Hearing Loss: Genetic Testing, Current Advances and the Situation in Latin America
Abstract
1. Introduction
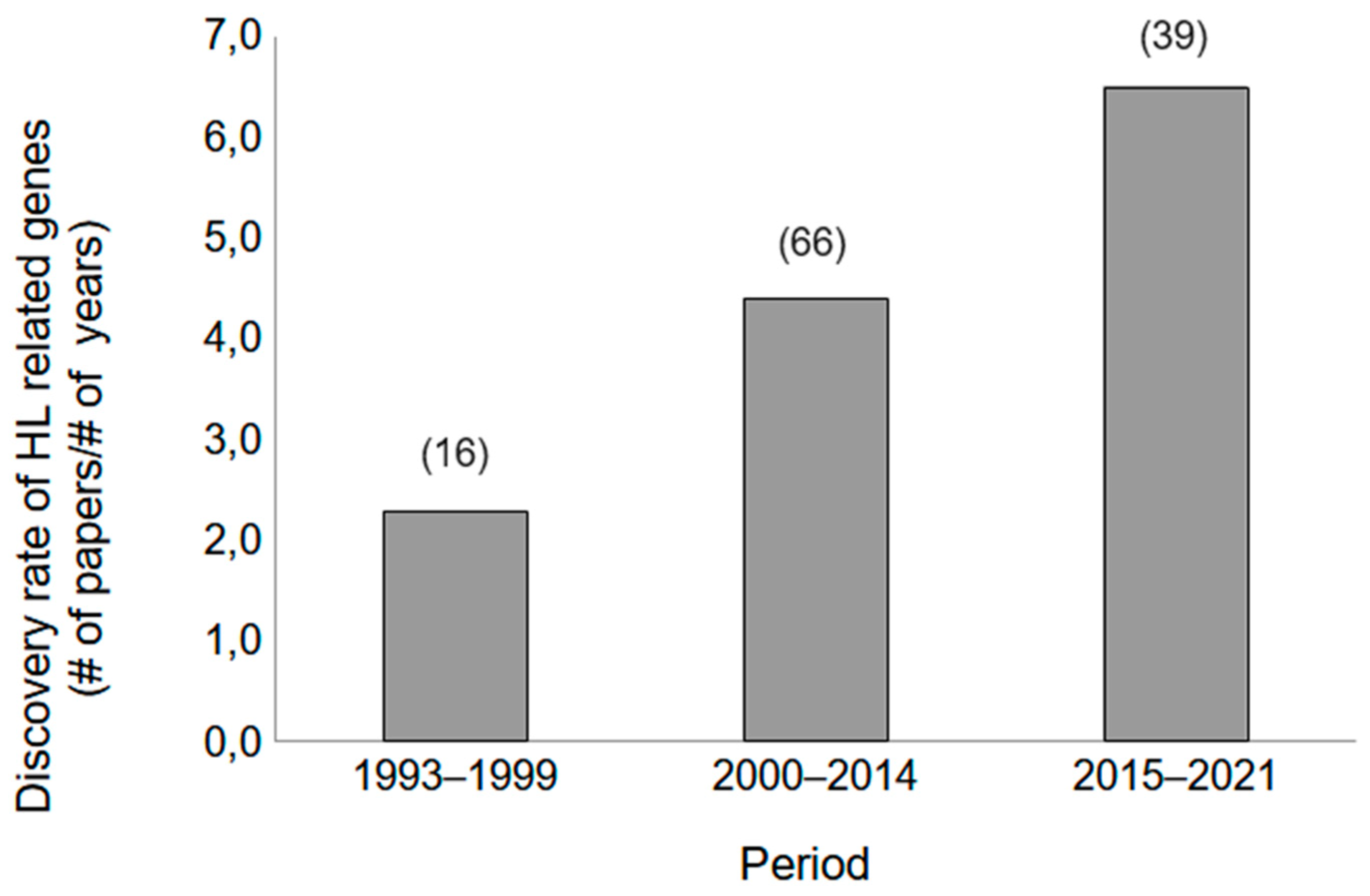
2. Genetic Discoveries Related to SNHL
3. Importance of Early Detection of Genetic Causes of Deafness
4. Individualized Prevention and Treatment for SNHL
5. Strengths and Barriers to Genetic Testing in HL
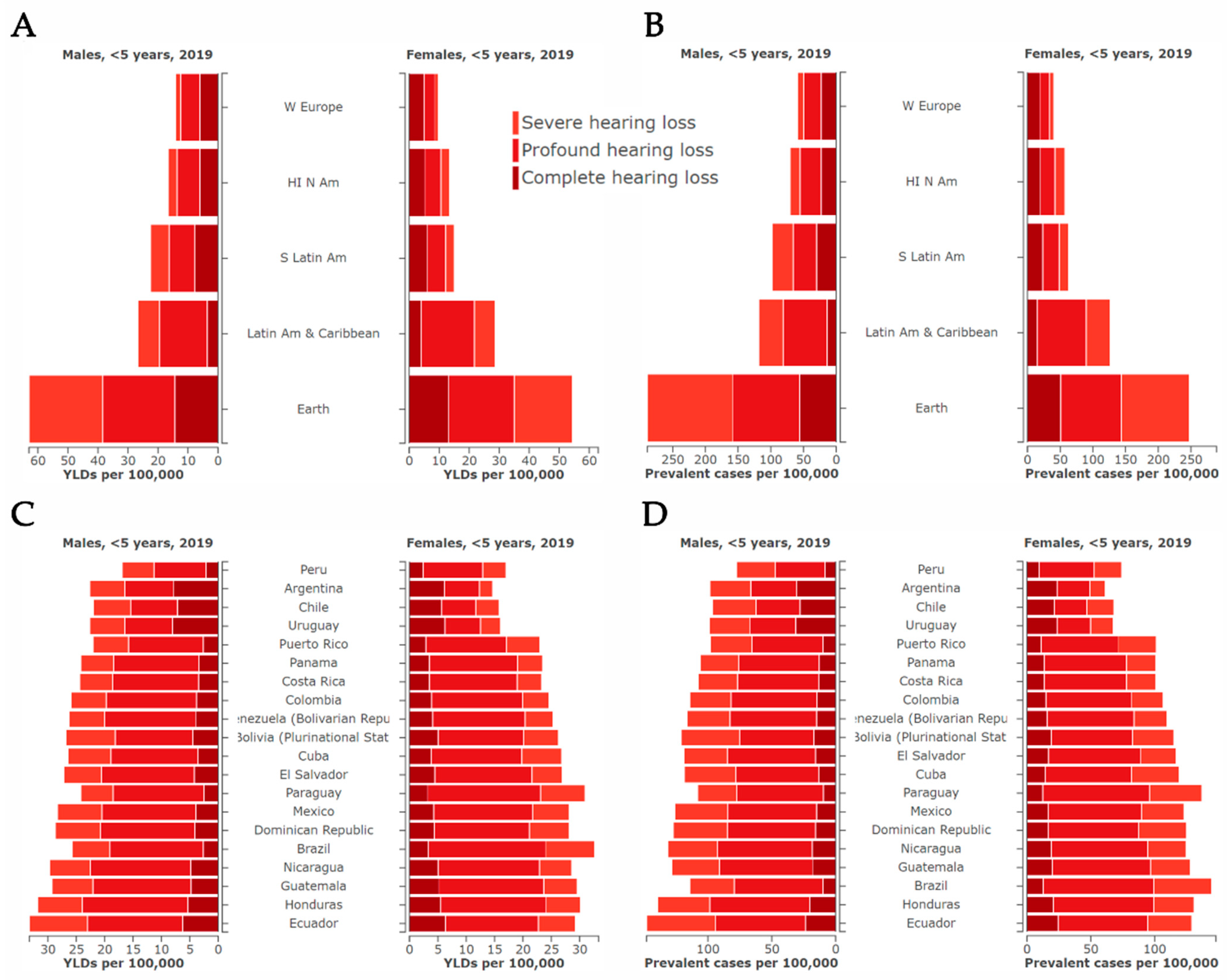
