Identification and Validation of Reference Genes for qRT-PCR Analysis of Petal-Color-Related Genes in Rosa praelucens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
2.2. Candidate Reference Genes, Petal-Color-Related Genes Selection, and Primer Design
2.3. RNA Extraction
2.4. Real-Time Quantitative RT-PCR
2.5. Analysis of Expression Stability
2.6. Validation of Reference Gene
3. Results
3.1. Isolation of Candidate Reference Genes from Transcriptome Analysis
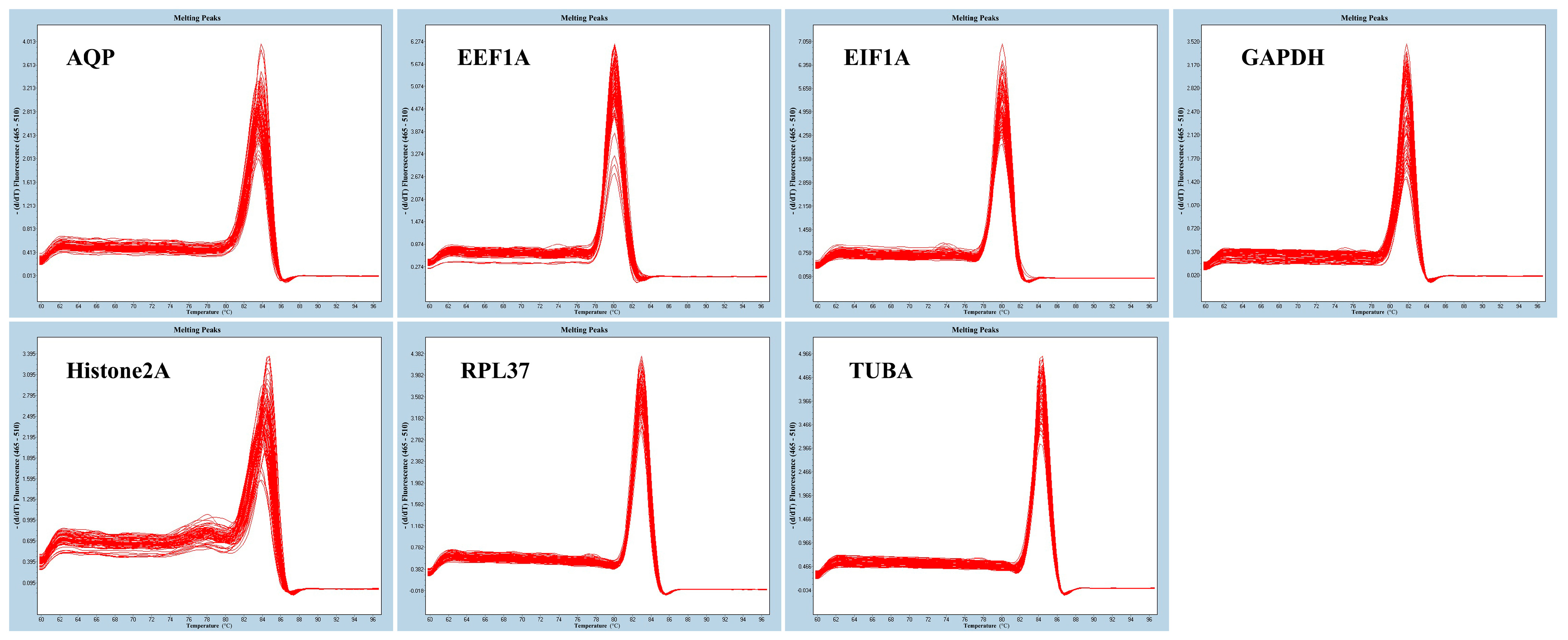
3.2. Primer Specificity and Expression Profile Analyses of Candidate Reference Genes
3.3. Expression Profile Analysis of Candidate Reference Genes
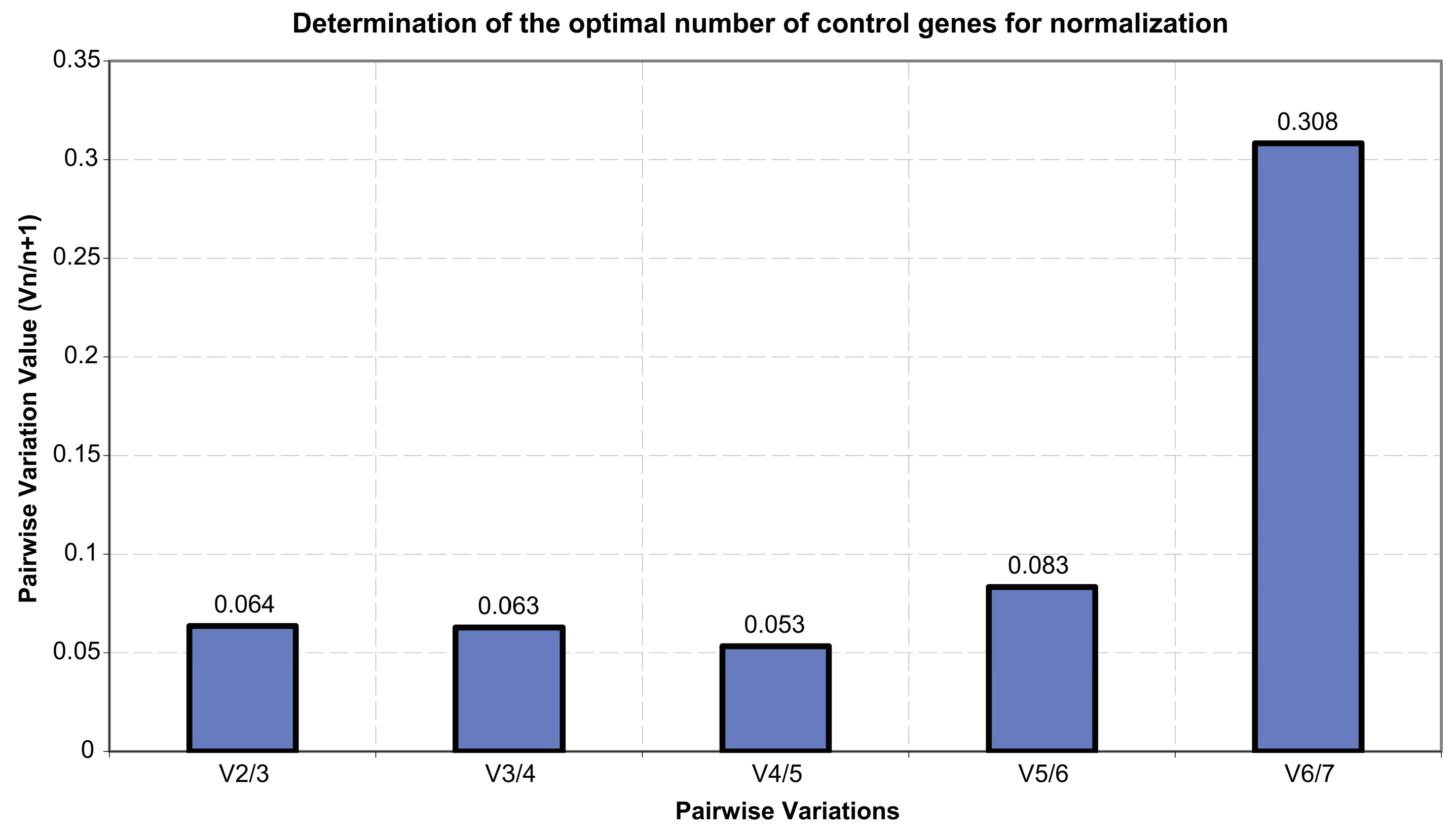
3.4. Stability Analysis of Candidate Reference Genes
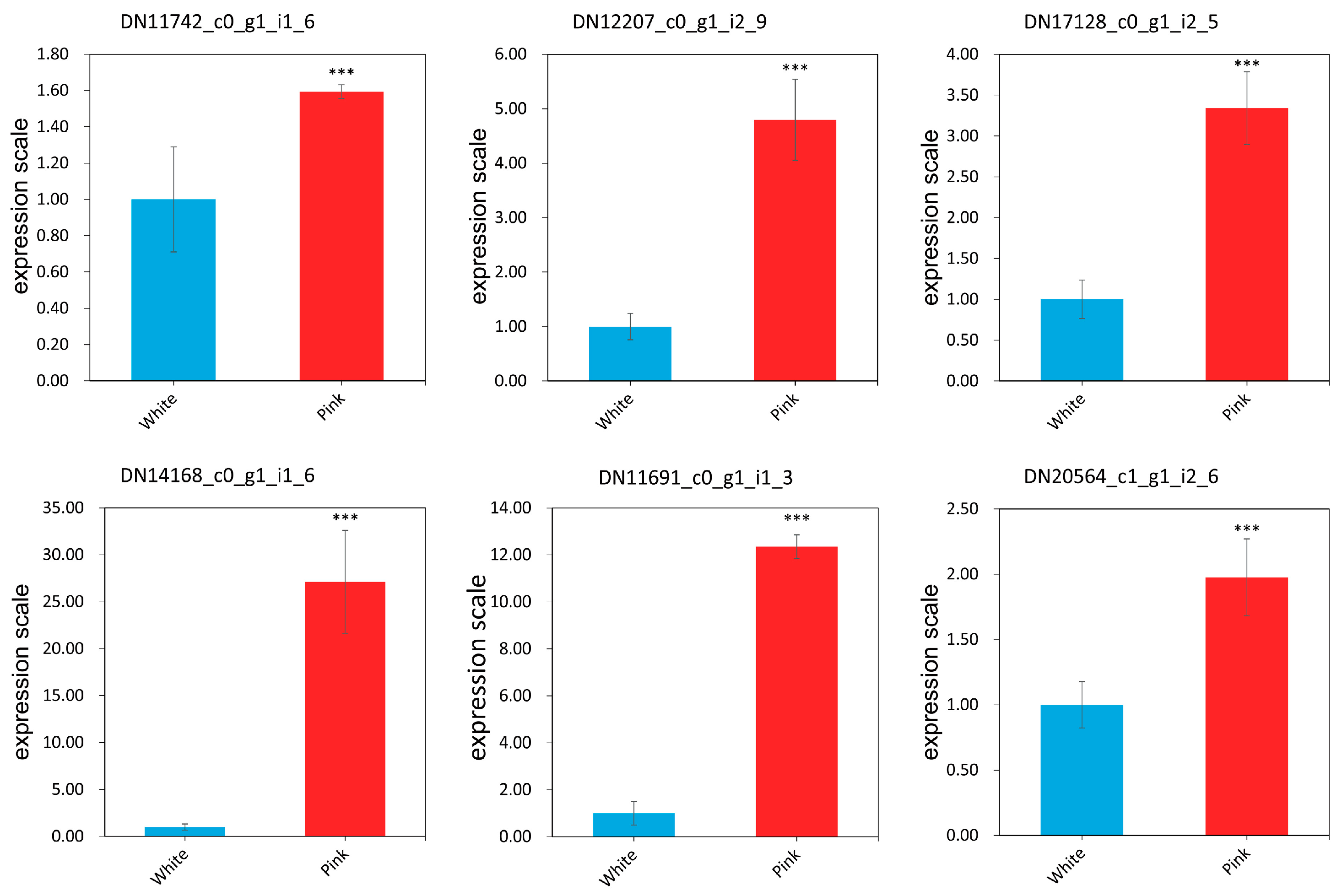
3.5. Validation of Selected Reference Gene EEF1A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ku, T.C.; Robertson, K.R. Rosa (Rosaceae). In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China, 2003; Volume 9, pp. 339–381. ISBN 978-1-93-564104-9. [Google Scholar]
- Qin, H.N.; Yang, Y.; Dong, S.Y.; He, Q.; Jia, Y.; Zhang, L.N.; Yu, S.X.; Liu, H.Y.; Liu, B.; Yan, Y.H.; et al. Threatened species list of China’s higher plants. Biodivers. Sci. 2017, 25, 696–744. [Google Scholar] [CrossRef]
- Li, X.X.; Zhou, Z.K. Endemic wild ornamental plants from North Western Yunnan. HortScience 2015, 40, 1612–1619. [Google Scholar] [CrossRef]
- Deng, J.Q.; Jian, H.Y.; Li, S.B.; Wang, Q.G.; Guo, Y.L.; Zhang, H. Cold tolerance of several wild Rosa resources endemic to Yunnan. Southwest. Chin. J. Agric. Sci. 2013, 26, 723–727. [Google Scholar]
