Effects of Soil Rhizobia Abundance on Interactions between a Vector, Pathogen, and Legume Plant Host
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Experimental Design and Data Collection
2.3. Data Analysis
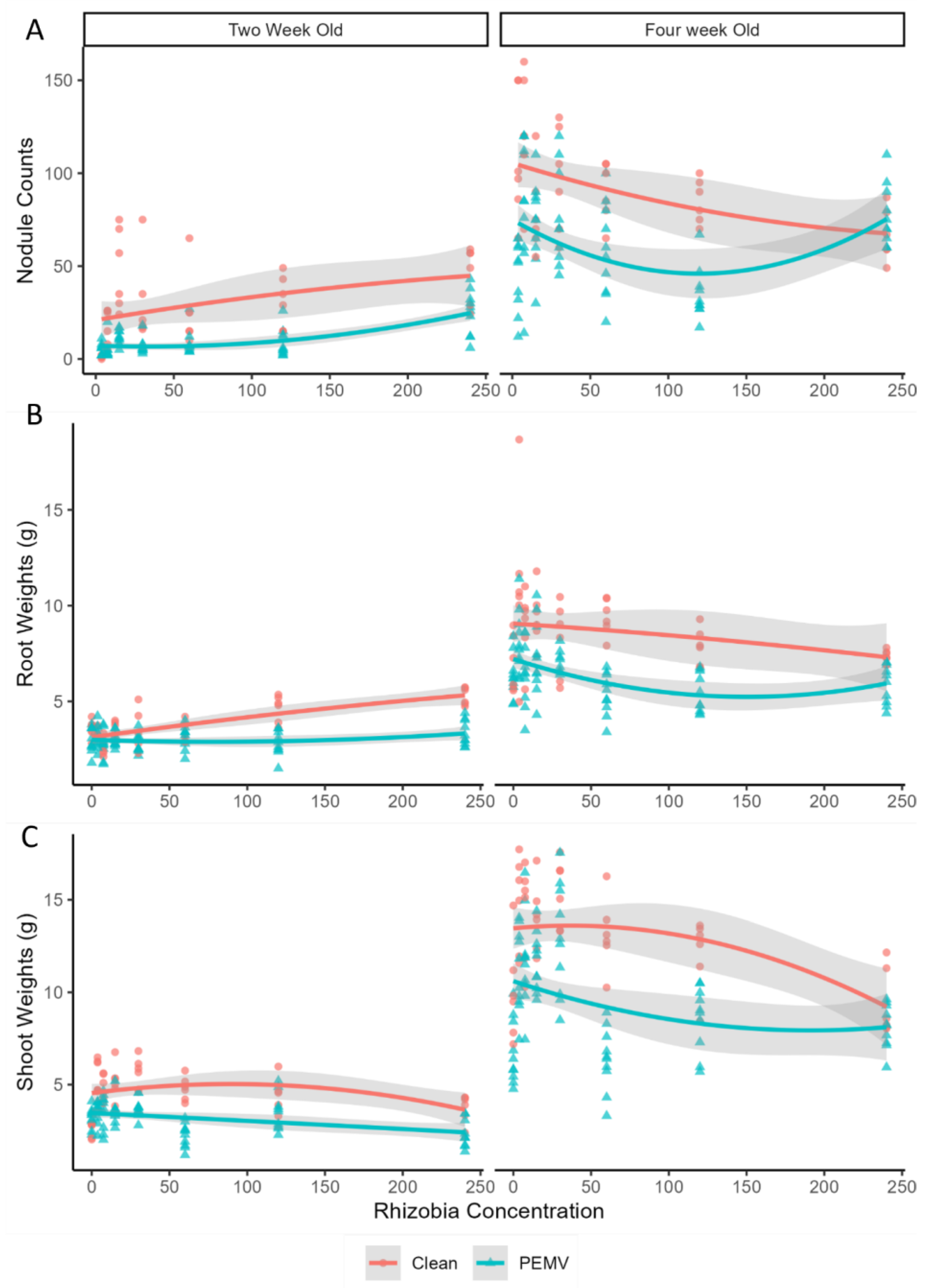
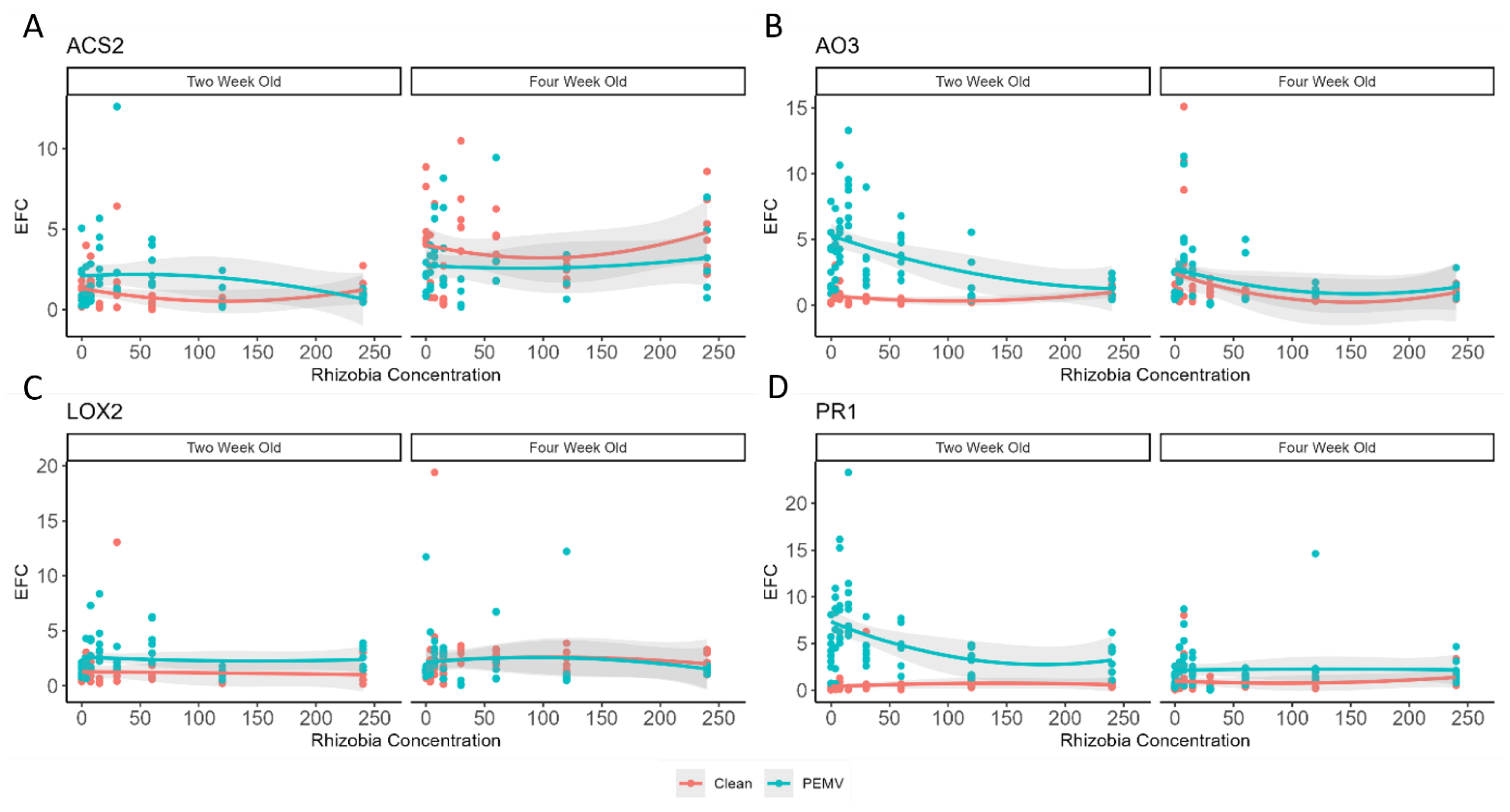
3. Results
3.1. Effects of Rhizobia Abundance and PEMV Infection on Plant Growth Traits
3.2. Effects of Rhizobia Abundance on PEMV Accumulation
3.3. Effects of Rhizobia Abundance and PEMV Infection on Defense Gene Expression in Peas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vander Heijden, M.G.A.; Bardgett, R.D.; Straalen, N.M.V. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.R.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130116. [Google Scholar] [CrossRef]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef]
- Vannette, R.L.; Hunter, M.D. Plant defence theory re-examined: Nonlinear expectations based on the costs and benefits of resource mutualisms. J. Ecol. 2011, 99, 66–76. [Google Scholar] [CrossRef]
- Gano-Cohen, K.A.; Wendlandt, C.E.; Al Moussawi, K.; Stokes, P.J.; Quides, K.W.; Weisberg, A.J.; Chang, J.H.; Sachs, J.L. Recurrent mutualism breakdown events in a legume rhizobia metapopulation. Proc. R. Soc. B 2020, 287, 20192549. [Google Scholar] [CrossRef]
- Brockwell, J.; Bottomley, P.J.; Thies, J.E. Manipulation of rhizobia microflora for improving legume productivity and soil fertility: A critical assessment. Plant Soil 1995, 174, 143–180. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Kumar, H.; Dubey, R.C.; Maheshwari, D.K. Effect of plant growth promoting rhizobia on seed germination, growth promotion and suppression of Fusarium wilt of fenugreek (Trigonella foenum-graecum L.). Crop Prot. 2011, 30, 396–403. [Google Scholar] [CrossRef]
- Basu, S.; Clark, R.E.; Bera, S.; Casteel, C.L.; Crowder, D.W. Plant responses to multiple antagonists are mediated by order of attack and phytohormone crosstalk. Mol. Ecol. 2021, 30, 4939–4948. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Muñoz, N.; Lascano, H.R.; Izaguirre-Mayoral, M.L. The seed-borne Southern bean mosaic virus hinders the early events of nodulation and growth in Rhizobium-inoculated Phaseolus vulgaris L. Funct. Plant Biol. 2017, 44, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, J.L. Conditional outcomes in mutualistic interactions. Trends Ecol. Evol. 1994, 9, 214–217. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Bronstein, J.L. The exploitation of mutualisms. Ecol. Lett. 2001, 4, 277–287. [Google Scholar] [CrossRef]