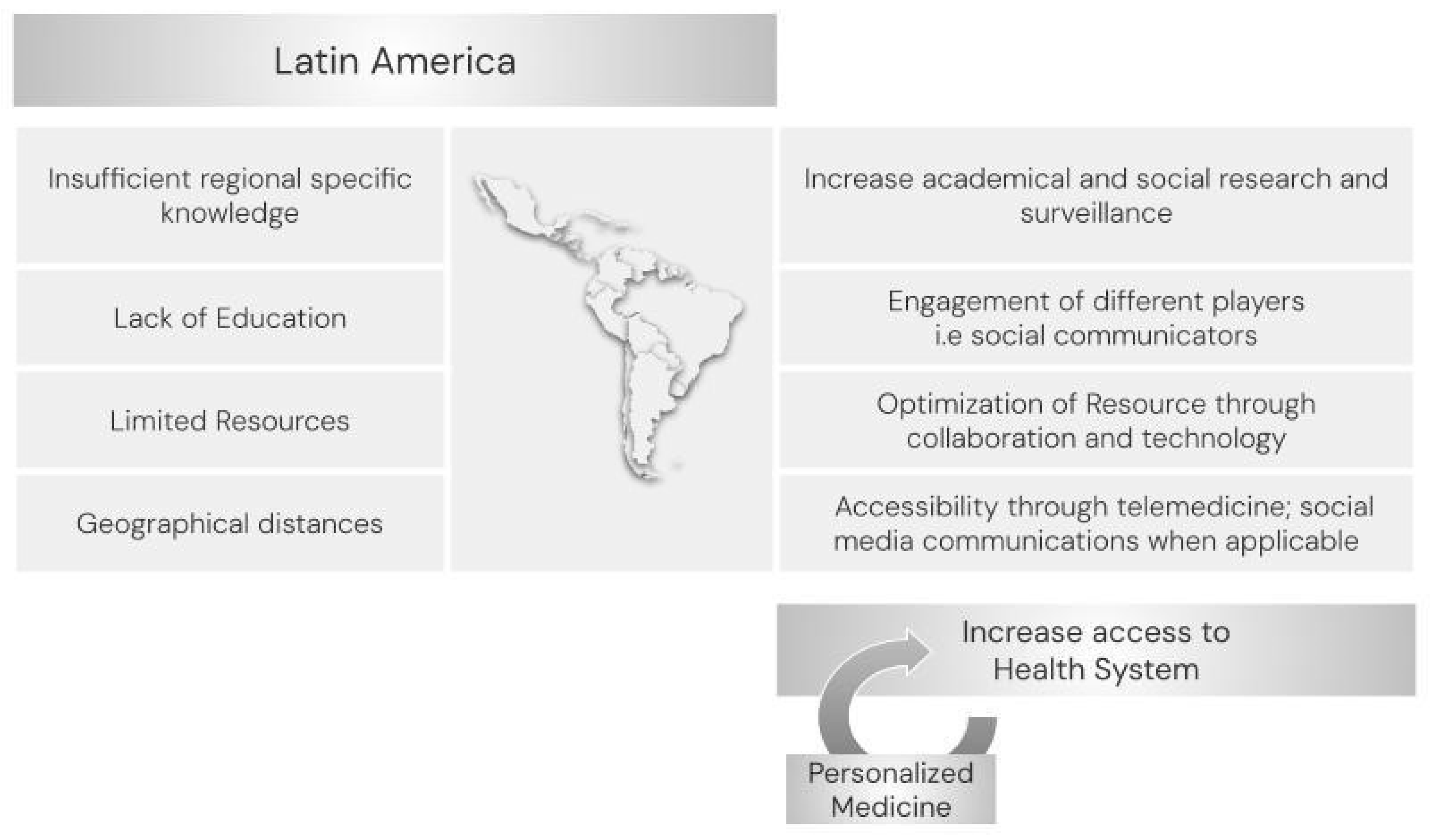
6. Current Situation in Latin America
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, C.C.; Nance, W.E. Newborn hearing screening—A silent revolution. N. Engl. J. Med. 2006, 354, 2151–2164. [Google Scholar] [CrossRef]
- Deafness and Hearing Loss|World Health Organization. Available online: https://www.who.int/health-topics/hearing-loss# (accessed on 10 December 2023).