- Fan, Y.L.; Chen, Y.C.; Jian, H.Y.; Yan, H.J.; Zhang, T.; Li, S.F.; Qiu, X.Q. Screening of Rosa germplasm resources with resistance to aphids. J. Yunnan Univ. (Nat. Sci. Ed.) 2021, 43, 619–628. [Google Scholar] [CrossRef]
- Jian, H.Y.; Li, S.F.; Guo, J.L.; Li, S.B.; Wang, Q.G.; Yan, H.J.; Qiu, X.Q.; Zhang, Y.H.; Cai, Z.Q.; Volis, S.; et al. High genetic diversity and differentiation of an extremely narrowly distributed and critically endangered decaploid rose (Rosa praelucens): Implications for its conservation. Conserv. Genet. 2018, 19, 761–776. [Google Scholar] [CrossRef]
- Wu, X.Y.; Chen, M.; Wang, Q.G.; Zhou, N.N.; Zhang, T.; Yan, H.J.; Qiu, X.Q.; Li, S.B.; Zhang, H.; Jian, H.Y.; et al. Comparative study on the breeding systems of Rosa praelucens and Rosa soulieana. Acta Hortic. Sin. 2014, 41, 2075–2084. [Google Scholar]
- Pan, L.J.; Guan, W.L.; LI, Y.H. Seed dormancy mechanism and its ecological significance of endangered species Rosa praelucens. Subtrop. Plant Sci. 2019, 48, 21–25. [Google Scholar]
- Wang, K.J.; Zhang, T.; Wang, Q.G.; Yan, H.J.; Qiu, X.Q.; Li, S.B.; Zhang, H.; Tang, K.X.; Jian, H.Y. The phylogenetic position and hybrid origination of Rosa praelucens Byhouwer. J. Plant Genet. Resour. 2018, 19, 1006–1015. [Google Scholar] [CrossRef]
- Fang, Q.; Tian, M.; Zhang, T.; Wang, Q.G.; Yan, H.J.; Qiu, X.Q.; Zhou, N.N.; Zhang, H.; Jian, H.Y.; Tang, K.X. Karyotype analysis of Rosa praelucens and its closely related congeneric species based on FISH. Acta Hortic. Sin. 2020, 47, 503–516. [Google Scholar] [CrossRef]
