A Study of Short-Chain Fatty Acids During the Canalicular and Early Saccular Phases of Fetal Lung Development and Childhood Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis Plan
- (1)
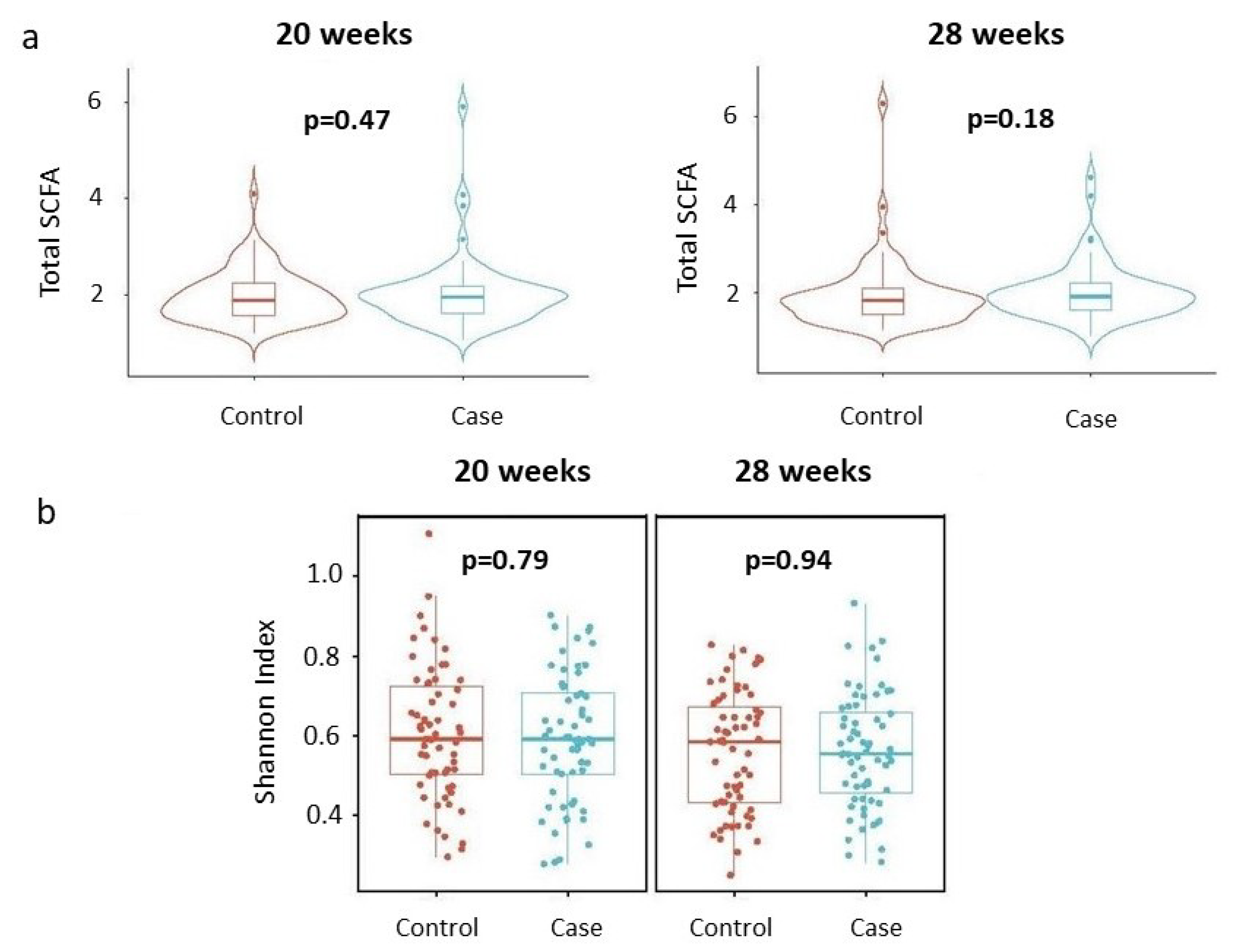
- Assessing whether total SCFA concentrations differ by disease status, stratified by gestational age (20 weeks and 28 weeks).