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Report of the Informal Working Group on Prevention of Deafness and Hearing Impairment Programme Planning|World Health Organization. Available online: http://www.who.int/iris/handle/10665/58839 (accessed on 20 December 2023).
- Zahner, T. The Differential Diagnosis of Hearing Loss. Dtsch. Arztebl. Int. 2011, 108, 433–444. [Google Scholar]
- Marie Tharpe, A.M. Unilateral and mild bilateral hearing loss in children: Past and current perspectives. Trends Amplif. 2008, 12, 7–15. [Google Scholar] [CrossRef]
- Roizen, N.J. Nongenetic causes of hearing loss. Ment. Retard. Dev. Disabil. Res. Rev. 2003, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Van Beeck Calkoen, E.A.; Engel, M.S.D.; van de Kamp, J.M.; Yntema, H.G.; Goverts, S.T.; Mulder, M.F.; Merkus, P.; Hensen, E.F. The etiological evaluation of sensorineural hearing loss in children. Eur. J. Pediatr. 2019, 178, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.H.; Bale, J.F., Jr.; White, K.R. Sensorineural hearing loss in children. Lancet 2005, 365, 879–890. [Google Scholar] [CrossRef]
- Morton, N.E. Genetic epidemiology of hearing impairment. Ann. N. Y. Acad. Sci. 1991, 630, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, G.; Willems, P.J.; Smith, R.J. Nonsyndromic hearing impairment: Unparalleled heterogeneity. Am. J. Hum. Genet. 1997, 60, 758–764. [Google Scholar]
- Hildebrand, M.S.; Kahrizi, K.; Bromhead, C.J.; Shearer, A.E.; Webster, J.A.; Khodaei, H.; Abtahi, R.; Bazazzadegan, N.; Babanejad, M.; Nikzat, N.; et al. Mutations in TMC1 are a common cause of DFNB7/11 hearing loss in the Iranian population. Ann. Otol. Rhinol. Laryngol. 2010, 119, 830–835. [Google Scholar] [CrossRef]
- Prezant, T.R.; Agapian, J.V.; Bohlman, M.C.; Bu, X.; Öztas, S.; Qiu, W.-Q.; Arnos, K.S.; Cortopassi, G.A.; Jaber, L.; Rotter, J.I.; et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat. Genet. 1993, 4, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Torroni, A.; Cruciani, F.; Rengo, C.; Sellitto, D.; López-Bigas, N.; Rabionet, R.; Govea, N.; López de Munain, A.; Sarduy, M.; Romero, L.; et al. The A1555G mutation in the 12S rRNA gene of human mtDNA: Recurrent origins and founder events in families affected by sensorineural deafness. Am. J. Hum. Genet. 1999, 65, 1349–1358. [Google Scholar] [CrossRef]
- De Kok, Y.J.; Van der Maarel, S.M.; Bitner-Glindzicz, M.; Huber, I.; Monaco, A.P.; Malcolm, S.; Pembrey, M.E.; Ropers, H.H.; Cremers, F.P. Association between X-linked mixed deafness and mutations in the POU domain gene POU3F4. Science 1995, 267, 685–688. [Google Scholar] [CrossRef]
- Phippard, D.; Lu, L.; Lee, D.; Saunders, J.C.; Crenshaw, E.B. Targeted mutagenesis of the POU-domain gene Brn4/Pou3f4 causes developmental defects in the inner ear. J. Neurosci. 1999, 19, 5980–5989. [Google Scholar] [CrossRef]
- Parzefall, T.; Shivatzki, S.; Lenz, D.R.; Rathkolb, B.; Ushakov, K.; Karfunkel, D.; Shapira, Y.; Wolf, M.; Mohr, M.; Wolf, E.; et al. Cytoplasmic mislocalization of POU3F4 due to novel mutations leads to deafness in humans and mice. Hum. Mutat. 2013, 34, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, E.; Roesch, S.; Simoni, E.; Marino, A.; Rasp, G.; Astolfi, L.; Sarikas, A.; Dossena, S. Novel POU3F4 variants identified in patients with inner ear malformations exhibit aberrant cellular distribution and lack of SLC6A20 transcriptional upregulation. Front. Mol. Neurosci. 2022, 15, 999833. [Google Scholar] [CrossRef] [PubMed]
- Denoyelle, F.; Weil, D.; Maw, M.A.; Wilcox, S.A.; Lench, N.J.; Allen-Powell, D.R.; Osborn, A.H.; Dahl, H.-H.M.; Middleton, A.; Houseman, M.J.; et al. Prelingual deafness: High prevalence of a 30delG mutation in the connexin 26 gene. Hum. Mol. Genet. 1997, 6, 2173–2177. [Google Scholar] [CrossRef]
- Zelante, L.; Gasparini, P.; Estivill, X.; Melchionda, S.; D’Agruma, L.; Govea, N.; Milá, M.; Monica, M.D.; Lutfi, J.; Shohat, M.; et al. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum. Mol. Genet. 1997, 6, 1605–1609. [Google Scholar] [CrossRef]
- Del Castillo, I.; Moreno-Pelayo, M.A.; del Castillo, F.J.; Brownstein, Z.; Marlin, S.; Adina, Q.; Cockburn, D.J.; Pandya, A.; Siemering, K.R.; Chamberlin, G.P.; et al. Prevalence and evolutionary origins of the del(GJB6-D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: A multicenter study. Am. J. Hum. Genet. 2003, 73, 1452–1458. [Google Scholar] [CrossRef]