- Jian, H.Y.; Zhang, H.; Tang, K.X.; Li, S.F.; Wang, Q.G.; Zhang, T.; Qiu, X.Q.; Yan, H.J. Decaploidy in Rosa praelucens Byhouwer (Rosaceae) endemic to Zhongdian Plateau, Yunnan, China. Caryologia 2010, 63, 162–167. [Google Scholar] [CrossRef]
- Pan, L.J.; Guan, W.L.; LI, Y.H. Population structure and spatial distribution pattern of endangered species Rosa praelucens. Subtrop. Plant Sci. 2018, 47, 229–234. [Google Scholar]
- Guan, W.L.; Li, S.F.; Song, J.; Pan, L.J.; Niu, H.B. Study on geographic distribution of Rosa praelucens endemic to Yunnan. J. W. Chin. For. Sci. 2012, 41, 88–93. [Google Scholar]
- Li, S.F.; Li, C.J.; Jian, H.Y.; Li, S.B.; Xiong, J.; Li, J.K.; Tang, K.X. Studies on phenotypic diversity of vulnerable Rosa praelucens endemic to Shangrila, Yunnan. Acta Hortic. Sin. 2013, 40, 924–932. [Google Scholar]
- Smulders, M.J.M.; Arens, P.; Bourke, P.M.; Debener, T.; Linde, M.; De RieK, J.; Leus, L.; Ruttink, T.; Baudino, S.; Saint-Oyant, H.L.; et al. In the name of the rose: A roadmap for rose research in the genome era. Hortic. Res. 2019, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, N.; Noda, N.; Chandler, S.; Tanaka, Y. Flower color and its engineering by genetic modification. In Ornamental Crops, Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 29–62. ISBN 978-3-319-90697-3. [Google Scholar]
- Han, Y.; Wan, H.H.; Cheng, T.R.; Wang, J.; Yang, W.R.; Pan, H.T.; Zhang, Q.X. Comparative RNA-seq analysis of transcriptome dynamics during petal development in Rosa chinensis. Sci. Rep. 2017, 7, 43382. [Google Scholar] [CrossRef]