- Neuhauser, C.; Fargione, J.E. A mutualism–parasitism continuum model and its application to plant–mycorrhizae interactions. Ecol. Model. 2004, 177, 337–352. [Google Scholar] [CrossRef]
- Díaz-Valle, A.; López-Calleja, A.C.; Alvarez-Venegas, R. Enhancement of pathogen resistance in common bean plants by inoculation with rhizobium etli. Front. Plant Sci. 2019, 10, 1317. [Google Scholar] [CrossRef]
- Smigielski, L.; Laubach, E.M.; Pesch, L.; Glock, J.M.L.; Albrecht, F.; Slusarenko, A.; Panstruga, R.; Kuhn, H. Nodulation induces systemic resistance of Medicago truncatula and Pisum sativum against Erysiphe pisi and primes for powdery mildew-triggered salicylic acid accumulation. Mol. Plant-Microbe Interact. 2019, 32, 1243–1255. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Finke, D. Plant-vector-pathogen interactions in the context of drought stress. Front. Ecol. Evol. 2019, 7, 262. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Boddy, L. Size matters: What have we learnt from microcosm studies of decomposer fungus–invertebrate interactions? Soil Biol. Biochem. 2014, 78, 274–283. [Google Scholar] [CrossRef]
- Davis, T.S.; Bosque-Pérez, N.A.; Popova, I.; Eigenbrode, S.D. Evidence for additive effects of virus infection and water availability on phytohormone induction in a staple crop. Front. Ecol. Evol. 2015, 3, 114. [Google Scholar] [CrossRef]
- Gupta, A.; Sinha, R.; Fernandes, J.L.; Abdelrahman, M.; Burritt, D.J.; Tran, L.S. Phytohormones regulate convergent and divergent responses between individual and combined drought and pathogen infection. Crit. Rev. Biotechnol. 2020, 40, 320–340. [Google Scholar] [CrossRef] [PubMed]
- Mutch, L.A.; Young, J.P.W. Diversity and specificity of Rhizobium leguminosarum biovar viciae on wild and cultivated legumes. Mol. Ecol. 2004, 13, 2435–2444. [Google Scholar] [CrossRef]
