiser Healthy Aging and Diverse Life Experience (KHANDLE) Study. Alzheimer Dis. Assoc. Disord. 2021, 35, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.S.; Pittman, C.A.; Price, C.L.; Nieman, C.L.; Oh, E.S. Telemedicine and Dementia Care: A Systematic Review of Barriers and Facilitators. J. Am. Med. Dir. Assoc. 2021, 22, 1396–1402.e18. [Google Scholar] [CrossRef]
- Sekhon, H.; Sekhon, K.; Launay, C.; Afililo, M.; Innocente, N.; Vahia, I.; Rej, S.; Beauchet, O. Telemedicine and the rural dementia population: A systematic review. Maturitas 2021, 143, 105–114. [Google Scholar] [CrossRef]
- Perry, B.L.; McConnell, W.R.; Coleman, M.E.; Roth, A.R.; Peng, S.; Apostolova, L.G. Why the cognitive “fountain of youth” may be upstream: Pathways to dementia risk and resilience through social connectedness. Alzheimers Dement. 2022, 18, 934–941. [Google Scholar] [CrossRef]
- Yu, K.; Siang Ng, T.K. Investigating Biological Pathways Underpinning the Longitudinal Association Between Loneliness and Cognitive Impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 1417–1426. [Google Scholar] [CrossRef]
- Vasefi, M.; Ghaboolian-Zare, E.; Abedelwahab, H.; Osu, A. Environmental toxins and Alzheimer’s disease progression. Neurochem. Int. 2020, 141, 104852. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Curtain, C.C.; Ali, F.; Volitakis, I.; Cherny, R.A.; Norton, R.S.; Beyreuther, K.; Barrow, C.J.; Masters, C.L.; Bush, A.I.; Barnham, K.J. Alzheimer’s disease amyloid-β binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J. Biol. Chem. 2001, 276, 20466–20473. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.-Y.; Zhu, Y.-Z.; Zhu, H.-L.; Fan, J.-B.; Chen, J.; Liang, Y. Low micromolar zinc accelerates the fibrillization of human tau via bridging of Cys-291 and Cys-322. J. Biol. Chem. 2009, 284, 34648–34657. [Google Scholar] [CrossRef]
- Mir, R.H.; Sawhney, G.; Pottoo, F.H.; Mohi-Ud-Din, R.; Madishetti, S.; Jachak, S.M.; Ahmed, Z.; Masoodi, M.H. Role of environmental pollutants in Alzheimer’s disease: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 44724–44742. [Google Scholar] [CrossRef]
- Kakeyama, M.; Tohyama, C. Developmental neurotoxicity of dioxin and its related compounds. Ind. Health 2003, 41, 215–230. [Google Scholar] [CrossRef]
- Tröster, A.I.; Ruff, R.M.; Watson, D.P. Dementia as a neuropsychological consequence of chronic occupational exposure to polychlorinated biphenyls (PCBs). Arch. Clin. Neuropsychol. 1991, 6, 301–318. [Google Scholar] [CrossRef]
- Hauser, R.; Williams, P.; Altshul, L.; Calafat, A.M. Evidence of interaction between polychlorinated biphenyls and phthalates in relation to human sperm motility. Environ. Health Perspect. 2005, 113, 425–430. [Google Scholar] [CrossRef]
- Negishi, T.; Ishii, Y.; Kyuwa, S.; Kuroda, Y.; Yoshikawa, Y. Inhibition of staurosporine-induced neuronal cell death by bisphenol A and nonylphenol in primary cultured rat hippocampal and cortical neurons. Neurosci. Lett. 2003, 353, 99–102. [Google Scholar] [CrossRef]
- Leranth, C.; Hajszan, T.; Szigeti-Buck, K.; Bober, J.; MacLusky, N.J. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc. Natl. Acad. Sci. USA 2008, 105, 14187–14191. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shao, X.; Zhang, Z.; Zou, Y.; Chen, Y.; Han, S.; Wang, S.; Wu, X.; Yang, L.; Chen, Z. Effects of di-n-butyl phthalate and diethyl phthalate on acetylcholinesterase activity and neurotoxicity related gene expression in embryonic zebrafish. Bull. Environ. Contam. Toxicol. 2013, 91, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Rojo, M.M.; Cantoral, A.; Cantonwine, D.E.; Schnaas, L.; Peterson, K.; Hu, H.; Meeker, J.D. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci. Total Environ. 2013, 461, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Viberg, H.; Fredriksson, A.; Eriksson, P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 2003, 192, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Viberg, H.; Jakobsson, E.; Örn, U.; Fredriksson, A. A brominated flame retardant, 2, 2, 4, 4, 5-pentabromodiphenyl ether: Uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol. Sci. 2002, 67, 98–103. [Google Scholar] [CrossRef]