- Del Castillo, F.J.; Rodríguez-Ballesteros, M.; Alvarez, A.; Hutchin, T.; Leonardi, E.; De Oliveira, C.A.; Azaiez, H.; Brownstein, Z.; Avenarius, M.R.; Marlin, S.; et al. A novel deletion involving the connexin-30 gene, del(GJB6-D13S1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 non-syndromic hearing impairment. J. Med. Genet. 2005, 42, 588–594. [Google Scholar] [CrossRef]
- Wilch, E.; Zhu, M.; Burkhart, K.B.; Regier, M.; Elfenbein, J.L.; Fisher, R.A.; Friderici, K.H. Expression of GJB2 and GJB6 is reduced in a novel DFNB1 allele. Am. J. Hum. Genet. 2006, 79, 174–179. [Google Scholar] [CrossRef]
- Feldmann, D.; Le Maréchal, C.; Jonard, L.; Thierry, P.; Czajka, C.; Couderc, R.; Ferec, C.; Denoyelle, F.; Marlin, S.; Fellmann, F. A new large deletion in the DFNB1 locus causes nonsyndromic hearing loss. Eur. J. Med. Genet. 2009, 52, 195–200. [Google Scholar] [CrossRef]
- DiStefano, M.T.; Hemphill, S.E.; Oza, A.M.; Siegert, R.K.; Grant, A.R.; Hughes, M.Y.; Cushman, B.J.; Azaiez, H.; Booth, K.T.; Chapin, A.; et al. ClinGen expert clinical validity curation of 164 hearing loss gene-disease pairs. Genet. Med. 2019, 21, 2239–2247, Erratum in Genet. Med. 2019, 21, 2409. [Google Scholar] [CrossRef]
- Dbouk, H.A.; Mroue, R.M.; El-Sabban, M.E.; Talhouk, R.S. Connexins: A myriad of functions extending beyond assembly of gap junction channels. Cell Commun. Signal. 2009, 7, 4. [Google Scholar] [CrossRef]
- Keats, B.J.B.; Savas, S. Genetic heterogeneity in Usher syndrome. Am. J. Med. Genet. A 2004, 130, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Hereditary Hearing Loss Homepage. Available online: https://hereditaryhearingloss.org/ (accessed on 25 November 2023).
- Shearer, A.E.; Hildebrand, M.S.; Schaefer, A.M.; Smith, R.J. Genetic Hearing Loss Overview; Updated 28 September 2023; GeneReviews®: Seattle, WA, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1434/ (accessed on 27 January 2024).
- Genetic Testing Registry|National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/gtr/ (accessed on 22 December 2023).
- McDermott, J.H.; Molina-Ramírez, L.P.; Bruce, I.A.; Mahaveer, A.; Turner, M.; Miele, G.; Body, R.; Mahood, R.; Ulph, F.; MacLeod, R.; et al. Diagnosing and Preventing Hearing Loss in the Genomic Age. Trends Hear. 2019, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Tayoun, A.A.; DiStefano, M.; Pandya, A.; Rehm, H.L.; Robin, N.H.; Schaefer, A.M.; Yoshinaga-Itano, C. Clinical evaluation and etiologic diagnosis of hearing loss: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2022, 24, 1392–1406. [Google Scholar] [CrossRef]
- Sloan-Heggen, C.M.; Bierer, A.O.; Shearer, A.E.; Kolbe, D.L.; Nishimura, C.J.; Frees, K.L.; Ephraim, S.S.; Shibata, S.B.; Booth, K.T.; Campbell, C.A.; et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum. Genet. 2016, 135, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Hoefsloot, L.H.; Feenstra, I.; Kunst, H.P.M.; Kremer, H. Genotype phenotype correlations for hearing impairment: Approaches to management. Clin. Genet. 2014, 85, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Meyts, I.; Bosch, B.; Bolze, A.; Boisson, B.; Itan, Y.; Belkadi, A.; Pedergnana, V.; Moens, L.; Picard, C.; Cobat, A.; et al. Exome and genome sequencing for inborn errors of immunity. J. Allergy Clin. Immunol. 2016, 138, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, H.; Booth, K.T.; Ephraim, S.S.; Crone, B.; Black-Ziegelbein, E.A.; Marini, R.J.; Shearer, A.E.; Sloan-Heggen, C.M.; Kolbe, D.; Casavant, T.; et al. Genomic Landscape and Mutational Signatures of Deafness-Associated Genes. Am. J. Hum. Genet. 2018, 103, 484–497. [Google Scholar] [CrossRef]
- Oza, A.M.; DiStefano, M.T.; Hemphill, S.E.; Cushman, B.J.; Grant, A.R.; Siegert, R.K.; Shen, J.; Chapin, A.; Boczek, N.J.; Schimmenti, L.A.; et al. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum. Mutat. 2018, 39, 1593–1613. [Google Scholar] [CrossRef]
- Rouse, S.L.; Florentine, M.M.; Taketa, E.; Chan, D.K. Racial and ethnic disparities in genetic testing for hearing loss: A systematic review and synthesis. Hum. Genet. 2022, 141, 485–494. [Google Scholar] [CrossRef]
- McDaid, D.; Park, A.-L.; Chadha, S. Estimating the global costs of hearing loss. Int. J. Audiol. 2021, 60, 162–170. [Google Scholar] [CrossRef]