- Jay, M.; Biolley, J.P.; Fiasson, J.L.; Fiasson, K.; Gonnet, J.F.; Grossi, C.; Raymond, O.; Viricel, M.R. Anthocyanins and other flavonoid pigments. In Encyclopedia of Rose Science; Roberts, A.V., Debener, T., Gudin, S., Eds.; Elsevier: Oxford/London, UK, 2003; pp. 248–255. ISBN 0-12-227620-5. [Google Scholar]
- Ogata, J.; Kanno, Y.; Itoh, Y.; Tsugawa, H.; Suzuki, M. Anthocyanin biosynthesis in roses. Nature 2005, 435, 757–758. [Google Scholar] [CrossRef]
- Luo, P.; Ning, G.G.; Wang, Z.; Shen, Y.X.; Jin, H.N.; Li, P.H.; Huang, S.S.; Zhao, J.; Bao, M.Z. Disequilibrium of flavonol synthase and dihydroflavonol-4-reductase expression associated tightly to white vs. red color flower formation in plants. Front. Plant Sci. 2015, 6, 1257. [Google Scholar] [CrossRef]
- Chen, Z.J. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W. Neopolyploidy in flowering plants. Annu. Rev. Ecol. Syst. 2002, 33, 589–639. [Google Scholar] [CrossRef]
- Wendel, J.F. Genome evolution in polyploids. Plant Mol. Biol. 2000, 42, 2. [Google Scholar] [CrossRef]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative realtime PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Volkov, R.A.; Panchuk, I.I.; Schoffl, F. Heat-stress-dependency and developmental modulation of gene expression: The potential of housekeeping genes as internal standards in mRNA expression profiling using real-time RT-PCR. J. Exp. Bot. 2003, 54, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.R. Genome-wide identification and testing of superior reference genes for transcript normalization in arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef]