- Al-Mousa, F.; Michelangeli, F. Some commonly used brominated flame retardants cause Ca2+-ATPase inhibition, β-amyloid peptide release and apoptosis in SH-SY5Y neuronal cells. PLoS ONE 2012, 7, e33059. [Google Scholar] [CrossRef]
- Trudeau, V.L.; Chiu, S.; Kennedy, S.W.; Brooks, R.J. Octylphenol (OP) alters the expression of members of the amyloid protein family in the hypothalamus of the snapping turtle, Chelydra serpentina serpentina. Environ. Health Perspect. 2002, 110, 269–275. [Google Scholar] [CrossRef]
- Pocar, P.; Augustin, R.; Gandolfi, F.; Fischer, B. Toxic effects of in vitro exposure to p-tert-octylphenol on bovine oocyte maturation and developmental competence. Biol. Reprod. 2003, 69, 462–468. [Google Scholar] [CrossRef]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS ONE 2012, 7, e32432. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 2010, 113, 187–197. [Google Scholar] [CrossRef]
- Kukull, W.A.; Larson, E.B.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Pfanschmidt, M.L.; Thompson, J.D.; O’meara, E.S.; Brenner, D.E.; Van Belle, G. Solvent exposure as a risk factor for Alzheimer’s disease: A case-control study. Am. J. Epidemiol. 1995, 141, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Edwards, M.; Menon, C.V.; Gong, G.; Barber, R. Long-term low-level arsenic exposure is associated with poorer neuropsychological functioning: A Project FRONTIER study. Int. J. Environ. Res. Public Health 2011, 8, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Claudio, L.; Markowitz, S.B.; Berkowitz, G.S.; Brenner, B.L.; Romero, H.; Wetmur, J.G.; Matte, T.D.; Gore, A.C.; Godbold, J.H. Pesticides and inner-city children: Exposures, risks, and prevention. Environ. Health Perspect. 1999, 107, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Tiwari, S.K.; Agarwal, S.; Sharma, V.P.; Chaturvedi, R.K. Prenatal carbofuran exposure inhibits hippocampal neurogenesis and causes learning and memory deficits in offspring. Toxicol. Sci. 2012, 127, 84–100. [Google Scholar] [CrossRef]
- Kamboj, S.S.; Kumar, V.; Kamboj, A.; Sandhir, R. Mitochondrial oxidative stress and dysfunction in rat brain induced by carbofuran exposure. Cell. Mol. Neurobiol. 2008, 28, 961–969. [Google Scholar] [CrossRef]
- Chen, N.-N.; Luo, D.-J.; Yao, X.-Q.; Yu, C.; Wang, Y.; Wang, Q.; Wang, J.-Z.; Liu, G.-P. Pesticides induce spatial memory deficits with synaptic impairments and an imbalanced tau phosphorylation in rats. J. Alzheimer’s Dis. 2012, 30, 585–594. [Google Scholar] [CrossRef]
- Bjørling-Poulsen, M.; Andersen, H.R.; Grandjean, P. Potential developmental neurotoxicity of pesticides used in Europe. Environ. Health 2008, 7, 1–22. [Google Scholar] [CrossRef]
- Karska, J.; Kowalski, S.; Gładka, A.; Brzecka, A.; Sochocka, M.; Kurpas, D.; Beszłej, J.A.; Leszek, J. Artificial light and neurodegeneration: Does light pollution impact the development of Alzheimer’s disease? Geroscience 2024, 46, 87–97. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, J.; Liu, Y.; Dong, G.H.; Yang, B.Y.; Li, N.; Wang, L.; Chen, G.; Li, S.; Guo, Y. Long-term exposure to outdoor light at night and mild cognitive impairment: A nationwide study in Chinese veterans. Sci. Total Environ. 2022, 847, 157441. [Google Scholar] [CrossRef]
