Genetic Risk Factors and Clinical Outcomes in Childhood Eye Cancers: A Review
Abstract
:1. Introduction
2. Types of Childhood Malignant Eye Cancers
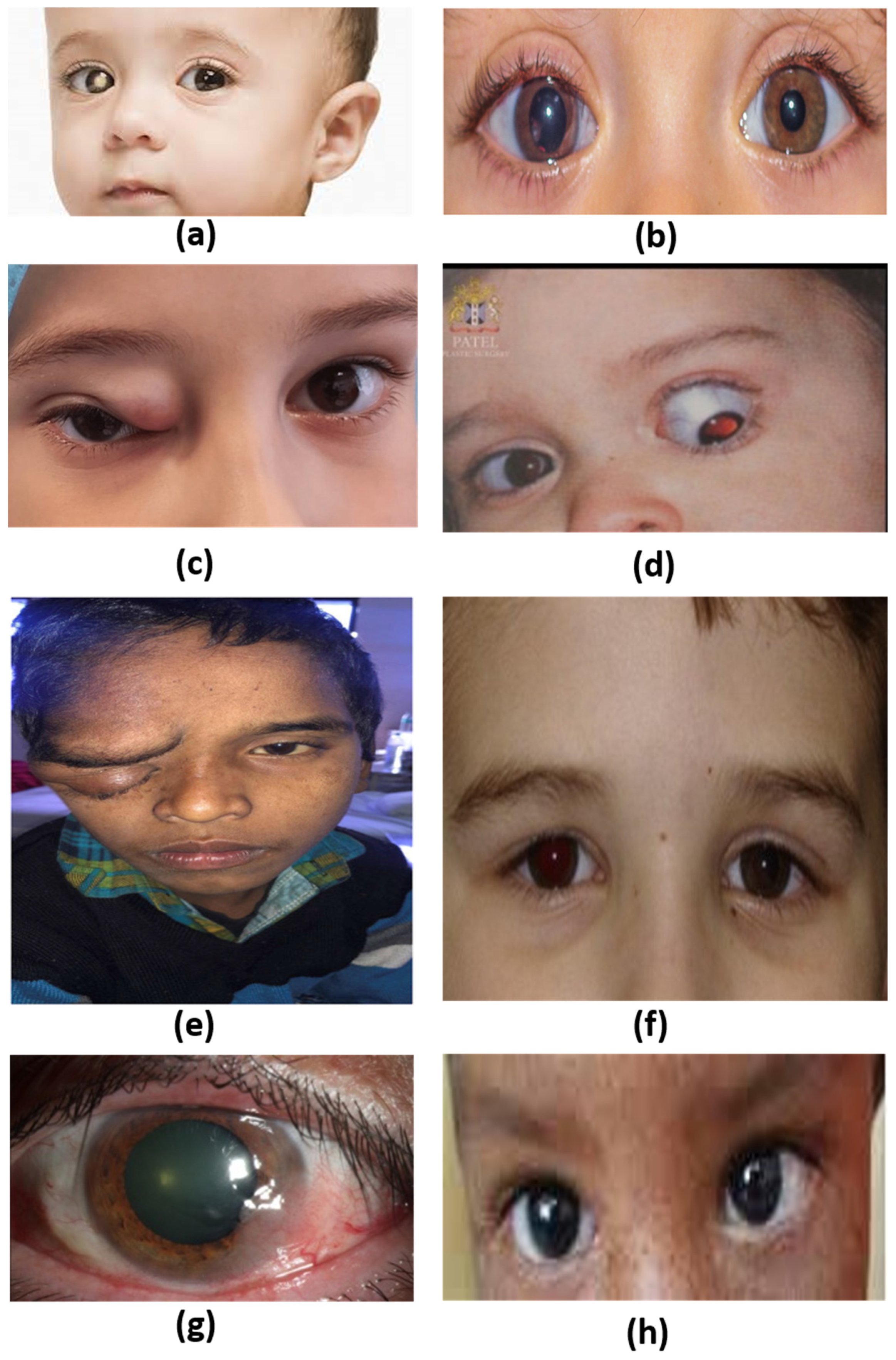
2.1. Retinoblastoma
2.2. Medulloepithelioma
2.3. Rhabdomyosarcoma
2.4. Optic Nerve Glioma
2.5. Plexiform Neurofibroma
2.6. Uveal Melanoma
2.7. Ocular Surface Squamous Neoplasia
2.8. Xeroderma Pigmentosa
2.9. Secondary Eye Cancers/Metastasis
3. Genetic Variants and Risk Factors in Childhood Eye Cancers
3.1. Genome-Wide Association Studies [GWAS]
3.2. Significance in Understanding Genetic Architecture
3.3. Notable GWAS Findings in Childhood Malignant Eye Cancers
3.4. Next-Generation Sequencing [NGS] Studies
3.5. Integrative Analysis of GWAS and NGS Studies
4. Clinical Applications and Outcomes
4.1. Clinical Presentation and Diagnosis
4.2. Treatment Options
4.3. Impact of Genetic Factors on Treatment Response and Outcomes
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oculoplastics & Ocular Oncology Deptartment; Chittagong Eye Infirmary, Bangladesh; Rani Roy, S.; Huque, F. A Review of Childhood Ocular, Orbital, and Surface Tumors with Updated Clinical Management. Int. J. Gen. Intern. Med. BIJGIM 2022, 1, 29–41. [Google Scholar] [CrossRef]
- Kivelä, T. The Epidemiological Challenge of the Most Frequent Eye Cancer: Retinoblastoma, an Issue of Birth and Death. Br. J. Ophthalmol. 2009, 93, 1129–1131. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L. Ocular Tumors of Childhood. Pediatr. Clin. N. Am. 1993, 40, 805–826. [Google Scholar] [CrossRef]
- Rao, A.A.; Naheedy, J.H.; Chen, J.Y.-Y.; Robbins, S.L.; Ramkumar, H.L. A Clinical Update and Radiologic Review of Pediatric Orbital and Ocular Tumors. J. Oncol. 2013, 2013, 975908. [Google Scholar] [CrossRef]
- Castillo, B.V.; Kaufman, L. Pediatric Tumors of the Eye and Orbit. Pediatr. Clin. N. Am. 2003, 50, 149–172. [Google Scholar] [CrossRef]
- Reschke, M.; Biewald, E.; Bronstein, L.; Brecht, I.B.; Dittner-Moormann, S.; Driever, F.; Ebinger, M.; Fleischhack, G.; Grabow, D.; Geismar, D.; et al. Eye Tumors in Childhood as First Sign of Tumor Predisposition Syndromes: Insights from an Observational Study Conducted in Germany and Austria. Cancers 2021, 13, 1876. [Google Scholar] [CrossRef]