- Wan, Y.L.; Hong, A.Y.; Zhang, Y.X.; Liu, Y. Selection and validation of reference genes of Paeonia lactiflora in growth development and light stress. Physiol. Mol. Biol. Plants 2019, 25, 1097–1105. [Google Scholar] [CrossRef]
- Klie, M.; Debener, T. Identification of superior reference genes for data normalisation of expression studies via quantitative PCR in hybrid roses (Rosa hybrid). BMC Res. Notes 2011, 4, 518. [Google Scholar] [CrossRef]
- Hruz, T.; Wyss, M.; Docquier, M.; Pfaffl, M.W.; Masanetz, S.; Borghi, L.; Verbrugghe, P.; Kalaydjieva, L.; Bleuler, S.; Laule, O.; et al. RefGenes: Identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC Genom. 2011, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.J.; Qian, Y.Q.; Han, L.; Sun, Z.Y.; Fan, C.M.; Liu, J.X.; Ju, G.S. Selection of reliable reference genes for gene expression studies in Rhododendron micranthum Turcz. Sci. Hortic. 2012, 138, 128–133. [Google Scholar] [CrossRef]
- Meng, Y.L.; Li, N.; Tian, J.; Gao, J.P.; Zhang, C.Q. Identification and validation of reference genes for gene expression studies in postharvest rose flower (Rosa hybrida). Sci. Hortic. 2013, 158, 16–21. [Google Scholar] [CrossRef]
- Yan, J.F. Studies on Metabolism and Variation Mechanism of Chimeric pigment in Chrysanthemum Flower Color. Master’s Thesis, Soochow University, Suzhou, China, 2014. [Google Scholar]
- Li, Y.P.; Zhang, Y.J.; Zhu, Z.Q.; Zhi, J.W.; Liu, M.X.; Zhang, J.W.; Guo, W.J.; Sun, Y.; Kong, J.J.; Sun, J.X. Study on the Quantitative Analysis of 11 Key Genes Expression of Flower Color in Different Color System of Rosa. Mol. Plant Breed. 2022. Available online: https://kns.cnki.net/kcms/detail/46.1068.S.20220413.1634.016.html (accessed on 4 April 2022).