- (2)
- Evaluating whether the Shannon index of the SCFA profile varies by disease status, stratified by gestational age.
- (3)
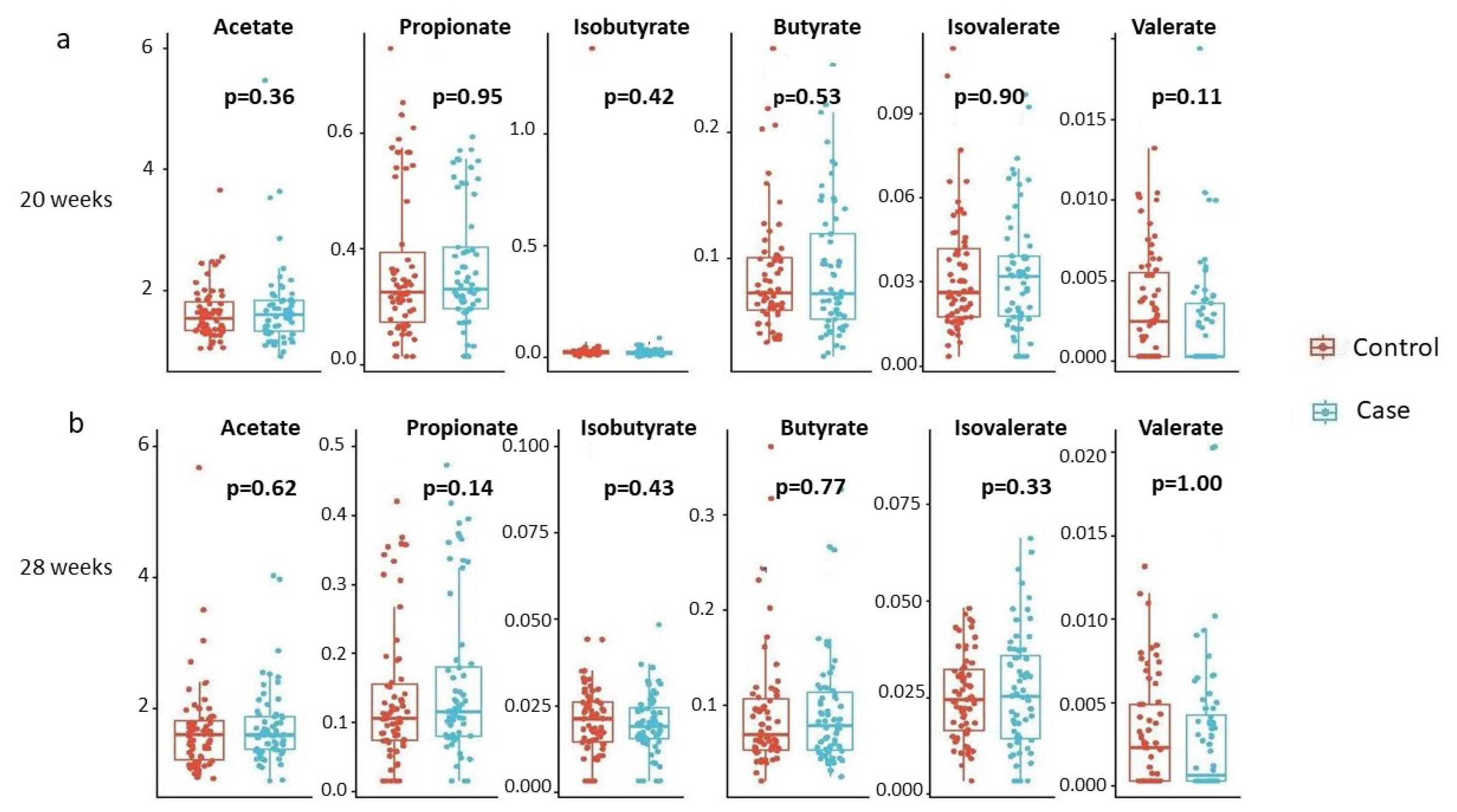
- Investigating whether individual SCFAs differ by disease status, stratified by gestational age.