- Kratz, C.P.; Jongmans, M.C.; Cavé, H.; Wimmer, K.; Behjati, S.; Guerrini-Rousseau, L.; Milde, T.; Pajtler, K.W.; Golmard, L.; Gauthier-Villars, M.; et al. Predisposition to Cancer in Children and Adolescents. Lancet Child Adolesc. Health 2021, 5, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. Cancer Predisposition Genes: Molecular Mechanisms and Clinical Impact on Personalized Cancer Care: Examples of Lynch and HBOC Syndromes. Acta Pharmacol. Sin. 2016, 37, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.C.; Paliwal, P.; Singh, K. Genetic Testing in Pediatric Ophthalmology. Indian J. Pediatr. 2018, 85, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Plon, S.E.; Lupo, P.J. Genetic Predisposition to Childhood Cancer in the Genomic Era. Annu. Rev. Genom. Hum. Genet. 2019, 20, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Byroju, V.V.; Nadukkandy, A.S.; Cordani, M.; Kumar, L.D. Retinoblastoma: Present Scenario and Future Challenges. Cell Commun. Signal 2023, 21, 226. [Google Scholar] [CrossRef]
- Rouhani, B.; Ramasubramanian, A. Pediatric Genetic Ocular Tumors. J. Pediatr. Genet. 2015, 3, 259–269. [Google Scholar] [CrossRef]
- Aerts, I.; Lumbroso-Le Rouic, L.; Gauthier-Villars, M.; Brisse, H.; Doz, F.; Desjardins, L. Retinoblastoma. Orphanet. J. Rare Dis. 2006, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Kamihara, J.; Bourdeaut, F.; Foulkes, W.D.; Molenaar, J.J.; Mossé, Y.P.; Nakagawara, A.; Parareda, A.; Scollon, S.R.; Schneider, K.W.; Skalet, A.H.; et al. Retinoblastoma and Neuroblastoma Predisposition and Surveillance. Clin. Cancer Res. 2017, 23, e98–e106. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gálvez, C.C.; Ordaz-Favila, J.C.; Villar-Calvo, V.M.; Cancino-Marentes, M.E.; Bosch-Canto, V. Retinoblastoma: Review and New Insights. Front. Oncol. 2022, 12, 963780. [Google Scholar] [CrossRef] [PubMed]
- Faranoush, M.; Mehrvar, N.; Tashvighi, M.; Fabian, I.D.; Zloto, O.; Bascaran, C.; Ghorbani, R.; Ghasemi, F.; Naseripour, M.; Sedaghat, A.; et al. Retinoblastoma Presentation, Treatment and Outcome in a Large Referral Centre in Tehran: A 10-Year Retrospective Analysis. Eye 2021, 35, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Assumpta Bella, L.; Afetane Evina, T.; Omgbwa Eballe, A. Les Tumeurs Oculaires Primitives de l’enfant: Aspects Épidémiologiques et Histopathologiques à l’hôpital Gynéco-Obstétrique et Pédiatrique de Yaoundé. Cah. Santé 2010, 20, 139–141. [Google Scholar] [CrossRef]
- Global Retinoblastoma Study Group. The Global Retinoblastoma Outcome Study: A Prospective, Cluster-Based Analysis of 4064 Patients from 149 Countries. Lancet Glob. Health 2022, 10, e1128–e1140. [Google Scholar] [CrossRef]
- Retinoblastoma. Available online: https://moorfields.ae/wp-content/uploads/2019/07/Retinoblastoma.jpg (accessed on 12 January 2024).