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Lalitha, S. Primer premier 5. Biotechnol. Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Chen, C.B.; Wu, J.Y.; Hua, Q.Z.; Tel-Zur, N.; Xie, F.F.; Zhang, Z.K.; Chen, J.Y.; Zhang, R.; Hu, G.B.; Zhao, J.T.; et al. Identification of reliable reference genes for quantitative real-time PCR normalization in pitaya. Plant Meth. 2019, 15, 70. [Google Scholar] [CrossRef]
- van Vandesompele, J.; Preter, K.; Pattyn, F.; Poppe, B.; Roy, N.; Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Chen, D.; Pan, X.; Xiao, P.; Farwell, M.A.; Zhang, B. Evaluation and identification of reliable reference genes for pharmacogenomics, toxicogenomics, and small RNA expression analysis. J. Cell Physiol. 2011, 226, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data processing system (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Wan, H.H. Identification and QTL Analysis of Flavonoids and Carotenoids in Rose Petals. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2018. [Google Scholar]
- Yoshihara, N.; Imayama, T.; Fukuchi-Mizutani, M.; Okuhara, H.; Tanaka, Y.; Ino, I.; Yabuya, T. cDNA cloning and characterization of UDP-glucose: Anthocyanidin 3-O-glucosyltransferase in Iris hollandica. Plant Sci. 2005, 169, 496–501. [Google Scholar] [CrossRef]
- Yoshihara, N.; Imayama, T.; Matsuo, Y.; Fukuchi-Mizutani, M.; Tanaka, Y.; Ino, I.; Yabuya, T. Characterization of cDNA clones encoding anthocyanin 3-p-coumaroyltransferase from Iris hollandica. Plant Sci. 2006, 171, 632–639. [Google Scholar] [CrossRef]
- Lou, Q.; Liu, Y.L.; Qi, Y.Y.; Jiao, S.Z.; Tian, F.F.; Jiang, L.; Wang, Y.J. Transcriptome sequencing and metabolite analysis reveals the role of delphinidin metabolism in flower color in grape hyacinth. J. Exp. Bot. 2014, 65, 3157–3164. [Google Scholar] [CrossRef]
- Kriangphan, N.; Vuttipongchaikij, S.; Kittiwongwattana, C.; Suttangkakul, A.; Pinmanee, P.; Sakulsathaporn, A.; Suwimon, R.; Suputtitada, S.; Chanvivattana, Y.; Apisitwanich, S. Effects of sequence and expression of eight anthocyanin biosynthesis genes on floral coloration in four Dendrobium hybrids. Hortic. J. 2015, 84, 83–92. [Google Scholar] [CrossRef]
- Xu, L.F.; Xu, H.; Cao, Y.W.; Yang, P.P.; Feng, Y.Y.; Tang, Y.C.; Yuan, S.X.; Ming, J. Validation of reference genes for quantitative real-time PCR during bicolor tepal development in asiatic hybrid lilies (Lilium spp.). Front. Plant Sci. 2017, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Duan, Q.; Cui, G.F.; Du, W.W.; Jia, W.J.; Wang, X.N.; Wang, J.H.; Chen, F.D. Selection and validation of reference genes for qRT-PCR analysis of the correlated genes in flower pigments biosynthesis pathway of Anemone obtusiloba. Acta Hortic. Sin. 2021, 48, 377–388. [Google Scholar] [CrossRef]
- Biolley, J.P.; Jay, M. Anthocyanins in modern roses: Chemical and colorimetric features in relation to the color range. J. Exp. Bot. 1993, 44, 1725–1734. [Google Scholar] [CrossRef]