- (4)
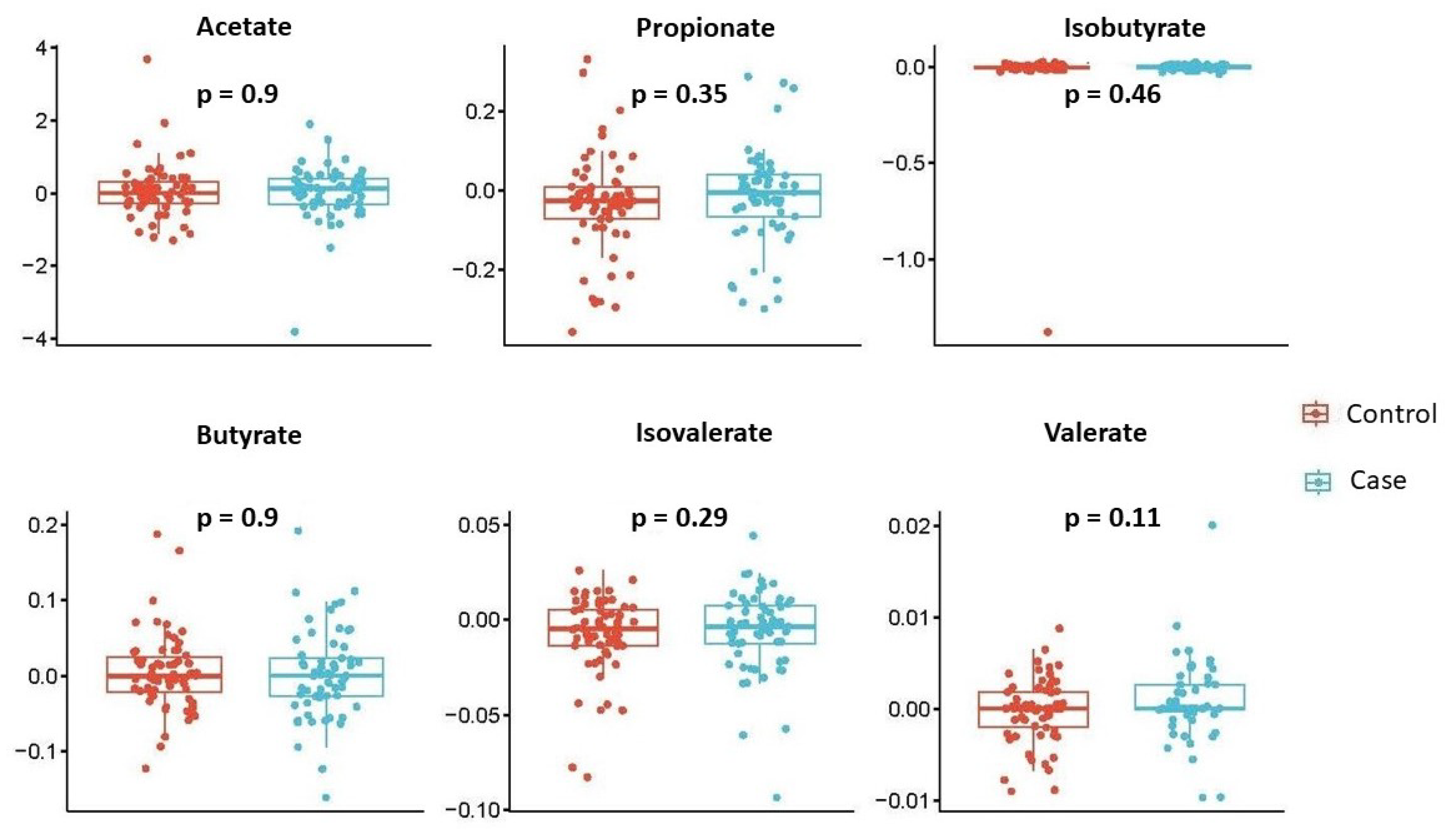
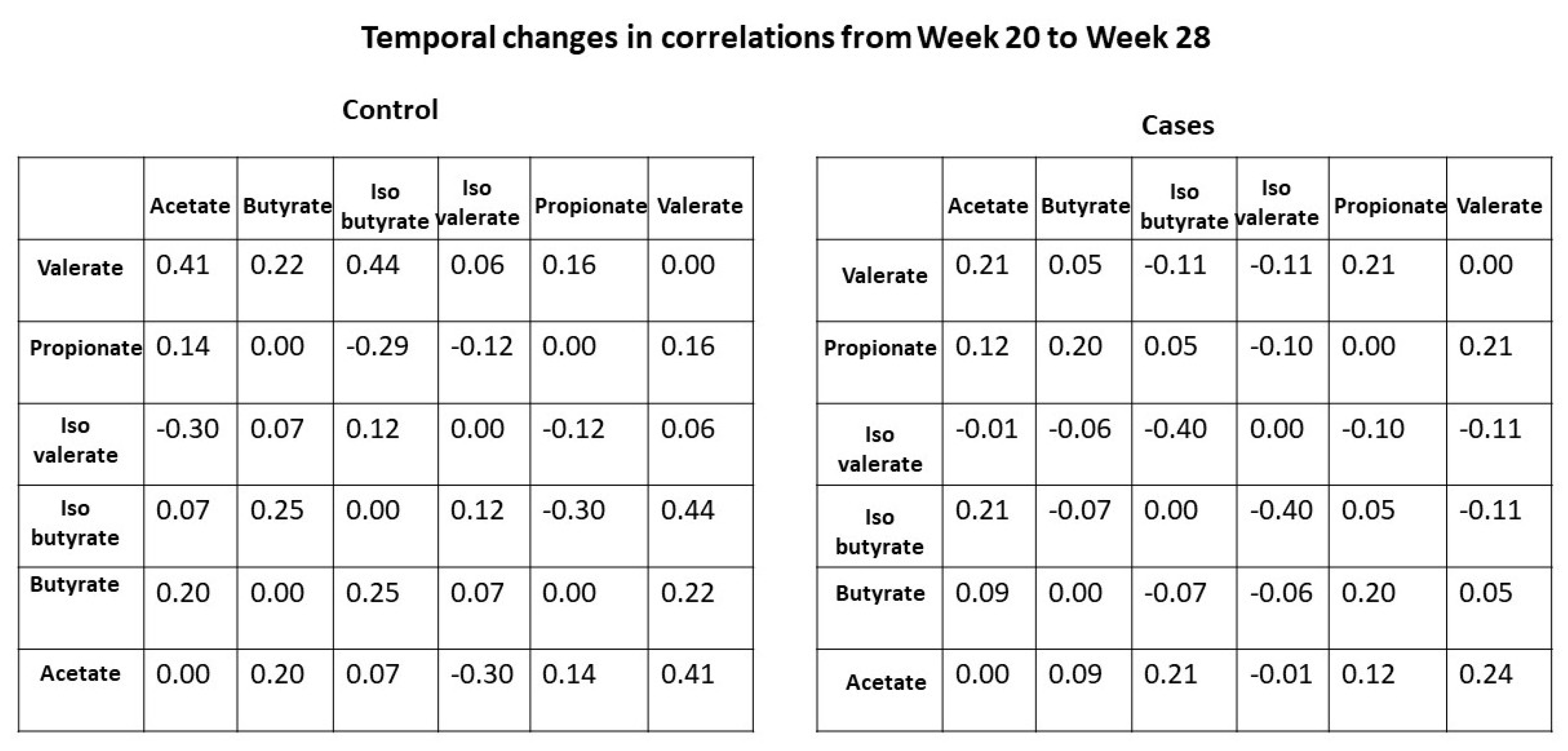
- Examining the temporal change (28 weeks–20 weeks) of each individual SCFA by disease status.