- Clement, S.L.; Husebye, D.S.; Eigenbrode, S.D. Ecological factors influencing pea aphid outbreaks in the US Pacific Northwest. In Aphid Biodiversity Under Environmental Change: Patterns and Processes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 107–128. [Google Scholar]
- Basu, S.; Lee, B.W.; Clark, R.E.; Bera, S.; Casteel, C.L.; Crowder, D.W. Legume plant defenses and nutrients mediate indirect interactions between soil rhizobia and chewing herbivores. Basic Appl. Ecol. 2022, 64, 57–67. [Google Scholar] [CrossRef]
- Chisholm, P.J.; Sertsuvalkul, N.; Casteel, C.L.; Crowder, D.W. Reciprocal plant-mediated interactions between a virus and a non-vector herbivore. Ecology 2018, 99, 2139–2144. [Google Scholar] [CrossRef]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef]
- Selim, S.; Sanssené, J.; Rossard, S.; Courtois, J. Systemic induction of the defensin and phytoalexin pisatin pathways in pea (Pisum sativum) against Aphanomyces euteiches by acetylated and nonacetylated oligogalacturonides. Molecules 2017, 22, 1017. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Poulaki, E.G.; Tsolakidou, M.D.; Gkizi, D.; Pantelides, I.S.; Tjamos, S.E. The ethylene biosynthesis genes ACS2 and ACS6 modulate disease severity of Verticillium dahliae. Plants 2020, 9, 907. [Google Scholar] [CrossRef]
- Sharma, K.; Gupta, S.; Sarma, S.; Rai, M.; Sreelakshmi, Y.; Sharma, R. Mutations in tomato 1-aminocyclopropane carboxylic acid synthase2 uncover its role in development beside fruit ripening. Plant J. 2021, 106, 95–112. [Google Scholar] [CrossRef]
- Fondevilla, S.; Küster, H.; Krajinski, F.; Cubero, J.I.; Rubiales, D. Identification of genes differentially expressed in a resistant reaction to Mycosphaerella pinodes in pea using microarray technology. BMC Genom. 2011, 12, 28. [Google Scholar] [CrossRef]
- Basu, S.; Clark, R.E.; Blundell, R.; Casteel, C.L.; Charlton, A.M.; Crowder, D.W. Reciprocal plant-mediated antagonism between a legume plant virus and soil rhizobia. Funct. Ecol. 2021, 35, 2045–2055. [Google Scholar] [CrossRef]
- Yergaliyev, T.M.; Nurbekova, Z.; Mukiyanova, G.; Akbassova, A.; Sutula, M.; Zhangazin, S.; Bari, A.; Tleukulova, Z.; Shamekova, M.; Masalimov, Z.K.; et al. The involvement of ROS producing aldehyde oxidase in plant response to Tombusvirus infection. Plant Physiol. Biochem. 2016, 109, 36–44. [Google Scholar] [CrossRef]