- Stathopoulos, C.; Gaillard, M.-C.; Schneider, J.; Munier, F.L. Successful Treatment of Ciliary Body Medulloepithelioma with Intraocular Melphalan Chemotherapy: A Case Report. BMC Ophthalmol. 2020, 20, 239. [Google Scholar] [CrossRef]
- Karakosta, C.; Liaskou, M.; Kattamis, A.; Rigatou, E.; Paraskevopoulos, K. Orbital Rhabdomyosarcoma Masquerading as a Dermoid Cyst: A Case Report and Review of the Literature. Cureus 2023, 15, e50332. [Google Scholar] [CrossRef]
- Mesfin, F.B.; Al-Dhahir, M.A. Gliomas. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Poswal, P.; Bhutani, N.; Arora, S.; Kumar, R. Plexiform Neurofibroma with Neurofibromatosis Type I/ von Recklinghausen’s Disease: A Rare Case Report. Ann. Med. Surg. 2020, 57, 346–350. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Arepalli, S.; Atalay, H.T.; Manjandavida, F.P.; Pieretti, G.; Shields, J.A. Uveal Melanoma in Children and Teenagers. Saudi J. Ophthalmol. 2013, 27, 197–201. [Google Scholar] [CrossRef]
- Ocular Surface Squamous Neoplasia-EyeWiki. Available online: https://eyewiki.aao.org/Ocular_Surface_Squamous_Neoplasia (accessed on 11 February 2024).
- Ghosh, U.K.; Das, P.K.; Shaila, K.N.; Razzaque, M.A.; Kaes, M.I.; Khatun, M.E. Xeroderma Pigmentosum in a Child: An Early Ocular Manifestation. Khwaja Yunus Ali Med. Coll. J. 2024, 14, 175–177. [Google Scholar] [CrossRef]
- Hanbazazh, M.; Dryja, T.P. Molecular Genetics of Intraocular Tumors. Semin. Ophthalmol. 2020, 35, 174–181. [Google Scholar] [CrossRef]
- Fabian, I.D.; Sagoo, M.S. Understanding Retinoblastoma: Epidemiology and Genetics. Community Eye Health 2018, 31, 7. [Google Scholar]
- Mendoza, P.R.; Grossniklaus, H.E. The Biology of Retinoblastoma. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 134, pp. 503–516. ISBN 9780128010594. [Google Scholar]
- Marković, L.; Bukovac, A.; Varošanec, A.M.; Šlaus, N.; Pećina-Šlaus, N. Genetics in Ophthalmology: Molecular Blueprints of Retinoblastoma. Hum. Genom. 2023, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Zugbi, S.; Ganiewich, D.; Bhattacharyya, A.; Aschero, R.; Ottaviani, D.; Sampor, C.; Cafferata, E.; Mena, M.; Sgroi, M.; Winter, U.; et al. Clinical, Genomic, and Pharmacological Study of MYCN-Amplified RB1 Wild-Type Metastatic Retinoblastoma. Cancers 2020, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.; Shields, C.; Shields, J.; Honavar, S. Intraocular Medulloepithelioma—A Review of Clinical Features, DICER 1 Mutation, and Management. Indian J. Ophthalmol. 2019, 67, 755. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L.; Eagle, R.C.; Vemuganti, G.K.; Almeida, A.; Manjandavida, F.P.; Mulay, K.; Honavar, S.G.; Shields, J.A. Ciliary Body Medulloepithelioma. Ophthalmology 2013, 120, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Nagarkatti-Gude, N.; Wang, Y.; Ali, M.J.; Honavar, S.G.; Jager, M.J.; Chan, C.-C. Genetics of Primary Intraocular Tumors. Ocul. Immunol. Inflamm. 2012, 20, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Thien, H.H.; Kim Hoa, N.T.; Duy, P.C.; Carlos, R.-G.; Son, N.H. Pediatric Primary Orbital Rhabdomyosarcoma. J. Pediatr. Surg. Case Rep. 2020, 59, 101475. [Google Scholar] [CrossRef]
- Jurdy, L.; Merks, J.H.M.; Pieters, B.R.; Mourits, M.P.; Kloos, R.J.H.M.; Strackee, S.D.; Saeed, P. Orbital Rhabdomyosarcomas: A Review. Saudi J. Ophthalmol. 2013, 27, 167–175. [Google Scholar] [CrossRef]
- Albalawi, E.D.; Alkatan, H.M.; Elkhamary, S.M.; Safieh, L.A.; Maktabi, A.M.Y. Genetic Profiling of Rhabdomyosarcoma with Clinicopathological and Radiological Correlation. Can. J. Ophthalmol. 2019, 54, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Rootman, J. Diseases of the Orbit, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; ISBN 9780781715126. [Google Scholar]