- Habert, M.O.; Horn, J.F.; Sarazin, M.; Lotterie, J.A.; Puel, M.; Onen, F.; Zanca, M.; Portet, F.; Touchon, J.; Verny, M.; et al. Brain perfusion SPECT with an automated quantitative tool can identify prodromal Alzheimer’s disease among patients with mild cognitive impairment. Neurobiol. Aging 2011, 32, 15–23. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.L. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol. 2000, 10, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Wright Willis, A.; Evanoff, B.A.; Lian, M.; Criswell, S.R.; Racette, B.A. Geographic and ethnic variation in Parkinson disease: A population-based study of US Medicare beneficiaries. Neuroepidemiology 2010, 34, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Viaggi, C.; Di Camillo, D.; Willis, A.W.; Lozzi, L.; Rocchi, C.; Capannolo, M.; Aloisi, G.; Vaglini, F.; Maccarone, R.; et al. Bright light exposure reduces TH-positive dopamine neurons: Implications of light pollution in Parkinson’s disease epidemiology. Sci. Rep. 2013, 3, 1395. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Yu, Y.; Huang, Y.; Wan, Y.H.; Su, P.Y.; Tao, F.B.; Sun, Y. Exposure to bedroom light pollution and cardiometabolic risk: A cohort study from Chinese young adults. Environ. Pollut. 2022, 294, 118628. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Coppedè, F. Gene-environment interactions in Alzheimer disease: The emerging role of epigenetics. Nat. Rev. Neurol. 2022, 18, 643–660. [Google Scholar] [CrossRef]
- Dunn, A.R.; O’Connell, K.M.S.; Kaczorowski, C.C. Gene-by-environment interactions in Alzheimer’s disease and Parkinson’s disease. Neurosci. Biobehav. Rev. 2019, 103, 73–80. [Google Scholar] [CrossRef]
- Baccarelli, A.; Dolinoy, D.C.; Walker, C.L. A precision environmental health approach to prevention of human disease. Nat. Commun. 2023, 14, 2449. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.; Guo, L.; Qin, W.; Liang, M.; Schumann, G.; Yu, C. Environmental neuroscience linking exposome to brain structure and function underlying cognition and behavior. Mol. Psychiatry 2023, 28, 17–27. [Google Scholar] [CrossRef]
- Rappaport, S.M.; Smith, M.T. Environment and disease risks. Science 2010, 330, 460–461. [Google Scholar] [CrossRef]
- Monti, C.; Pangallo, M.; De Francisci Morales, G.; Bonchi, F. On learning agent-based models from data. Sci. Rep. 2023, 13, 9268. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, U.; Rand, W. An Introduction to Agent-Based Modeling: Modeling Natural, Social, and Engineered Complex Systems with NetLogo; MIT Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Railsback, S.F.; Grimm, V. Agent-Based and Individual-Based Modeling: A Practical Introduction; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- An, G.; Mi, Q.; Dutta-Moscato, J.; Vodovotz, Y. Agent-based models in translational systems biology. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Bankes, S.C. Agent-based modeling: A revolution? Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl. S3), 7199–7200. [Google Scholar] [CrossRef]
- Grimm, V.; Revilla, E.; Berger, U.; Jeltsch, F.; Mooij, W.M.; Railsback, S.F.; Thulke, H.H.; Weiner, J.; Wiegand, T.; DeAngelis, D.L. Pattern-oriented modeling of agent-based complex systems: Lessons from ecology. Science 2005, 310, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Stephan, S.; Galland, S.; Labbani Narsis, O.; Shoji, K.; Vachenc, S.; Gerart, S.; Nicolle, C. Agent-based approaches for biological modeling in oncology: A literature review. Artif. Intell. Med. 2024, 152, 102884. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Grefenstette, J.J.; Galloway, D.; Albert, S.M.; Burke, D.S. Policies to reduce influenza in the workplace: Impact assessments using an agent-based model. Am. J. Public Health 2013, 103, 1406–1411. [Google Scholar] [CrossRef]