- (1)
- model without adjusting for confounders: SCFAs∼outcome;
- (2)
- model with centered log-ratio (CLR)-transformed SCFAs without adjusting for confounders: clr-SCFAs∼outcome.
3. Results
3.1. Primary Analysis
3.2. Secondary Analysis
3.3. Sensitivity Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCFA | Short-chain fatty acid |
| HDACs | Histone deacetylases |
| LOQ | Limit of quantitation |
Appendix A
| 20 Weeks | 28 Weeks | Total | |
|---|---|---|---|
| Acetate | |||
| Above LOQ | |||
| Below LOQ | |||
| Propionate | |||
| Above LOQ | 115 (93) | ||
| Below LOQ | |||
| Isobutyrate | |||
| Above LOQ | 121 (98) | 237 (94) | |
| Below LOQ | 11 (9) | ||
| Butyrate | |||
| Above LOQ | |||
| Below LOQ | |||
| Isovalerate | |||
| Above LOQ | |||
| Below LOQ | |||
| Valerate | |||
| Above LOQ | |||
| Below LOQ | |||
| Hexanoate | |||
| Above LOQ | 1 (1) | ||
| Below LOQ | 123 (99) | ||
References
- Serebrisky, D.; Wiznia, A. Pediatric asthma: A global epidemic. Ann. Glob. Health 2019, 85, 6. [Google Scholar] [CrossRef]
- Pearce, N.; Aït-Khaled, N.; Beasley, R.; Mallol, J.; Keil, U.; Mitchell, E.; Robertson, C. Worldwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007, 62, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Havstad, S.L.; Ownby, D.R.; Joseph, C.L.; Sitarik, A.R.; Myers, J.B.; Gebretsadik, T.; Hartert, T.V.; Hershey, G.K.K.; Jackson, D.J.; et al. Pediatric asthma incidence rates in the United States from 1980 to 2017. J. Allergy Clin. Immunol. 2021, 148, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Hacquard, S.; Garrido-Oter, R.; González, A.; Spaepen, S.; Ackermann, G.; Lebeis, S.; McHardy, A.C.; Dangl, J.L.; Knight, R.; Ley, R.; et al. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe 2015, 17, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Wang, Q.; Li, F.; Liang, B.; Liang, Y.; Chen, S.; Mo, X.; Ju, Y.; Zhao, H.; Jia, H.; Spector, T.D.; et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 2018, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, M.; Zhou, H.; Yang, Y.; Shen, S.; You, Y.; Xue, Z. The role of gut microbiome in the complex relationship between respiratory tract infection and asthma. Front. Microbiol. 2023, 14, 1219942. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Vuillermin, P.J.; O’Hely, M.; Collier, F.; Allen, K.J.; Tang, M.L.; Harrison, L.C.; Carlin, J.B.; Saffery, R.; Ranganathan, S.; Sly, P.D.; et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat. Commun. 2020, 11, 1452. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.A.; Kelly, R.S.; Lasky-Su, J.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Rifas-Shiman, S.L.; Carey, V.J.; et al. Fecal short-chain fatty acids in pregnancy and offspring asthma and allergic outcomes. J. Allergy Clin. Immunol. Pract. 2020, 8, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef]