- Young, N.M.; Reilly, B.K.; Burke, L. Limitations of universal newborn hearing screening in early identification of pediatric cochlear implant candidates. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 230–234. [Google Scholar] [CrossRef]
- Dai, P.; Huang, L.H.; Wang, G.J.; Gao, X.; Qu, C.Y.; Chen, X.W.; Ma, F.R.; Zhang, J.; Xing, W.L.; Xi, S.Y.; et al. Concurrent Hearing and Genetic Screening of 180,469 Neonates with Follow-up in Beijing, China. Am. J. Hum. Genet. 2019, 105, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Fischer, C.; Tran, K.; Rachakonda, T.; Kallogjeri, D.; Lieu, J.E.C. Quality of Life in Children with Hearing Impairment: Systematic Review and Meta-analysis. Arch. Otolaryngol. Head Neck Surg. 2016, 155, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Leroux-Roels, G.; Ofori-Anyinam, O.; Moris, P.; De Kock, E.; Clement, F.; Dubois, M.-C.; Koutsoukos, M.; Demoitié, M.-A.; Cohen, J.; et al. Evaluation of the safety and immunogenicity of two antigen concentrations of the Mtb72F/AS02(A) candidate tuberculosis vaccine in purified protein derivative-negative adults. Clin. Vaccine Immunol. 2010, 17, 1763–1771. [Google Scholar] [CrossRef]
- Laszig, R.; Aschendorff, A.; Beck, R.; Schild, C.; Kröger, S.; Wesarg, T.; Arndt, S. Long-term functional outcomes of cochlear implants in children. HNO 2009, 57, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Remjasz-Jurek, A.; Clarós, P.; Clarós-Pujol, A.; Pujol, C.; Clarós, A. Outcomes of cochlear implantation in children with Usher syndrome: A long-term observation. Eur. Arch. Otorhinolaryngol. 2023, 280, 2119–2132. [Google Scholar] [CrossRef]
- Lyu, J.; Kong, Y.; Xu, T.-Q.; Dong, R.-J.; Qi, B.-E.; Wang, S.; Li, Y.-X.; Liu, H.-H.; Chen, X.-Q. Long-term follow-up of auditory performance and speech perception and effects of age on cochlear implantation in children with pre-lingual deafness. Chin. Med. J. 2019, 132, 1925–1934. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, J.; Du, H.; Geng, L.; Li, A.; Zhao, N.; Xu, Y.; Liu, X.; Qian, X.; Gao, X. Influence of cochlear implants on hearing-related quality of life: Results from Chinese children with cochlear implants entering mainstream education. Int. J. Pediatr. Otorhinolaryngol. 2022, 160, 111228. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglio, P.; Bruque, C.D.; Luce, L.; Giliberto, F.; Lotersztein, V.; Menazzi, S.; Paoli, B.; Elgoyhen, A.B.; Dalamón, V. GJB2 and GJB6 Genetic Variant Curation in an Argentinean Non-Syndromic Hearing-Impaired Cohort. Genes 2020, 11, 1233. [Google Scholar] [CrossRef]
- Smith, R.J.H.; Azaiez, H.; Booth, K. GJB2-Related Autosomal Recessive Nonsyndromic Hearing Loss; Updated 20 July 2023; GeneReviews®: Seattle, WA, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1272/ (accessed on 27 January 2024).
- Mitchell, C.O.; Morton, C.C. Genetics of Childhood Hearing Loss. Otolaryngol. Clin. N. Am. 2021, 54, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Chari, D.A.; Chan, D.K. Diagnosis and Treatment of Congenital Sensorineural Hearing Loss. Curr. Otorhinolaryngol. Rep. 2017, 5, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, G.S.; Phillips, K.A. Precision Medicine: From Science To Value. Health Aff. 2018, 37, 694–701. [Google Scholar] [CrossRef]
- Garofalo, D.C.; Rosenblum, H.A.; Zhang, Y.; Chen, Y.; Appelbaum, P.S.; Sabatello, M. Increasing inclusivity in precision medicine research: Views of deaf and hard of hearing individuals. Genet. Med. 2022, 24, 712–721. [Google Scholar] [CrossRef]
- Doo-Yi, O.; Byung, Y.C. Genetic Information and Precision Medicine in Hearing Loss. Clin. Exp. Otorhinolaryngol. 2020, 13, 315–317. [Google Scholar]
- Rudman, J.R.; Mei, C.; Bressler, S.E.; Blanton, S.H.; Liu, X.-Z. Precision medicine in hearing loss. J. Genet. Genom. 2018, 45, 99–109. [Google Scholar] [CrossRef]
- Kenna, M.A. Genetic testing for pediatric hearing loss: No time to waste. Hum. Genet. 2022, 141, 315–317. [Google Scholar] [CrossRef]
- Prayle, A.; Smyth, A.R. Aminoglycoside use in cystic fibrosis: Therapeutic strategies and toxicity. Curr. Opin. Pulm. Med. 2010, 16, 604–610. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.H.; Mahood, R.; Stoddard, D.; Mahaveer, A.; Turner, M.A.; Corry, R.; Garlick, J.; Miele, G.; Ainsworth, S.; Kemp, L.; et al. Pharmacogenetics to Avoid Loss of Hearing (PALOH) trial: A protocol for a prospective observational implementation trial. BMJ Open 2021, 11, e044457. [Google Scholar] [CrossRef] [PubMed]