- Martin-Giacalone, B.A.; Weinstein, P.A.; Plon, S.E.; Lupo, P.J. Pediatric Rhabdomyosarcoma: Epidemiology and Genetic Susceptibility. J. Clin. Med. JCM 2021, 10, 2028. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Light, N.; Subasri, V.; Young, E.L.; Wegman-Ostrosky, T.; Barkauskas, D.A.; Hall, D.; Lupo, P.J.; Patidar, R.; Maese, L.D.; et al. Pathogenic Germline Variants in Cancer Susceptibility Genes in Children and Young Adults With Rhabdomyosarcoma. JCO Precis. Oncol. 2021, 5, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Fliney, G.; Fisayo, A.; An, Y.; Gopal, P.P.; Omuro, A.; Pointdujour-Lim, R.; Erson-Omay, E.Z.; Omay, S.B. Genetic Characterization of an Aggressive Optic Nerve Pilocytic Glioma. Brain Tumor Pathol. 2021, 38, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lena, G.; Pech-Gourg, G.; Scavarda, D.; Klein, O.; Paz-Paredes, A. Gliome du nerf optique chez l’enfant. Neurochirurgie 2010, 56, 249–256. [Google Scholar] [CrossRef]
- Fried, I.; Tabori, U.; Tihan, T.; Reginald, A.; Bouffet, E. Optic Pathway Gliomas: A Review. CNS Oncol. 2013, 2, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Patel, J.; Patel, B.C. Optic Nerve Glioma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wladis, E.J.; Adamo, M.A.; Weintraub, L. Optic Nerve Gliomas. J. Neurol. Surg. B Skull Base 2021, 82, 091–095. [Google Scholar] [CrossRef]
- Van Poppelen, N.M.; De Bruyn, D.P.; Bicer, T.; Verdijk, R.; Naus, N.; Mensink, H.; Paridaens, D.; De Klein, A.; Brosens, E.; Kiliҫ, E. Genetics of Ocular Melanoma: Insights into Genetics, Inheritance and Testing. Int. J. Mol. Sci. IJMS 2020, 22, 336. [Google Scholar] [CrossRef]
- Singh, A.D.; Topham, A. Incidence of Uveal Melanoma in the United States: 1973–1997. Ophthalmology 2003, 110, 956–961. [Google Scholar] [CrossRef]
- Tara, S.; Prabu, R.; Muralidhar, V. Congenital Uveal Malignant Melanoma- A Rare Case Report. Am. J. Ophthalmol. Case Rep. 2022, 26, 101539. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal Melanoma: Relatively Rare but Deadly Cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Betancourt, N.; Field, M.G.; Davila-Alquisiras, J.H.; Karp, C.L.; Hernández-Zimbrón, L.F.; García-Vázquez, R.; Vazquez-Romo, K.A.; Wang, G.; Fromow-Guerra, J.; Hernandez-Quintela, E.; et al. Whole Exome Profiling and Mutational Analysis of Ocular Surface Squamous Neoplasia. Ocul. Surf. 2020, 18, 627–632. [Google Scholar] [CrossRef]
- Abeti, R.; Zeitlberger, A.; Peelo, C.; Fassihi, H.; Sarkany, R.P.E.; Lehmann, A.R.; Giunti, P. Xeroderma Pigmentosum: Overview of Pharmacology and Novel Therapeutic Strategies for Neurological Symptoms. Br. J. Pharmacol. 2019, 176, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B. (Ed.) Paediatric Ophthalmology, Neuro-Ophthalmology, Genetics: With 25 Tables; Essentials in Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 9783540225942. [Google Scholar]
- Kaliki, S.; Das, A.V. Ocular and Periocular Tumors in Asian Indian Children and Adolescents. Indian Pediatr. 2020, 57, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Sthapit, P.R.; Saiju, R. Ocular Involvement in Metastatic and Systemic Malignancies Is Not Rare. Cancer Rep. 2021, 4, e1347. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tyagi, S.C. Genes and Genetics in Eye Diseases: A Genomic Medicine Approach for Investigating Hereditary and Inflammatory Ocular Disorders. Int. J. Ophthalmol. 2018, 11, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Capasso, M.; Montella, A.; Tirelli, M.; Maiorino, T.; Cantalupo, S.; Iolascon, A. Genetic Predisposition to Solid Pediatric Cancers. Front. Oncol. 2020, 10, 590033. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.R.; Huque, F. An Overview of Childhood Ocular, Orbital and Surface Tumor with Clinical Updates. J. Natl. Inst. Ophthalmol. 2022, 5, 51–68. [Google Scholar] [CrossRef]