- Halloran, M.E.; Longini, I.M., Jr.; Nizam, A.; Yang, Y. Containing bioterrorist smallpox. Science 2002, 298, 1428–1432. [Google Scholar] [CrossRef]
- Nianogo, R.A.; Arah, O.A. Agent-based modeling of noncommunicable diseases: A systematic review. Am. J. Public Health 2015, 105, e20–e31. [Google Scholar] [CrossRef]
- Goldstick, J.E.; Jay, J. Agent-Based Modeling: An Underutilized Tool in Community Violence Research. Curr. Epidemiol. Rep. 2022, 9, 135–141. [Google Scholar] [CrossRef]
- Winkler, M.R.; Mui, Y.; Hunt, S.L.; Laska, M.N.; Gittelsohn, J.; Tracy, M. Applications of Complex Systems Models to Improve Retail Food Environments for Population Health: A Scoping Review. Adv. Nutr. 2022, 13, 1028–1043. [Google Scholar] [CrossRef]
- Speybroeck, N.; Van Malderen, C.; Harper, S.; Müller, B.; Devleesschauwer, B. Simulation models for socioeconomic inequalities in health: A systematic review. Int. J. Environ. Res. Public Health 2013, 10, 5750–5780. [Google Scholar] [CrossRef] [PubMed]
- Auchincloss, A.H.; Diez Roux, A.V. A new tool for epidemiology: The usefulness of dynamic-agent models in understanding place effects on health. Am. J. Epidemiol. 2008, 168, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.M.; Axtell, R. Growing Artificial Societies: Social Science from the Bottom Up; Brookings Institution Press: Washington, DC, USA, 1996. [Google Scholar]
- Luke, D.A.; Stamatakis, K.A. Systems science methods in public health: Dynamics, networks, and agents. Annu. Rev. Public Health 2012, 33, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.; Gordis, E.; Strully, K.; Marshall, B.D.L.; Cerdá, M. Applications of agent-based modeling in trauma research. Psychol. Trauma 2023, 15, 939–950. [Google Scholar] [CrossRef]
- Squires, H.; Kelly, M.P.; Gilbert, N.; Sniehotta, F.; Purshouse, R.C. The long-term effectiveness and cost-effectiveness of public health interventions; how can we model behavior? A review. Health Econ. 2023, 32, 2836–2854. [Google Scholar] [CrossRef]
- Tracy, M.; Cerdá, M.; Keyes, K.M. Agent-Based Modeling in Public Health: Current Applications and Future Directions. Annu. Rev. Public Health 2018, 39, 77–94. [Google Scholar] [CrossRef]
- Taucare, G.; Chan, G.; Nilsson, S.; Toms, L.L.; Zhang, X.; Mueller, J.F.; Jolliet, O. Temporal trends of per- and polyfluoroalkyl substances concentrations: Insights from Australian human biomonitoring 2002–2021 and the U.S. NHANES programs 2003–2018. Environ. Res. 2024, 262, 119777. [Google Scholar] [CrossRef]
- Lund, A.M.; Gouripeddi, R.; Facelli, J.C. STHAM: An agent based model for simulating human exposure across high resolution spatiotemporal domains. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 459–468. [Google Scholar] [CrossRef]
- Brandon, N.; Price, P.S. Calibrating an agent-based model of longitudinal human activity patterns using the Consolidated Human Activity Database. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 194–204. [Google Scholar] [CrossRef]
- Brandon, N.; Dionisio, K.L.; Isaacs, K.; Tornero-Velez, R.; Kapraun, D.; Setzer, R.W.; Price, P.S. Simulating exposure-related behaviors using agent-based models embedded with needs-based artificial intelligence. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Chapizanis, D.; Karakitsios, S.; Gotti, A.; Sarigiannis, D.A. Assessing personal exposure using Agent Based Modelling informed by sensors technology. Environ. Res. 2021, 192, 110141. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.; Robinson, J.A.; Kanduč, T.; Sarigiannis, D.; Kocman, D. Simulating the impact of particulate matter exposure on health-related behaviour: A comparative study of stochastic modelling and personal monitoring data. Health Place 2023, 83, 103111. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, J.R.; Strike, D.G.; Simonson, D.A.; Broccard, A.F.; Crooke, P.S. An agent-based and spatially explicit model of pathogen dissemination in the intensive care unit. Crit. Care Med. 2005, 33, 168–176; discussion 253–254. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W.; Portelli, I.; Narzisi, G.; Nelson, L.S.; Menges, F.; Rekow, E.D.; Mincer, J.S.; Mishra, B.; Goldfrank, L.R. A novel approach to multihazard modeling and simulation. Disaster Med. Public Health Prep. 2009, 3, 75–87. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Shu, Y.; Sun, L.; Jin, Z.; Ma, Z.; Liu, M.; Bi, J.; Kinney, P.L. A stochastic exposure model integrating random forest and agent-based approaches: Evaluation for PM(2.5) in Jiangsu, China. J. Hazard. Mater. 2022, 431, 128639. [Google Scholar] [CrossRef]
- Zechman, E.M. Agent-based modeling to simulate contamination events and evaluate threat management strategies in water distribution systems. Risk Anal. 2011, 31, 758–772. [Google Scholar] [CrossRef]
- Shin, H. Quantifying the health effects of exposure to non-exhaust road emissions using agent-based modelling (ABM). MethodsX 2022, 9, 101673. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Battistoni, C.; McNulty, R.; Morales, F.; Gorky, J.; Foley, H.; Dhurjati, P. An agent-based model to investigate microbial initiation of Alzheimer’s via the olfactory system. Theor. Biol. Med. Model. 2020, 17, 5. [Google Scholar] [CrossRef]
- Hoffman, T.E.; Hanneman, W.H.; Moreno, J.A. Network Simulations Reveal Molecular Signatures of Vulnerability to Age-Dependent Stress and Tau Accumulation. Front. Mol. Biosci. 2020, 7, 590045. [Google Scholar] [CrossRef]
- Burke, J.F.; Copeland, L.L.; Sussman, J.B.; Hayward, R.A.; Gross, A.L.; Briceño, E.M.; Whitney, R.; Giordani, B.J.; Elkind, M.S.V.; Manly, J.J.; et al. Development and validation of the Michigan Chronic Disease Simulation Model (MICROSIM). PLoS ONE 2024, 19, e0300005. [Google Scholar] [CrossRef]
- Sonnenschein, T.; Scheider, S.; de Wit, G.A.; Tonne, C.C.; Vermeulen, R. Agent-based modeling of urban exposome interventions: Prospects, model architectures, and methodological challenges. Exposome 2022, 2, osac009. [Google Scholar] [CrossRef] [PubMed]
- Diez Roux, A.V. Complex systems thinking and current impasses in health disparities research. Am. J. Public Health 2011, 101, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, M.; Jung, D. Policy evaluation of economic—Environmental tradeoffs in regulating industrial water use: An agent-based model. J. Environ. Manag. 2023, 346, 118988. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; Ma, H.W. An agent-based model for an air emissions cap and trade program: A case study in Taiwan. J. Environ. Manag. 2016, 183, 613–621. [Google Scholar] [CrossRef]
- Shi, H.; Wang, S.; Li, J.; Zhang, L. Modeling the impacts of policy measures on resident’s PM2.5 reduction behavior: An agent-based simulation analysis. Environ. Geochem. Health 2020, 42, 895–913. [Google Scholar] [CrossRef]
- Burke, D.S.; Epstein, J.M.; Cummings, D.A.; Parker, J.I.; Cline, K.C.; Singa, R.M.; Chakravarty, S. Individual-based computational modeling of smallpox epidemic control strategies. Acad. Emerg. Med. 2006, 13, 1142–1149. [Google Scholar] [CrossRef]
- Wilson, A.M.; Verhougstraete, M.P.; Donskey, C.J.; Reynolds, K.A. An agent-based modeling approach to estimate pathogen exposure risks from wheelchairs. Am. J. Infect. Control 2021, 49, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M. Systems approaches to understanding how the environment influences population health and population health interventions. In Systems Science and Population Health; Oxford University Press: Oxford, UK, 2017; pp. 151–165. [Google Scholar]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Siroux, V.; Agier, L.; Slama, R. The exposome concept: A challenge and a potential driver for environmental health research. Eur. Respir. Rev. 2016, 25, 124–129. [Google Scholar] [CrossRef]