- Omichi, R.; Shibata, S.B.; Morton, C.C.; Smith, R.J.H. Gene therapy for hearing loss. Hum. Mol. Genet. 2019, 28, R65–R79. [Google Scholar] [CrossRef]
- Shibata, S.B.; Ranum, P.T.; Moteki, H.; Pan, B.; Goodwin, A.T.; Goodman, S.S.; Abbas, P.J.; Holt, J.R.; Smith, R.J.H. RNA Interference Prevents Autosomal-Dominant Hearing Loss. Am. J. Hum. Genet. 2016, 98, 1101–1113. [Google Scholar] [CrossRef]
- Yoshimura, H.; Shibata, S.B.; Ranum, P.T.; Moteki, H.; Smith, R.J.H. Targeted Allele Suppression Prevents Progressive Hearing Loss in the Mature Murine Model of Human TMC1 Deafness. Mol. Ther. 2019, 27, 681–690. [Google Scholar] [CrossRef]
- Maeda, Y.; Fukushima, K. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum. Mol. Genet. Hum. Mol. Genet. 2005, 14, 1641–1650. [Google Scholar] [CrossRef]
- Yin, G.; Wang, X.H.; Sun, Y. Recent advances in CRISPR-Cas system for the treatment of genetic hearing loss. Am. J. Stem Cells 2023, 12, 37–50. [Google Scholar]
- Gao, X.; Tao, Y.; Lamas, V.; Huang, M.; Yeh, W.-H.; Pan, B.; Hu, Y.-J.; Hu, J.H.; Thompson, D.B.; Shu, Y.; et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature 2018, 553, 217–221. [Google Scholar] [CrossRef]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Cradick, J.T.; Fine, E.J.; Antico, C.J.; Bao, G. CRISPR/Cas9 systems targeting β-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 2013, 41, 9584–9592. [Google Scholar] [CrossRef]
- Tao, Y.; Lamas, V.; Du, W.; Zhu, W.; Li, Y.; Whittaker, M.N.; Zuris, J.A.; Thompson, D.B.; Rameshbabu, A.P.; Shu, Y.; et al. Treatment of monogenic and digenic dominant genetic hearing loss by CRISPR-Cas9 ribonucleoprotein delivery in vivo. Nat. Commun. 2023, 14, 4928. [Google Scholar] [CrossRef]
- Amariutei, A.E.; Jeng, J.-Y.; Safieddine, S.; Marcotti, W. Recent advances and future challenges in gene therapy for hearing loss. R. Soc. Open Sci. 2023, 10, 230644. [Google Scholar] [CrossRef]
- Develop Innovative Therapeutic Solutions for Inner Ear Disorders|Sensorion. Available online: https://www.sensorion.com/en/our-approach/restore-treat-prevent/ (accessed on 18 November 2023).
- Gene Therapy Improves Auditory Response for Child with Profound Genetic Hearing Loss|Contemporary Pediatrics. Available online: https://www.contemporarypediatrics.com/view/gene-therapy-improves-auditory-response-for-child-with-profound-genetic-hearing-loss (accessed on 19 November 2023).
- Terry Sharrer, G. Personalized Medicine: Ethical Aspects. Methods Mol. Biol. 2017, 1606, 37–50. [Google Scholar]
- Cordeiro, J.V. Ethical and legal challenges of personalized medicine: Paradigmatic examples of research, prevention, diagnosis and treatment. Rev. Port. Saúde Pública 2014, 32, 164–180. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, A.P.; Nguyen, D.; Pan, D.R.; Jhaveri, V.M.; Rudman, J.R.; Dharmaraja, A.; Yan, D.; Feng, Y.; Chapagain, P.; et al. Genetic basis of hearing loss in Spanish, Hispanic and Latino populations. Gene 2018, 647, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Hearing Loss Is on the Rise|World Health Organization. Available online: https://www.who.int/docs/default-source/documents/world-hearing-day-2018-infographic.pdf?sfvrsn=54ccef8d_12 (accessed on 15 December 2023).
- Población con Discapacidad|Instituto Nacional de Estadística y Censos. Available online: https://www.indec.gob.ar/indec/web/Nivel4-Tema-2-21-143 (accessed on 17 December 2023).
- Estudio Nacional Sobre el Perfil de las Personas con Discapacidad|Instituto Nacional de Estadística y Censos. Available online: https://www.indec.gob.ar/ftp/cuadros/poblacion/estudio_discapacidad_12_18.pdf (accessed on 7 December 2023).
- Cordeiro-SilvaI, M.D.F.; Barbosa, A. Prevalence of 35delG/GJB2 and del (GJB6-D13S1830) mutations in patients with non-syndromic deafness from a population of Espírito Santo—Brazil. Braz. J. Otorhinolaryngol. 2010, 76, 428–432. [Google Scholar]
- Schüffner, R.d.O.A.; Nascimento, K.L.; Dias, F.A.; Silva, P.H.T.d.; Pires, W.G.B.; Cipriano Junior, N.M.; Santos, L.L. Molecular study of hearing loss in Minas Gerais, Brazil. Braz. J. Otorhinolaryngol. 2020, 86, 327–331. [Google Scholar] [CrossRef] [PubMed]
- En Colombia, Menos del 7% de los Niños Acceden al Tamizaje Auditivo Neonatal|Sociedad Colombiana de Pediatría. Available online: https://scp.com.co/en-colombia-menos-del-7-de-los-ninos-acceden-al-tamizaje-auditivo-neonatal/ (accessed on 17 December 2023).