- Rathore, S.; Verma, A.; Ratna, R.; Marwa, N.; Ghiya, Y.; Honavar, S.G.; Tiwari, A.; Das, S.; Varshney, A. Retinoblastoma: A Review of the Molecular Basis of Tumor Development and Its Clinical Correlation in Shaping Future Targeted Treatment Strategies. Indian J. Ophthalmol. 2023, 71, 2662–2676. [Google Scholar] [CrossRef]
- Gupta, A.K.; Meena, J.P. A Narrative Review of Retinoblastoma and Recent Advances in Its Management. Pediatr. Med. 2020, 3, 20. [Google Scholar] [CrossRef]
- Romani, A.; Zauli, E.; Zauli, G.; AlMesfer, S.; Al-Swailem, S.; Voltan, R. MDM2 Inhibitors-Mediated Disruption of Mitochondrial Metabolism: A Novel Therapeutic Strategy for Retinoblastoma. Front. Oncol. 2022, 12, 1000677. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Li, J.; Sheng, Y.; Wang, Y.; Dou, X. Synthetic Molecular Sensors Based on CRISPR-Cas9 Redirect Anticancer Signal Flows to Treat Retinoblastomas. Clin. Transl. Med. 2021, 11, e618. [Google Scholar] [CrossRef] [PubMed]
- Clarissa, A.; Sutandi, N.; Fath, A.A. Stem-Cell Therapy Following High-Dose Chemotherapy in Advanced Retinoblastoma: A Systematic Review. Asia-Pac. J. Ophthalmol. 2021, 10, 397–407. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, Z.; Wu, Y.; Chen, X.; Xiao, X.; Yu, H. A Functional Polymorphism (Rs937283) in the MDM2 Promoter Region Is Associated with Poor Prognosis of Retinoblastoma in Chinese Han Population. Sci. Rep. 2016, 6, 31240. [Google Scholar] [CrossRef]
- Carvalho, I.N.S.R.; Reis, A.H.D.O.; Cabello, P.H.; Vargas, F.R. Polymorphisms of CDKN1A Gene and Risk of Retinoblastoma. Carcinogenesis 2013, 34, 2774–2777. [Google Scholar] [CrossRef]
- Mobuchon, L.; Battistella, A.; Bardel, C.; Scelo, G.; Renoud, A.; Houy, A.; Cassoux, N.; Milder, M.; Cancel-Tassin, G.; Cussenot, O.; et al. A GWAS in Uveal Melanoma Identifies Risk Polymorphisms in the CLPTM1L Locus. NPJ Genom. Med. 2017, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Mobuchon, L.; Derrien, A.-C.; Houy, A.; Verrier, T.; Pierron, G.; Cassoux, N.; Milder, M.; Deleuze, J.-F.; Boland, A.; Scelo, G.; et al. Different Pigmentation Risk Loci for High-Risk Monosomy 3 and Low-Risk Disomy 3 Uveal Melanomas. J. Natl. Cancer Inst. 2022, 114, 302–309. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Zhang, J.; Tang, Z.; Chen, X.; Huang, L.; Liu, S.; Chen, H.; Wang, Y. Pertinence of Glioma and Single Nucleotide Polymorphism of TERT, CCDC26, CDKN2A/B and RTEL1 Genes in Glioma: A Meta-Analysis. Front. Oncol. 2023, 13, 1180099. [Google Scholar] [CrossRef]
- Pemov, A.; Sung, H.; Hyland, P.L.; Sloan, J.L.; Ruppert, S.L.; Baldwin, A.M.; Boland, J.F.; Bass, S.E.; Lee, H.J.; Jones, K.M.; et al. Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-Au-Lait Macule Count Identified Using Multi-Platform Analysis. PLoS Genet. 2014, 10, e1004575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, Y.-S.; Shen, R.; Jiang, H.-C.; Tan, L.; Li, R.-Q.; Yang, X.-H.; Gu, H.-Y.; He, W.-J.; Ma, J. Next Generation Sequencing of RB1gene for the Molecular Diagnosis of Ethnic Minority with Retinoblastoma in Yunnan. BMC Med. Genet. 2020, 21, 230. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, B.; Prakash, L.; Kannan, T.R.; Abraham, A.A.; Kim, U.; Muthukkaruppan, V.; Vanniarajan, A. Targeted next Generation Sequencing of RB1 Gene for the Molecular Diagnosis of Retinoblastoma. BMC Cancer 2015, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.R.; Pekmezci, M.; Bloomer, M.M.; Cadenas, N.J.; Stevers, M.; Banerjee, A.; Roy, R.; Olshen, A.B.; Van Ziffle, J.; Onodera, C.; et al. Next-Generation Sequencing of Retinoblastoma Identifies Pathogenic Alterations beyond RB1 Inactivation That Correlate with Aggressive Histopathologic Features. Ophthalmology 2020, 127, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.; Coupland, S.E.; Olohan, L.; Sibbring, J.S.; Kenny, J.G.; Hertz-Fowler, C.; Liu, X.; Haldenby, S.; Heimann, H.; Hussain, R.; et al. Targeted Next-Generation Sequencing of 117 Routine Clinical Samples Provides Further Insights into the Molecular Landscape of Uveal Melanoma. Cancers 2020, 12, 1039. [Google Scholar] [CrossRef]
- Lucas, C.-H.G.; Sloan, E.A.; Gupta, R.; Wu, J.; Pratt, D.; Vasudevan, H.N.; Ravindranathan, A.; Barreto, J.; Williams, E.A.; Shai, A.; et al. Multiplatform Molecular Analyses Refine Classification of Gliomas Arising in Patients with Neurofibromatosis Type 1. Acta Neuropathol. 2022, 144, 747–765. [Google Scholar] [CrossRef]
- De Traux De Wardin, H.; Dermawan, J.K.; Merlin, M.-S.; Wexler, L.H.; Orbach, D.; Vanoli, F.; Schleiermacher, G.; Geoerger, B.; Ballet, S.; Guillemot, D.; et al. Sequential Genomic Analysis Using a Multisample/Multiplatform Approach to Better Define Rhabdomyosarcoma Progression and Relapse. npj Precis. Oncol. 2023, 7, 96. [Google Scholar] [CrossRef]
- Wu-Chou, Y.-H.; Hung, T.-C.; Lin, Y.-T.; Cheng, H.-W.; Lin, J.-L.; Lin, C.-H.; Yu, C.-C.; Chen, K.-T.; Yeh, T.-H.; Chen, Y.-R. Genetic Diagnosis of Neurofibromatosis Type 1: Targeted next- Generation Sequencing with Multiple Ligation-Dependent Probe Amplification Analysis. J. Biomed. Sci. 2018, 25, 72. [Google Scholar] [CrossRef]
| Malignant Tumor | Age of Onset | Predominantly Affected Gender | Number of Cases Reported Annually Worldwide |
|---|---|---|---|
| Retinoblastoma | Infancy and early childhood | No significant difference | 8000–9000 |
| Ocular medulloepithelioma | Infancy and early childhood | No significant difference | Extremely rare |
| Rhabdomyosarcoma | Infancy and early childhood | Male | 250–350 |
| Optic nerve glioma | Late childhood | Female | Rare |
| Plexiform neurofibroma | Childhood | No significant difference | Rare |
| Uveal melanoma | Late childhood and young adulthood | No significant difference | Rare |
| Ocular surface squamous neoplasia | Childhood | Male | Rare |
| Xeroderma pigmentosa | Childhood | No significant difference | Rare |
| Secondary eye cancers | Varies depending on the original cancer | Varies depending on the original cancer | Rare |
| Gene | Function | Advantages | Disadvantages and Potential Impact of Gene Mutations | Associated Ocular Tumor |
|---|---|---|---|---|
| MDM2 | Negative regulator of p53 | Inhibits p53 activity, prevents apoptosis | Overexpression leads to p53 inhibition, thereby promoting tumor growth and therapy resistance | Retinoblastoma |
| CDKN1A | Cyclin-dependent kinase inhibitor | Regulates and promotes cell cycle progression | Loss of function leads to cell cycle dysregulation, uncontrolled cell division and tumor growth | |
| CCND1 | Encodes cyclin D1 | Overexpression leads to increased cell proliferation | ||
| RB1 | Retinoblastoma protein | Loss of function leads to uncontrolled cell division | ||
| MYCN | Encodes transcription factor MYCN | Regulates cell growth and proliferation | Amplification and overexpression of the gene leads to aggressive tumor growth | |
| DICER1 | RNA processing | Regulates gene expression | Mutations likely disrupt normal RNA processing pathways, contributing to tumorigenesis | Ocular medulloepithelioma |
| KMT2D | Histone methylation | Suppresses tumor formation | Mutations can lead to altered gene expression profiles, contributing to tumorigenesis | |