- Yuki, M.; Yokoi, M.; Ueda, Y.; Saito, N. Anthocyanins in flowers of genus Rosa, sections Cinnamomeae (=Rosa), Chinenses, Gallicanae and some modern garden roses. Biochem. Syst. Ecol. 2000, 28, 887–902. [Google Scholar] [CrossRef]
- Sarangowa, O.; Kanazawa, T.; Nishizawa, M.; Yamagishi, T. Flavonol glycosides in the petal of Rosa species as chemotaxonomic markers. Phytochemistry 2014, 107, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Xing, J.; Sun, M.; Corke, H. Phenolic antioxidants (hydrolyzable tannins, flavonols, and anthocyanins) identified by LC-ESI-MS and MALDI-QIT-TOF MS from Rosa chinensis flowers. J. Agric. Food Chem. 2005, 53, 9940–9948. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhang, P.Y.; Jin, J.F.; Zhang, L.Y.; Xu, Z.D. Cloning and bioinformatics analysis of flavonol synthase gene in Rosa rugosa. Shandong Forest. Sci. Technol. 2018, 3, 1–6. [Google Scholar]
- Meng, J.X.; Gao, Y.; Han, M.L.; Liu, P.Y.; Yang, C.; Shen, T.; Li, H.H. In Vitro anthocyanin induction and metabolite analysis in Malus spectabilis leaves under low nitrogen conditions. Hortic. Plant J. 2020, 6, 284–292. [Google Scholar] [CrossRef]
- Fu, Z.Z.; Shang, H.Q.; Jiang, H.; Gao, J.; Dong, X.Y.; Wang, H.J.; Li, Y.M.; Wang, L.M.; Zhang, J.; Shu, Q.Y.; et al. Systematic identification of the light-quality responding anthocyanin synthesis-related transcripts in petunia petals. Hortic. Plant J. 2020, 6, 428–438. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Glover, B.J. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.R.; Xiong, Y.H.; Cheng, Z.Q.; Huang, X.Q. Full length cloning and expression analysis of MYB gene cDNA of Rosa chinensis. Chin. Agric. Sci. 2008, 41, 4173–4179. [Google Scholar]
- He, G.R.; Zhang, R.; Jiang, S.H.; Wang, H.H.; Ming, F. The MYB transcription factor RcMYB1 plays a central role in rose anthocyanin biosynthesis. Hortic. Res. 2023, 10, uhad080. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, R.; Yang, F.; Li, X.; Liu, H.S.; Yan, Q.; Xiao, Y.H. Cloning and expression analyses of R 2R3-MYB genes related to anthocyanin biosynthesis in Rose. Chin. Agric. Sci. 2015, 48, 1392–1404. [Google Scholar] [CrossRef]
- Li, M.F.; Yang, Y.; Wang, H.; Fan, Y.W.; Sun, P.; Jin, W.M. Analysis the function of R2R3 MYB transcription factor RhMYB113 on regulating anthocyanin synthesis in Rosa hybrida. Acta Hortic. Sin. 2022, 49, 1957–1966. [Google Scholar] [CrossRef]


| Genes | FPKM | Nr Annotation |
|---|---|---|
| TUBA | 48.88 ± 6.10 | tubulin α chain |
| GAPDH | 44.27 ± 16.74 | glyeraldehyde 3-phosphate dehydrogenase |
| Histone2A | 104.97 ± 34.30 | histone H2B |
| RPL37 | 164.71 ± 37.83 | 60S ribosomal protein L37-3-like |
| EEF1A | 113.08 ± 60.23 | elongation factor 1-α |
| EIF1A | 157.89 ± 39.51 | eukaryotic translation initiation factor 1A |
| AQP | 276.73 ± 197.22 | aquaporin PIP2-1-like |
| Gene ID | White | Pink | p-Value # | Gene Expression |
|---|---|---|---|---|
| DN11742_c0_g1_i1_6 | 0.981 ± 0.416 | 24.618 ± 5.141 | 0.0014 ** | Up-regulated |