- Caracterización de Ciudadanos, Usuarios y Grupos de Interés|Instituto Nacional Para Sordos. Available online: https://www.insor.gov.co/home/descargar/Caracterizacio%CC%81n-Ciudadanos-2020.pdf (accessed on 17 December 2023).
- CENSO Nacional de Población y Vivienda 2018|Gov.Co. Available online: https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/censo-nacional-de-poblacion-y-vivenda-2018 (accessed on 16 December 2023).
- Socorro Peña, A.; Contreras-Rivas, A.I. Prevalencia de hipoacusia en recién nacidos sanos en un hospital de tercer nivel de atención. Detección mediante tamiz auditivo neonatal. Rev. Mex. Pediatr. 2018, 85, 130–134. [Google Scholar]
- Madriz, J.J. Hearing Impairment in Latin America: An Inventory of Limited Options and Resources. Audiology 2000, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Gerner de Garcia, B.; Gaffney, C.; Chacon, S.; Gaffney, M. Overview of newborn hearing screening activities in Latin America. Rev. Panam. Salud Publica 2011, 29, 145–152. [Google Scholar]
- Neumann, K.; Euler, H.A. A Survey on the Global Status of Newborn and Infant Hearing Screening. J. Early Hear. Detect. Interv. 2020, 5, 63–84. [Google Scholar]
- World Hearing Day|World Health Organization. Available online: https://cdn.who.int/media/docs/default-source/documents/health-topics/deafness-and-hearing-loss/world-hearing-day-2021-activity-report.pdf?sfvrsn=5710dabd_5&download=true (accessed on 17 December 2023).
- Lezirovitz, K.; Mingroni-Netto, R.C. Genetic etiology of non-syndromic hearing loss in Latin America. Hum. Genet. 2022, 141, 539–581. [Google Scholar] [CrossRef] [PubMed]
- Estivill, X.; Fortina, P.; Surrey, S.; Rabionet, R.; Melchionda, S.; D’Agruma, L.; Mansfield, E.; Rappaport, E.; Govea, N.; Milà, M.; et al. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet 1998, 351, 394–398. [Google Scholar] [CrossRef]
- Gasparini, P.; Rabionet, R.; Barbujani, G.; Melchionda, S.; Petersen, M.; Brøndum-Nielsen, K.; Metspalu, A.; Oitmaa, E.; Pisano, M.; Fortina, P.; et al. High carrier frequency of the 35delG deafness mutation in European populations. Genetic Analysis Consortium of GJB2 35delG. Eur. J. Hum. Genet. 2000, 8, 19–23. [Google Scholar] [CrossRef]
- Tekin, M.; Arnos, K.S.; Pandya, A. Advances in hereditary deafness. Lancet 2001, 358, 1082–1090. [Google Scholar] [CrossRef]
- Van Laer, L. A common founder for the 35delG GJB2 gene mutation in connexin 26 hearing impairment. J. Med. Genet. 2001, 38, 515–518. [Google Scholar] [CrossRef]
- Gravina, L.P.; Foncuberta, M.E.; Estrada, R.C.; Barreiro, C.; Chertkoff, L. Carrier frequency of the 35delG and A1555G deafness mutations in the Argentinean population. Impact on the newborn hearing screening. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Gravina, L.P.; Foncuberta, M.E.; Prieto, M.E.; Garrido, J.; Barreiro, C.; Chertkoff, L. Prevalence of DFNB1 mutations in Argentinean children with non-syndromic deafness. Report of a novel mutation in GJB2. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 250–254. [Google Scholar] [CrossRef]
- Dalamón, V.; Béhèran, A.; Diamante, F.; Pallares, N.; Diamante, V.; Elgoyhen, A.B. Prevalence of GJB2 mutations and the del(GJB6-D13S1830) in Argentinean non-syndromic deaf patients. Hear. Res. 2005, 207, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, P.; Gasparini, P.; Estivill, X.; Volpini, V.; Castellvi-Bel, S.; Govea, N.; Mila, M.; Della Monica, M.; Ventruto, V.; De Benedetto, M.; et al. Linkage of DFNB1 to non-syndromic neurosensory autosomal-recessive deafness in Mediterranean families. Eur. J. Hum. Genet. 1997, 5, 83–88. [Google Scholar] [PubMed]
- Sartorato, E.L.; Gottardi, E.; De Oliveira, C.A.; Magna, L.A.; Annichino-Bizzacchi, J.M.; Seixas, C.A.; Maciel-Guerra, A.T. Determination of the frequency of the 35delG allele in Brazilian neonates. Clin. Genet. 2000, 58, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Batissoco, A.C.; Abreu-Silva, R.S.; Braga, M.C.C.; Lezirovitz, K.; Della-Rosa, V.; Alfredo, T.; Otto, P.A.; Mingroni-Netto, R.C. Prevalence of GJB2 (connexin-26) and GJB6 (connexin-30) mutations in a cohort of 300 Brazilian hearing-impaired individuals: Implications for diagnosis and genetic counseling. Ear Hear. 2009, 30, 1–7. [Google Scholar] [CrossRef]