| PAX3 | Transcription factor | Involved in embryonic development | Overexpression linked to tumorigenesis | Rhabdomyosarcoma |
| PAX7 | Involved in muscle development | |||
| FOXO1 | Regulates cell cycle and apoptosis | Overexpression linked to cell death and growth inhibition | ||
| TP53 | Tumor suppressor | Protects against cancer development | Mutations may lead to cancer susceptibility | |
| TERT | Telomerase activity | Maintains telomere length | Overexpression linked to cellular immortality | Optic nerve glioma |
| CCDC26 | Potential involvement in cilia formation | May regulate cell signaling pathways | Dysregulation of the gene can contribute to tumorigenesis | |
| CDKN2A/B | Cyclin-dependent kinase inhibitor 2A/B | Suppresses tumor formation | Loss of function promotes cell cycle progression | |
| KIAA1549 | Cellular processes | May play a role in photoreceptor function | Gene fusions have been implicated in gliomas | |
| RTEL | Regulator of telomere length | Important for telomere maintenance | Mutation may lead to telomere dysfunction and genomic instability | |
| BRAF | Serine/threonine-protein kinase B-Raf | Part of the RAS/RAF/MEK/ERK pathway | Mutations lead to cell proliferation | Optic nerve glioma Plexiform neurofibroma |
| NF1 | Neurofibromin 1 | Regulates RAS signaling pathway | Loss of function promotes cell proliferation | |
| CDKN2A | Cell cycle regulation | Functions as a tumor suppressor | Mutation may lead to uncontrolled cell proliferation | Plexiform neurofibroma Ocular surface squamous neoplasia |
| DPH2 | Essential for diphthamide biosynthesis | Vital for protein translation | Deficiency linked to susceptibility to bacterial toxins | |
| MSH6 | DNA mismatch repair | Maintains genomic stability | Mutations may lead to microsatellite instability and tumorigenesis | |
| CLPTM1L | Promotes cell survival and proliferation | Regulation of cell growth and proliferation | Dysregulation of gene expression may contribute to tumorigenesis | Uveal melanoma |
| HERC2 | Regulates protein degradation | May help in maintaining cellular homeostasis | Mutation may disrupt protein degradation pathways | |
| IRF4 | Transcriptional regulation | Plays a role in immune response | Overexpression linked to inflammation and autoimmunity | |
| SF3B1 | Splicing factor 3B subunit 1 | Involved in RNA splicing | Mutations impact RNA splicing processes and tumor progression | |
| EIF1AX | Translation initiation factor | Essential for translation initiation | Mutations contributes to altered translation initiation and tumorigenesis | |
| BAP1 | Tumor suppressor | Protects against cancer development | Germline mutations associated with BAP1 cancer predisposition syndrome | |
| GNAQ | G protein subunit | Activates signaling pathways | Mutations result in activation of downstream signaling pathways, contributing to tumor development and progression. | |
| GNA11 | ||||
| HGF | Growth factor | May promote tissue regeneration | May promote tumor growth | Ocular surface squamous neoplasia |
| CREBB | Transcriptional regulation | Plays a role in cellular response to various stimuli | Dysregulation of the gene can contribute to tumorigenesis | |
| BRCA1 | DNA repair | Confers DNA repair capability | Mutations increase cancer risk | |
| BRCA2 | ||||
| APC | Tumor suppressor | Suppresses tumor formation | Mutations lead to tumorigenesis | |
| PTCH1 | Loss-of-function mutations lead to basal cell carcinoma | |||
| PDGFRA | Platelet-derived growth factor receptor A | Regulates cell growth and proliferation | Mutations may influence tumor growth and response to targeted therapies | |
| XPA | Nucleotide excision repair | Facilitates DNA repair | Mutations increase cancer susceptibility | Xeroderma pigmentosa |