- Caffarelli, C.; Gracci, S.; Giannì, G.; Bernardini, R. Are Babies Born Preterm High-Risk Asthma Candidates? J. Clin. Med. 2023, 12, 5400. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Silver, R.M.; Perkins, N.J.; Mumford, S.L.; Whitcomb, B.W.; Stanford, J.B.; Lesher, L.L.; Faraggi, D.; Wactawski-Wende, J.; Browne, R.W.; et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: Design and baseline characteristics. Paediatr. Perinat. Epidemiol. 2013, 27, 598–609. [Google Scholar] [CrossRef]
- Shaaban, M.; Shepelak, Z.D.; Stanford, J.B.; Silver, R.M.; Mumford, S.L.; Schisterman, E.F.; Hinkle, S.N.; Nkoy, F.L.; Theilen, L.; Page, J.; et al. Low-dose aspirin, maternal cardiometabolic health, and offspring respiratory health 9 to 14 years after delivery: Findings from the EAGeR Follow-up Study. Paediatr. Perinat. Epidemiol. 2024, 38, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.e.; Keil, U.; Anderson, H.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.; Pearce, N.; Sibbald, B.; Stewart, A.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Cait, A.; Hughes, M.; Antignano, F.; Cait, J.; Dimitriu, P.; Maas, K.; Reynolds, L.; Hacker, L.; Mohr, J.; Finlay, B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef] [PubMed]
| Control | Case | Total | |
|---|---|---|---|
| BMI | |||
| min | 15.71 | 15.81 | 15.71 |
| max | 45.9 | 42.89 | 45.9 |
| mean (sd) | 25.68 (5.77) | 25.93 (5.75) | 25.81 (5.74) |
| Age | |||
| min | 21.18 | 19.51 | 19.51 |
| max | 37.11 | 38.39 | 38.39 |
| mean (sd) | 25.68 (5.77) | 25.93 (5.75) | 25.81 (5.74) |
| Income (%) | |||
| Annual less than | 2 (3) | 2 (3) | 4 (3) |
| – | 13 (21) | 16 (25) | 29 (23) |
| – | 8 (13) | 7 (11) | 15 (12) |
| – | 10 (16) | 5 (8) | 15 (12) |
| 30 (48) | 34 (53) | 64 (50) | |
| First fetus sex (%) | |||
| Female | 29 (46) | 30 (47) | 59 (46) |
| Male | 34 (54) | 34 (53) | 68 (54) |
| Education (%) | |||
| Not high-school graduate | 1 (2) | 1 (2) | 2 (2) |
| High-school graduate | 3 (5) | 8 (12) | 11 (9) |
| >High school | 59 (94) | 55 (86) | 114 (90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Perkins, N.J.; Nkoy, F.; Stanford, J.B.; Schliep, K.C.; Peddada, S.D. A Study of Short-Chain Fatty Acids During the Canalicular and Early Saccular Phases of Fetal Lung Development and Childhood Asthma. Genes 2024, 15, 1595. https://doi.org/10.3390/genes15121595
Lin H, Perkins NJ, Nkoy F, Stanford JB, Schliep KC, Peddada SD. A Study of Short-Chain Fatty Acids During the Canalicular and Early Saccular Phases of Fetal Lung Development and Childhood Asthma. Genes. 2024; 15(12):1595. https://doi.org/10.3390/genes15121595
Chicago/Turabian StyleLin, Huang, Neil J. Perkins, Flory Nkoy, Joseph B. Stanford, Karen C. Schliep, and Shyamal D. Peddada. 2024. "A Study of Short-Chain Fatty Acids During the Canalicular and Early Saccular Phases of Fetal Lung Development and Childhood Asthma" Genes 15, no. 12: 1595. https://doi.org/10.3390/genes15121595
APA StyleLin, H., Perkins, N. J., Nkoy, F., Stanford, J. B., Schliep, K. C., & Peddada, S. D. (2024). A Study of Short-Chain Fatty Acids During the Canalicular and Early Saccular Phases of Fetal Lung Development and Childhood Asthma. Genes, 15(12), 1595. https://doi.org/10.3390/genes15121595