- Hu, H.; Liu, X.; Zheng, Y.; He, X.; Hart, J.; James, P.; Laden, F.; Chen, Y.; Bian, J. Methodological Challenges in Spatial and Contextual Exposome-Health Studies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 827–846. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, Y.; Kuiper, J.R.; Ho, E.; Buckley, J.P.; Feuerstahler, L. Applying Latent Variable Models to Estimate Cumulative Exposure Burden to Chemical Mixtures and Identify Latent Exposure Subgroups: A Critical Review and Future Directions. Stat. Biosci. 2024, 16, 482–502. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D. The HEALS project. In Unraveling the Exposome: A Practical View; Springer: Cham, Switzerland, 2019; pp. 405–422. [Google Scholar]
- Vlaanderen, J.; de Hoogh, K.; Hoek, G.; Peters, A.; Probst-Hensch, N.; Scalbert, A.; Melén, E.; Tonne, C.; de Wit, G.A.; Chadeau-Hyam, M.; et al. Developing the building blocks to elucidate the impact of the urban exposome on cardiometabolic-pulmonary disease: The EU EXPANSE project. Environ. Epidemiol. 2021, 5, e162. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Yang, Y.-X.; Chen, S.-D.; Li, H.-Q.; Zhang, X.-Q.; Kuo, K.; Tan, L.; Feng, L.; Dong, Q.; Zhang, C.; et al. Investigating causal relationships between exposome and human longevity: A Mendelian randomization analysis. BMC Med. 2021, 19, 150. [Google Scholar] [CrossRef]
- Avery, C.L.; Howard, A.G.; Ballou, A.F.; Buchanan, V.L.; Collins, J.M.; Downie, C.G.; Engel, S.M.; Graff, M.; Highland, H.M.; Lee, M.P.; et al. Strengthening Causal Inference in Exposomics Research: Application of Genetic Data and Methods. Environ. Health Perspect. 2022, 130, 055001. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Li, D.; Zhou, L.; Cao, Z.; Wang, J.; Yang, H.; Lyu, M.; Zhang, Y.; Yang, R.; Wang, J.; Bian, Y.; et al. Associations of environmental factors with neurodegeneration: An exposome-wide Mendelian randomization investigation. Ageing Res. Rev. 2024, 95, 102254. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, J.; Shirkey, G.; John, R.; Wu, S.R.; Park, H.; Shao, C. Applications of structural equation modeling (SEM) in ecological studies: An updated review. Ecol. Process. 2016, 5, 19. [Google Scholar] [CrossRef]
- Bae, E.B.; Han, K.-M. A structural equation modeling approach using behavioral and neuroimaging markers in major depressive disorder. J. Psychiatr. Res. 2024, 171, 246–255. [Google Scholar] [CrossRef]
- Moore, T.M.; Visoki, E.; Argabright, S.T.; Didomenico, G.E.; Sotelo, I.; Wortzel, J.D.; Naeem, A.; Gur, R.C.; Gur, R.E.; Warrier, V.; et al. Modeling environment through a general exposome factor in two independent adolescent cohorts. Exposome 2022, 2, osac010. [Google Scholar] [CrossRef]
- Younan, D.; Petkus, A.J.; Widaman, K.F.; Wang, X.; Casanova, R.; Espeland, M.A.; Gatz, M.; Henderson, V.W.; Manson, J.E.; Rapp, S.R.; et al. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 2020, 143, 289–302. [Google Scholar] [CrossRef]
- Petkus, A.J.; Younan, D.; Wang, X.; Beavers, D.P.; Espeland, M.A.; Gatz, M.; Gruenewald, T.; Kaufman, J.D.; Chui, H.C.; Millstein, J.; et al. Associations Between Air Pollution Exposure and Empirically Derived Profiles of Cognitive Performance in Older Women. J. Alzheimers Dis. 2021, 84, 1691–1707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Drouard, G.; Whipp, A.M.; Heinonen-Guzejev, M.; Bolte, G.; Kaprio, J. Association between trajectories of the neighborhood social exposome and mental health in late adolescence: A FinnTwin12 cohort study. J. Affect. Disord. 2024, 358, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 047004. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Li, K.; Ye, H.; Dong, Z.; Amujilite; Zhao, M.; Xu, Q.; Xu, J. The health and economic burden of ozone pollution on Alzheimer’s disease and mild cognitive impairment in China. Environ. Res. 2024, 259, 119506. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, W.; Gao, X.; He, Y.; Lin, C.; Zhou, J.; Yang, L. Impact of airborne particulate matter exposure on hospital admission for Alzheimer’s disease and the attributable economic burden: Evidence from a time-series study in Sichuan, China. Environ. Sci. Eur. 2024, 36, 12. [Google Scholar] [CrossRef]
- Biasutti, M.; Dufour, N.; Ferroud, C.; Dab, W.; Temime, L. Cost-effectiveness of magnetic resonance imaging with a new contrast agent for the early diagnosis of Alzheimer’s disease. PLoS ONE 2012, 7, e35559. [Google Scholar] [CrossRef]
- Ross, E.L.; Weinberg, M.S.; Arnold, S.E. Cost-effectiveness of Aducanumab and Donanemab for Early Alzheimer Disease in the US. JAMA Neurol. 2022, 79, 478–487. [Google Scholar] [CrossRef]
- Brockmann, R.; Nixon, J.; Love, B.L.; Yunusa, I. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer’s disease: Patient outcomes, healthcare costs, and drug development. Lancet Reg. Health Am. 2023, 20, 100467. [Google Scholar] [CrossRef]
- Kuntz, K.M.; Russell, L.B.; Owens, D.K.; Sanders, G.D.; Trikalinos, T.A.; Salomon, J.A. Decision models in cost-effectiveness analysis. In Cost-Effectiveness in Health and Medicine; Oxford University Press: New York, NY, USA, 2016; pp. 105–136. [Google Scholar]
- Rudmik, L.; Drummond, M. Health economic evaluation: Important principles and methodology. Laryngoscope 2013, 123, 1341–1347. [Google Scholar] [CrossRef]
- Baio, G. survHE: Survival analysis for health economic evaluation and cost-effectiveness modeling. J. Stat. Softw. 2020, 95, 1–47. [Google Scholar] [CrossRef]
- Briggs, A.; Sculpher, M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998, 13, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, E.; Jones, A.M.; Rice, N. The evaluation of health policies through microsimulation methods. Health Econom. Data Group. (HEDG) Work. Pap. 2010, 10, 2. [Google Scholar] [CrossRef]
- Chhatwal, J.; He, T. Economic evaluations with agent-based modelling: An introduction. Pharmacoeconomics 2015, 33, 423–433. [Google Scholar] [CrossRef]
- Hernandez, L.; Ozen, A.; DosSantos, R.; Getsios, D. Systematic Review of Model-Based Economic Evaluations of Treatments for Alzheimer’s Disease. Pharmacoeconomics 2016, 34, 681–707. [Google Scholar] [CrossRef]
- Brück, C.C.; Wolters, F.J.; Ikram, M.A.; de Kok, I.M.C.M. Projections of costs and quality adjusted life years lost due to dementia from 2020 to 2050: A population-based microsimulation study. Alzheimer’s Dement. 2023, 19, 4532–4541. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Kuiper, J.; Chen, Y.; Feuerstahler, L.; Teresi, J.A.; Buckley, J.P. Developing an exposure burden score for chemical mixtures using item response theory, with applications to PFAS mixtures. Environ. Health Perspect. 2022, 130, 117001. [Google Scholar] [CrossRef]
- Liu, S.H.; Feuerstahler, L.; Chen, Y.; Braun, J.M.; Buckley, J.P. Toward Advancing Precision Environmental Health: Developing a Customized Exposure Burden Score to PFAS Mixtures to Enable Equitable Comparisons Across Population Subgroups, Using Mixture Item Response Theory. Environ. Sci. Technol. 2023, 57, 18104–18115. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, Y.; Feuerstahler, L.; Chen, A.; Starling, A.; Dabelea, D.; Wang, X.; Cecil, K.; Lanphear, B.; Yolton, K.; et al. The U.S. PFAS exposure burden calculator for 2017–2018: Application to the HOME Study, with comparison of epidemiological findings from NHANES. Neurotoxicol. Teratol. 2024, 102, 107321. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feuerstahler, L.; Martinez Steele, E.; Buckley, J.P.; Liu, S.H. Phthalate mixtures and insulin resistance: An item response theory approach to quantify exposure burden to phthalate mixtures. J. Expo. Sci. Environ. Epidemiol. 2023, 34, 581–590. [Google Scholar] [CrossRef]