- Dalamón, V.; Lotersztein, V.; Béhèran, A.; Lipovsek, M.; Diamante, F.; Pallares, N.; Francipane, L.; Frechtel, G.; Paoli, B.; Mansilla, E.; et al. GJB2 and GJB6 genes: Molecular study and identification of novel GJB2 mutations in the hearing-impaired Argentinean population. Audiol. Neurootol. 2010, 15, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Silva, R.S.; Lezirovitz, K.; Braga, M.C.C.; Spinelli, M.; Pirana, S.; Della-Rosa, V.A.; Otto, P.A.; Mingroni-Netto, R.C. Prevalence of the A1555G (12S rRNA) and tRNASer(UCN) mitochondrial mutations in hearing-impaired Brazilian patients. Braz. J. Med. Biol. Res. 2006, 39, 219–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bezerra Salomão, K.; Salomão, K.; Ayo, C. Investigation of the A1555G mutation in mitochondrial DNA (MT-RNR1) in groups of Brazilian individuals with nonsyndromic deafness and normal-hearing. Indian J. Hum. Genet. 2013, 19, 54–57. [Google Scholar]
- Batissoco, A.C.; Pedroso-Campos, V.; Pardono, E.; Sampaio-Silva, J.; Sonoda, C.Y.; Vieira-Silva, G.A.; da Silva de Oliveira Longati, E.U.; Mariano, D.; Hoshino, A.C.H.; Tsuji, R.K.; et al. Molecular and genetic characterization of a large Brazilian cohort presenting hearing loss. Hum. Genet. 2022, 141, 519–538. [Google Scholar] [CrossRef]
- Lezirovitz, K.; Pardono, E.; de Mello Auricchio, M.T.B.; de Carvalho e Silva, F.L.; Lopes, J.J.; Abreu-Silva, R.S.; Romanos, J.; Batissoco, A.C.; Mingroni-Netto, R.C. Unexpected genetic heterogeneity in a large consanguineous Brazilian pedigree presenting deafness. Eur. J. Hum. Genet. 2008, 16, 89–96. [Google Scholar] [CrossRef]
- Lezirovitz, K.; Nicastro, F.S.; Pardono, E.; Abreu-Silva, R.S.; Batissoco, A.C.; Neustein, I.; Spinelli, M.; Mingroni-Netto, R.C. Is autosomal recessive deafness associated with oculocutaneous albinism a “coincidence syndrome”. J. Hum. Genet. 2006, 51, 716–720. [Google Scholar] [CrossRef][Green Version]
- Dantas, V.G.L.; Raval, M.H.; Ballesteros, A.; Cui, R.; Gunther, L.K.; Yamamoto, G.L.; Alves, L.U.; Bueno, A.S.; Lezirovitz, K.; Pirana, S.; et al. Characterization of a novel MYO3A missense mutation associated with a dominant form of late onset hearing loss. Sci. Rep. 2018, 8, 8706. [Google Scholar] [CrossRef]
- Bueno, A.S.; Nunes, K.; Dias, A.M.M.; Alves, L.U.; Mendes, B.C.A.; Sampaio-Silva, J.; Smits, J.; Yntema, H.G.; Meyer, D.; Lezirovitz, K.; et al. Frequency and origin of the c.2090T>G p.(Leu697Trp) MYO3A variant associated with autosomal dominant hearing loss. Eur. J. Hum. Genet. 2022, 30, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Silva, R.; Dantas, V.L.G.; Alves, L.U.; Batissoco, A.C.; Oiticica, J.; Lawrence, E.A.; Kawafi, A.; Yang, Y.; Nicastro, F.S.; Novaes, B.C.; et al. NCOA3 identified as a new candidate to explain autosomal dominant progressive hearing loss. Hum. Mol. Genet. 2021, 29, 3691–3705. [Google Scholar] [CrossRef] [PubMed]
- World Report on Hearing|World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/339956/9789240021570-eng.pdf?sequence= (accessed on 18 December 2023).
- Mitropoulos, K.; Cooper, D.N.; Mitropoulou, C.; Agathos, S.; Reichardt, J.K.V.; Al-Maskari, F.; Chantratita, W.; Wonkam, A.; Dandara, C.; Katsila, T.; et al. Genomic Medicine without Borders: Which Strategies Should Developing Countries Employ to Invest in Precision Medicine? A New “Fast-Second Winner” Strategy. OMICS A J. Integr. Biol. 2017, 21, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Peart, L.S.; Gonzalez, J.; Morel Swols, D.; Duman, D.; Saridogan, T.; Ramzan, M.; Zafeer, M.F.; Liu, X.Z.; Eshraghi, A.A.; Hoffer, M.E.; et al. Dispersed DNA variants underlie hearing loss in South Florida’s minority population. Hum. Genom. 2023, 17, 103. [Google Scholar] [CrossRef]
- Florentine, M.M.; Rouse, S.L.; Stephans, J.; Conrad, D.; Czechowicz, J.; Matthews, I.R.; Meyer, A.K.; Nadaraja, G.S.; Parikh, R.; Virbalas, J.; et al. Racial and ethnic disparities in diagnostic efficacy of comprehensive genetic testing for sensorineural hearing loss. Hum. Genet. 2022, 141, 495–504. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rosa, M.A.; Bernardi, M.T.; Kleppe, S.; Walz, K. Hearing Loss: Genetic Testing, Current Advances and the Situation in Latin America. Genes 2024, 15, 178. https://doi.org/10.3390/genes15020178
De Rosa MA, Bernardi MT, Kleppe S, Walz K. Hearing Loss: Genetic Testing, Current Advances and the Situation in Latin America. Genes. 2024; 15(2):178. https://doi.org/10.3390/genes15020178
Chicago/Turabian StyleDe Rosa, Maria Agustina, Maria T. Bernardi, Soledad Kleppe, and Katherina Walz. 2024. "Hearing Loss: Genetic Testing, Current Advances and the Situation in Latin America" Genes 15, no. 2: 178. https://doi.org/10.3390/genes15020178
APA StyleDe Rosa, M. A., Bernardi, M. T., Kleppe, S., & Walz, K. (2024). Hearing Loss: Genetic Testing, Current Advances and the Situation in Latin America. Genes, 15(2), 178. https://doi.org/10.3390/genes15020178