| XPB | ||||
| XPC | ||||
| XPD | ||||
| XPE | ||||
| XPF | ||||
| XPG | ||||
| XPV | DNA polymerase | Facilitates translesion synthesis |
| Disease | Gene | Chromosome No. | SNP ID | Variant Allele | Patients (n) | Controls (n) | OR | 95%CI | p Value | Study Reference No. |
|---|---|---|---|---|---|---|---|---|---|---|
| Retinoblastoma | MDM2 | 12 | rs937283 | G | 95 | 70 | 1.74 | 1.21–2.51 | 0.01 | [63] |
| CDKN1A | 6 | rs1801270 | A | 85 | 90 | 1.98 | 1.16–3.37 | 0.0117 | [64] | |
| CCND1 | 6 | rs1059234 | T | 90 | 95 | 2.15 | 1.23–3.76 | 0.0065 | ||
| Uveal melanoma | CLPTM1L | 5 | rs421284 | C | * | * | 1.95 | 1.11–3.44 | - | [65] |
| CLPTM1L | 5 | rs452932 | C | * | * | 1.91 | 1.10–3.30 | - | ||
| HERC2 | 15 | rs1129038 | T | 1142 | 882 | 0.56 | 0.48–0.66 | 5.97 × 10−12 | [66] | |
| HERC2 | 15 | rs12913832 | G | 244 | 882 | 2.43 | 1.79–3.29 | 1.13 × 10−8 | ||
| IRF4 | 6 | rs12203592 | T | 137 | 881 | 1.01 | 0.70–1.47 | 1.78 × 10−7 | ||
| Optic nerve glioma | TERT | 5 | rs2736100 | G | * | * | 1.29 | 1.25–1.34 | - | [67] |
| CCDC26 | 8 | rs4295627 | T | * | * | 1.32 | 1.26–1.38 | - | ||
| CDKN2A/B | 9 | rs4977756 | T | * | * | 1.26 | 1.22–1.31 | - | ||
| RTEL | 20 | rs6010620 | T | * | * | 1.34 | 1.28–1.39 | - | ||
| Plexiform neurofibroma | DPH2 | 1 | rs7161 | C | * | * | - | - | - | [68] |
| DPH2 | 1 | rs4660761 | G | * | * | - | - | - | ||
| MSH6 | 2 | rs1800934 | T | * | * | - | - | - |
| Disease | Gene | Chromosome No. | Exon | Amino Acid Change | Allele | Cosegregation in Family | Study Reference No. |
|---|---|---|---|---|---|---|---|
| Retinoblastoma | RB1 | 13 | 11 | p.R355Nfs*6 | Heterozygous | De novo | [69] |
| RB1 | 13 | 22 | p.E746X | Heterozygous | Heterozygous mother | ||
| RB1 | 13 | 24 | Splice site | Heterozygous | De novo | ||
| RB1 | 13 | 2 | Splice site | Heterozygous | De novo | ||
| RB1 | 13 | 27 | p.R255X | Homozygous | De novo | [70] | |
| MYCN | 2 | - | Amplification | Amplification | * | [71] | |
| Uveal melanoma | BAP1 | 3 | 17 | nBAP1 | Heterozygous | * | [72] |
| SF3B1 | 3 | 15 | p.Lys653_Ser657del | Heterozygous | * | ||
| EIF1AX | 3 | 9 | p.Arg14_Gly15del | Heterozygous | * | ||
| Optic nerve glioma | NF1 | 17 | 4 | p.R1276* | Heterozygous | * | [73] |
| CDKN2A | 9 | 4 | Homozygous deletion | Homozygous | * | ||
| Rhabdomyosarcoma | PAX3::FOXO1 | 2 | - | PAX3::FOXO1 Fusion | Heterozygous | * | [74] |
| PAX7::FOXO1 | 1 | - | PAX7::FOXO1 Fusion | Heterozygous | * | ||
| Plexiform neurofibroma | NF1 | 17 | 5 | p.Val166fs | Heterozygous | Heterozygous | [75] |
| NF1 | 17 | 28 | p.Glu1266Ter | Heterozygous | Heterozygous | ||
| NF1 | 17 | 39 | p.Phe1884Cys | Heterozygous | Heterozygous | ||
| BRAF | 7 | 13 | p.Pro25Leu | Heterozygous | Heterozygous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, S.; Yu, A.C.; Almadani, B.; Abualkhair, S.; Ahmad, K.; Zauli, G. Genetic Risk Factors and Clinical Outcomes in Childhood Eye Cancers: A Review. Genes 2024, 15, 276. https://doi.org/10.3390/genes15030276
Hameed S, Yu AC, Almadani B, Abualkhair S, Ahmad K, Zauli G. Genetic Risk Factors and Clinical Outcomes in Childhood Eye Cancers: A Review. Genes. 2024; 15(3):276. https://doi.org/10.3390/genes15030276
Chicago/Turabian StyleHameed, Syed, Angeli Christy Yu, Bashaer Almadani, Shereen Abualkhair, Khabir Ahmad, and Giorgio Zauli. 2024. "Genetic Risk Factors and Clinical Outcomes in Childhood Eye Cancers: A Review" Genes 15, no. 3: 276. https://doi.org/10.3390/genes15030276
APA StyleHameed, S., Yu, A. C., Almadani, B., Abualkhair, S., Ahmad, K., & Zauli, G. (2024). Genetic Risk Factors and Clinical Outcomes in Childhood Eye Cancers: A Review. Genes, 15(3), 276. https://doi.org/10.3390/genes15030276