- Kulaeva, O.A.; Zhernakov, A.I.; Afonin, A.M.; Boikov, S.S.; Sulima, A.S.; Tikhonovich, I.A.; Zhukov, V.A. Pea Marker Database (PMD)—A new online database combining known pea (Pisum sativum L.) gene-based markers. PLoS ONE 2017, 12, e0186713. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Jaber, L.R.; Vidal, S. Interactions between an endophytic fungus, aphids and extrafloral nectaries: Do endophytes induce extrafloral-mediated defences in Vicia faba? Funct. Ecol. 2009, 23, 707–714. [Google Scholar] [CrossRef]
- Santos, F.; Peñaflor, M.F.G.V.; Paré, P.W.; Sanches, P.A.; Kamiya, A.C.; Tonelli, M.; Nardi, C.; Bento, J.M.S. A novel interaction between plant-beneficial rhizobacteria and roots: Colonization induces corn resistance against the root herbivore Diabrotica speciosa. PLoS ONE 2014, 9, e113280. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved drought stress response in alfalfa plants nodulated by an IAA over-producing Rhizobium strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef]
- Mabrouk, Y.; Hemissi, I.; Salem, I.B.; Mejri, S.; Saidi, M.; Belhadj, O. Potential of rhizobia in improving nitrogen fixation and yields of legumes. Symbiosis 2018, 107, 1–16. [Google Scholar]
- Igiehon, N.O.; Babalola, O.O.; Aremu, B.R. Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 2019, 19, 159. [Google Scholar] [CrossRef]
- Dutta, S.; Mishra, A.K.; Dileep Kumar, B.S. Induction of systemic resistance against fusarial wilt in pigeon pea through interaction of plant growth promoting rhizobacteria and rhizobia. Soil Biol. Biochem. 2008, 40, 452–461. [Google Scholar] [CrossRef]
- Ranjbar Sistani, N.; Kaul, H.P.; Desalegn, G.; Wienkoop, S. Rhizobium impacts on seed productivity, quality, and protection of Pisum sativum upon disease stress caused by Didymella pinodes: Phenotypic, proteomic, and metabolomic traits. Front. Plant Sci. 2017, 8, 1961. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef]
- Shahid, M.; Saghir Khan, M.; Kumar, M. Kitazin-pea interaction: Understanding the fungicide induced nodule alteration, cytotoxicity, oxidative damage and toxicity alleviation by Rhizobium leguminosarum. RSC Adv. 2019, 9, 16929–16947. [Google Scholar] [CrossRef]