- Liu, S.H.; Dams-O’Connor, K.; Spicer, J. Building an allostatic load scale using item response theory. In Proceedings of the International Biometric Society ENAR, Nashville, TN, USA, 22–25 March 2020. [Google Scholar]
- Balestriero, R.; Ibrahim, M.; Sobal, V.; Morcos, A.S.; Shekhar, S.; Goldstein, T.; Bordes, F.; Bardes, A.; Mialon, G.; Tian, Y.; et al. A Cookbook of Self-Supervised Learning. arXiv 2023, arXiv:abs/2304.12210. [Google Scholar]
- Krishnan, R.; Rajpurkar, P.; Topol, E.J. Self-supervised learning in medicine and healthcare. Nat. Biomed. Eng. 2022, 6, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, H.; Gu, Y. Dive into the details of self-supervised learning for medical image analysis. Med. Image Anal. 2023, 89, 102879. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ganoe, C.H.; Sieberg, R.T.; Cheung, Y.Y.; Hassanpour, S. Self-Supervised Contextual Language Representation of Radiology Reports to Improve the Identification of Communication Urgency. AMIA Jt. Summits Transl. Sci. Proc. 2020, 2020, 413–421. [Google Scholar] [PubMed]
- Liu, W.; Teng, Z.; Li, Z.; Chen, J. CVGAE: A Self-Supervised Generative Method for Gene Regulatory Network Inference Using Single-Cell RNA Sequencing Data. Interdiscip. Sci. Comput. Life Sci. 2024, 16, 990–1004. [Google Scholar] [CrossRef]
- Wei, Q.; Islam, M.T.; Zhou, Y.; Xing, L. Self-supervised deep learning of gene–gene interactions for improved gene expression recovery. Brief. Bioinform. 2024, 25, bba1031. [Google Scholar] [CrossRef] [PubMed]
- Padegal, G.; Rao, M.K.; Boggaram Ravishankar, O.A.; Acharya, S.; Athri, P.; Srinivasa, G. Analysis of RNA-Seq data using self-supervised learning for vital status prediction of colorectal cancer patients. BMC Bioinform. 2023, 24, 241. [Google Scholar] [CrossRef]
- Kostas, D.; Aroca-Ouellette, S.; Rudzicz, F. BENDR: Using Transformers and a Contrastive Self-Supervised Learning Task to Learn From Massive Amounts of EEG Data. Front. Hum. Neurosci. 2021, 15, 653659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.H.; Weber, E.S.; Manz, K.E.; McCarthy, K.J.; Chen, Y.; Schüffler, P.J.; Zhu, C.W.; Tracy, M. Assessing the Impact and Cost-Effectiveness of Exposome Interventions on Alzheimer’s Disease: A Review of Agent-Based Modeling and Other Data Science Methods for Causal Inference. Genes 2024, 15, 1457. https://doi.org/10.3390/genes15111457
Liu SH, Weber ES, Manz KE, McCarthy KJ, Chen Y, Schüffler PJ, Zhu CW, Tracy M. Assessing the Impact and Cost-Effectiveness of Exposome Interventions on Alzheimer’s Disease: A Review of Agent-Based Modeling and Other Data Science Methods for Causal Inference. Genes. 2024; 15(11):1457. https://doi.org/10.3390/genes15111457
Chicago/Turabian StyleLiu, Shelley H., Ellerie S. Weber, Katherine E. Manz, Katharine J. McCarthy, Yitong Chen, Peter J. Schüffler, Carolyn W. Zhu, and Melissa Tracy. 2024. "Assessing the Impact and Cost-Effectiveness of Exposome Interventions on Alzheimer’s Disease: A Review of Agent-Based Modeling and Other Data Science Methods for Causal Inference" Genes 15, no. 11: 1457. https://doi.org/10.3390/genes15111457
APA StyleLiu, S. H., Weber, E. S., Manz, K. E., McCarthy, K. J., Chen, Y., Schüffler, P. J., Zhu, C. W., & Tracy, M. (2024). Assessing the Impact and Cost-Effectiveness of Exposome Interventions on Alzheimer’s Disease: A Review of Agent-Based Modeling and Other Data Science Methods for Causal Inference. Genes, 15(11), 1457. https://doi.org/10.3390/genes15111457