| DN12207_c0_g1_i2_9 | 1.591 ± 0.096 | 46.562 ± 8.123 | 0.00067 *** | Up-regulated |
| DN17128_c0_g1_i2_5 | 3.281 ± 0.398 | 37.732 ± 1.513 | 2.825 × 10−6 *** | Up-regulated |
| DN14168_c0_g1_i1_6 | 0.185 ± 0.095 | 7.524 ± 0.312 | 2.582 × 10−6 *** | Up-regulated |
| DN11691_c0_g1_i1_3 | 0.282 ± 0.164 | 18.862 ± 0.395 | 1.875 × 10−7 *** | Up-regulated |
| DN20564_c1_g1_i2_6 | 0.344 ± 0.300 | 2.380 ± 0.452 | 0.0029 ** | Up-regulated |
| Type | Gene | Forward Primer (5->3) | Reverse Primer (5->3) | Length (bp) | Tm (°C) |
|---|---|---|---|---|---|
| Candidate reference genes | GAPDH | GATGAGGATGTTGTGTCAACC | GCTTGACAAACTTGTCGTTC | 97 | 60 |
| TUBA | CAGCCTGATGGCCAAATG | CAACAAAGATTGCGCGAG | 115 | 60 | |
| Histone 2A | TTACTTCCCGGGAGATCCAG | CTTAGTCCCCTCAGAAACGG | 83 | 60 | |
| EEF1A | ATCCTCACCAAGATTGACAG | TTGGTGGGAAGCATCTTAAC | 101 | 60 | |
| RPL37 | TGAGGTACCTTCGCCATGTG | TTCCCTTGCTCCTTGGCTTA | 81 | 60 | |
| EIF1A | CTTCCAGAGAATACACGTCTTA | CTCGATGTAGTCATCAGCAC | 84 | 60 | |
| AQP | TCTCAGCCAAGGACTACC | AGCACAGTGATGTAGAGAAAC | 133 | 60 | |
| Petal-color-related genes | DN11742_c0_g1_i1_6 | CTAAAGAGGTTGAGAGCGCTA | TACAAAGGATAAGACCTGCAC | 91 | 60 |
| DN12207_c0_g1_i2_9 | CCAAGGAGAAGATCATCCTCA | GGATATGCTCGGAGAAAGTC | 93 | 60 | |
| DN17128_c0_g1_i2_5 | TTGACCAAGGCATGTGTGGA | TAATTGTACCCGGATCTGTGT | 115 | 60 | |
| DN14168_c0_g1_i1_6 | GTGATGACTTGGTGTACCTT | GTTTGGGACGAGAATGGTGA | 119 | 60 | |
| DN11691_c0_g1_i1_3 | TGCATGGACTAAAGAGGAAGA | AAAGGAACCTGATGCCATT | 81 | 60 | |
| DN20564_c1_g1_i2_6 | CAAGCTACCGATAGCCACA | CCTTATTACATCCGGGAACCA | 100 | 60 |
| Gene | geNorm | NormFinder | BestKeeper | Geometric Mean | ||||
|---|---|---|---|---|---|---|---|---|
| M Value | Ranking | SV | Ranking | SD | Ranking | Geomean | Comprehensive Ranking | |
| EEF1A | 0.576 | 1 | 0.109 | 1 | 1.114 | 4 | 1.587 | 1 |
| EIF1A | 0.585 | 2 | 0.122 | 2 | 1.127 | 6 | 2.884 | 2 |
| RPL37 | 0.608 | 3 | 0.144 | 3 | 1.120 | 5 | 3.557 | 3 |
| GAPDH | 0.656 | 4 | 0.205 | 5 | 1.086 | 3 | 3.915 | 4 |
| Histone2A | 0.714 | 5 | 0.399 | 6 | 1.353 | 7 | 5.944 | 6 |
| TUBA | 0.728 | 6 | 0.151 | 4 | 0.730 | 1 | 2.884 | 7 |
| AQP | 2.162 | 8 | 1.494 | 7 | 0.968 | 2 | 4.610 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, H.; Wang, H.; Qiu, X.; Yan, H.; Ma, L. Identification and Validation of Reference Genes for qRT-PCR Analysis of Petal-Color-Related Genes in Rosa praelucens. Genes 2024, 15, 277. https://doi.org/10.3390/genes15030277
Jian H, Wang H, Qiu X, Yan H, Ma L. Identification and Validation of Reference Genes for qRT-PCR Analysis of Petal-Color-Related Genes in Rosa praelucens. Genes. 2024; 15(3):277. https://doi.org/10.3390/genes15030277
Chicago/Turabian StyleJian, Hongying, Huichun Wang, Xianqin Qiu, Huijun Yan, and Lulin Ma. 2024. "Identification and Validation of Reference Genes for qRT-PCR Analysis of Petal-Color-Related Genes in Rosa praelucens" Genes 15, no. 3: 277. https://doi.org/10.3390/genes15030277