- Tankari, M.; Wang, C.; Zhang, X.; Li, L.; Soothar, R.K.; Ma, H.; Xing, H.; Yan, C.; Zhang, Y.; Liu, F.; et al. Leaf gas exchange, plant water relations and water use efficiency of Vigna Unguiculata L. Walp. inoculated with rhizobia under different soil water regimes. Water 2019, 11, 498. [Google Scholar] [CrossRef]
- Duffy, B.; Schouten, A.; Raaijmakers, J.M. Pathogen self-defense: Mechanisms to counteract microbial antagonism. Annu. Rev. Phytopathol. 2003, 41, 501–538. [Google Scholar] [CrossRef] [PubMed]
- Constantin, G.D.; Grønlund, M.; Johansen, I.E.; Stougaard, J.; Lund, O.S. Virus-induced gene silencing (VIGS) as a reverse genetic tool to study development of symbiotic root nodules. Mol. Plant-Microbe Interact. 2008, 21, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Orellana, R.G.; Fan, F.F. Nodule infection by bean yellow mosaic virus in Phaseolus vulgaris. Appl. Environ. Microbiol. 1978, 36, 814–818. [Google Scholar] [CrossRef]
- Ohki, S.T.; Leps, W.T.; Hiruki, C. Effects of alfalfa mosaic virus infection on factors associated with symbiotic N2 fixation in alfalfa. Can. J. Plant Pathol. 1986, 8, 277–281. [Google Scholar] [CrossRef]
- Rao, G.P.; Shukla, K.; Gupta, S.N. Effect of cucumber mosaic virus infection on nodulation, nodular physiology and nitrogen fixation of pea plants. J. Plant Dis. Prot. 1987, 94, 606–613. [Google Scholar]
- Concha, C.; Doerner, P. The impact of the rhizobia-legume symbiosis on host root system architecture. J. Exp. Bot. 2020, 71, 3902–3921. [Google Scholar] [CrossRef]
- Mendoza-Suárez, M.A.; Geddes, B.A.; Sánchez-Cañizares, C.; Ramírez-González, R.H.; Kirchhelle, C.; Jorrin, B.; Poole, P.S. Optimizing Rhizobium-legume symbioses by simultaneous measurement of rhizobial competitiveness and N2 fixation in nodules. Proc. Natl. Acad. Sci. USA 2020, 117, 9822–9831. [Google Scholar] [CrossRef]
- Dean, J.M.; Mescher, M.C.; De Moraes, C.M. Plant dependence on rhizobia for nitrogen influences induced plant defenses and herbivore performance. Integr. J. Mol. Sci. 2014, 15, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Aoki, S.; Kawaguchi, M. Evolutionary dynamics of nitrogen fixation in the legume–rhizobia symbiosis. PLoS ONE 2014, 9, e93670. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Werner, G.D.; Cornwell, W.K.; Cornelissen, J.H.; Kiers, E.T. Evolutionary signals of symbiotic persistence in the legume–rhizobia mutualism. Proc. Natl. Acad. Sci. USA 2015, 112, 10262–10269. [Google Scholar] [CrossRef]
- Meade, J.; Higgins, P.; O’Gara, F. Studies on the inoculation and competitiveness of a Rhizobium leguminosarum strain in soils containing indigenous rhizobia. Appl. Environ. Microbiol. 1985, 49, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, P.J.; Eigenbrode, S.D.; Clark, R.E.; Basu, S.; Crowder, D.W. Plant-mediated interactions between a vector and a non-vector herbivore promote the spread of a plant virus. Proc. R. Soc. B 2019, 286, 20191383. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Basu, S.; Lee, B.W.; Crowder, D.W. Tri-trophic interactions mediate the spread of a vector-borne plant pathogen. Ecology 2019, 100, e02879. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequences | NCBI Accession No. | Amplicon Size (bp) |
|---|---|---|---|
| PEMV-1 FP PEMV-1 RP | GCAATCCTACAGGACCTTCATA CTCATCGTCTTCCGTGTCATC | HM439775.1 | 121 bp |
| PEMV-2 FP PEMV-2 RP | TGCTAGGAGAGGTGGAGATATG GCAATTGAGTAGGGTGGGTAAA | JF713436.1 | 130 bp |
| PsPR1 F PsPR1 R | TGGGGCAGTGGTGACATAAC TGCGCCAAACAACCTGAGTA | LT635896 | 178 |
| Lox2 F Lox2 R | GCAACCAAGTGACGAAGTCTA GGAGACCCGATTGTAAGGTATTT | PsCam 059875 | 95 |
| PsAO3 F PsAO3 R | TTATAGGACACAGGCTAGCTCAGCA TGACACAAGCTTATTCAGCATGACA | EF491600.1 | 127 |
| PsACS2 F PsACS2 R | GGCATAGTAATTTGAGGTTGAGCC GCCCCAACATTTAAAGGACCTATTA | AF016459 | 103 |
| β-tubulin FP β-tubulin RP | GTAACCCAAGCTTTGGTGATC ACTGAGAGTCCTGTACTGCT | X54844.1 | 203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, P.; Basu, S.; Lee, B.W.; Oeller, L.; Crowder, D.W. Effects of Soil Rhizobia Abundance on Interactions between a Vector, Pathogen, and Legume Plant Host. Genes 2024, 15, 273. https://doi.org/10.3390/genes15030273
Malhotra P, Basu S, Lee BW, Oeller L, Crowder DW. Effects of Soil Rhizobia Abundance on Interactions between a Vector, Pathogen, and Legume Plant Host. Genes. 2024; 15(3):273. https://doi.org/10.3390/genes15030273
Chicago/Turabian StyleMalhotra, Pooja, Saumik Basu, Benjamin W. Lee, Liesl Oeller, and David W. Crowder. 2024. "Effects of Soil Rhizobia Abundance on Interactions between a Vector, Pathogen, and Legume Plant Host" Genes 15, no. 3: 273. https://doi.org/10.3390/genes15030273






