A Scalable and Robust Chloroplast Genotyping Solution: Development and Application of SNP and InDel Markers in the Maize Chloroplast Genome
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. DNA Extraction, Library Construction, and Sequencing
2.3. Chloroplast Genome Assembly and Annotation
2.4. Phylogenetic Analyses
2.5. Development and Validation of Maize Chloroplast Markers Using the KASP Assay
2.6. Development and Verification of Intraspecific Loci
3. Results
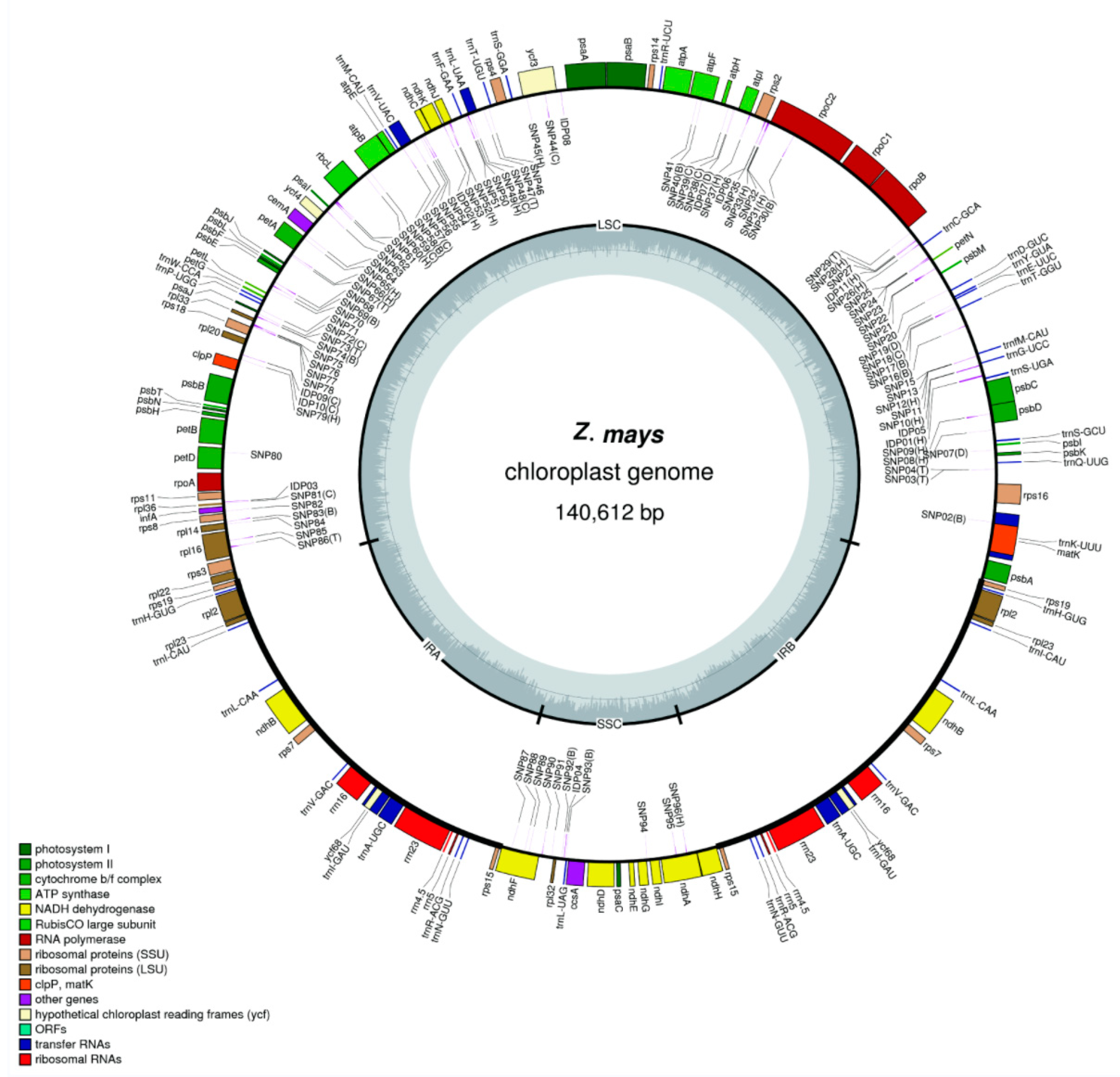
3.1. Annotation and Analysis of the Chloroplast Genome of Maize
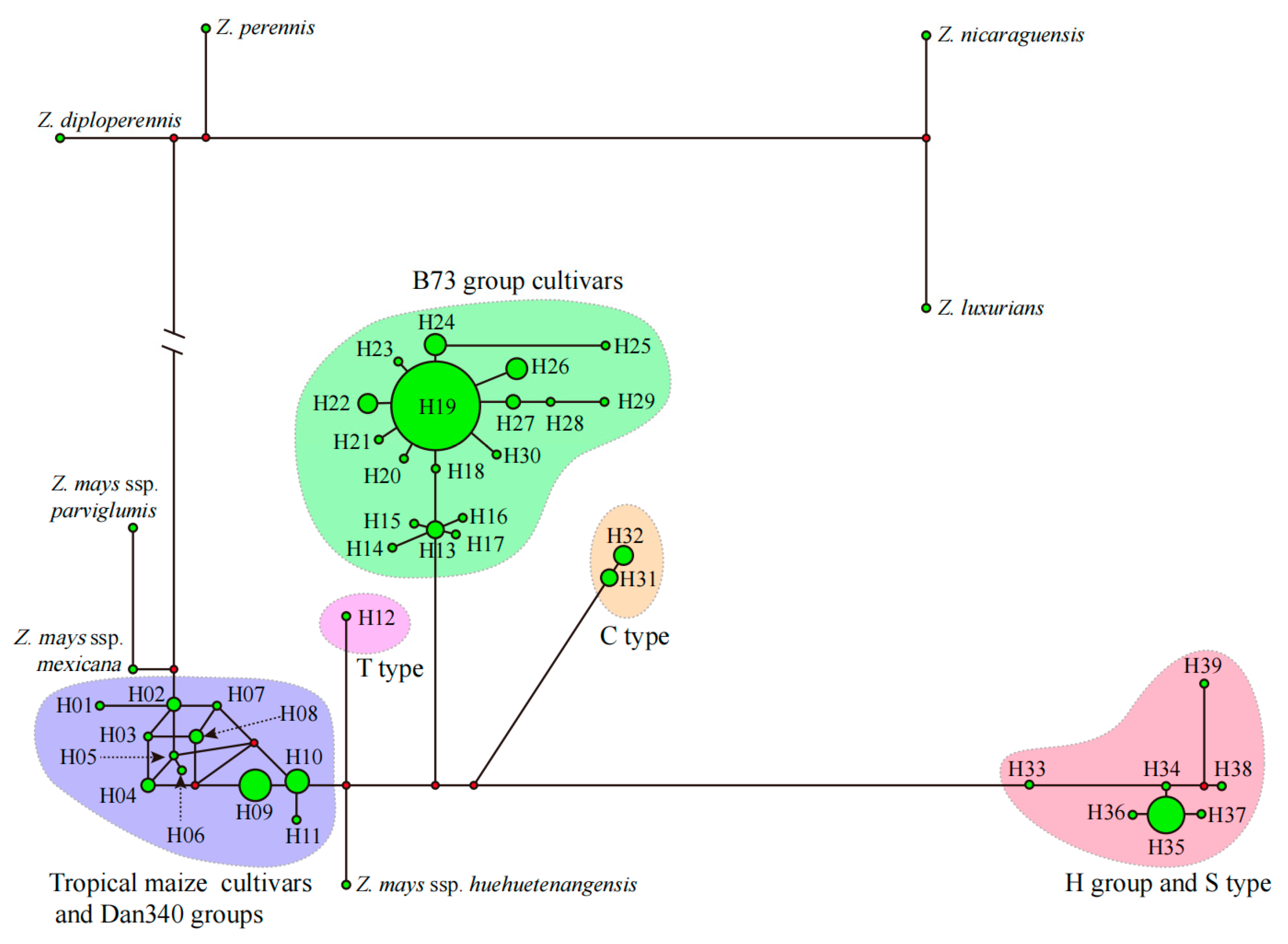
3.2. Typing Maize Varieties Based on the Chloroplast Genome
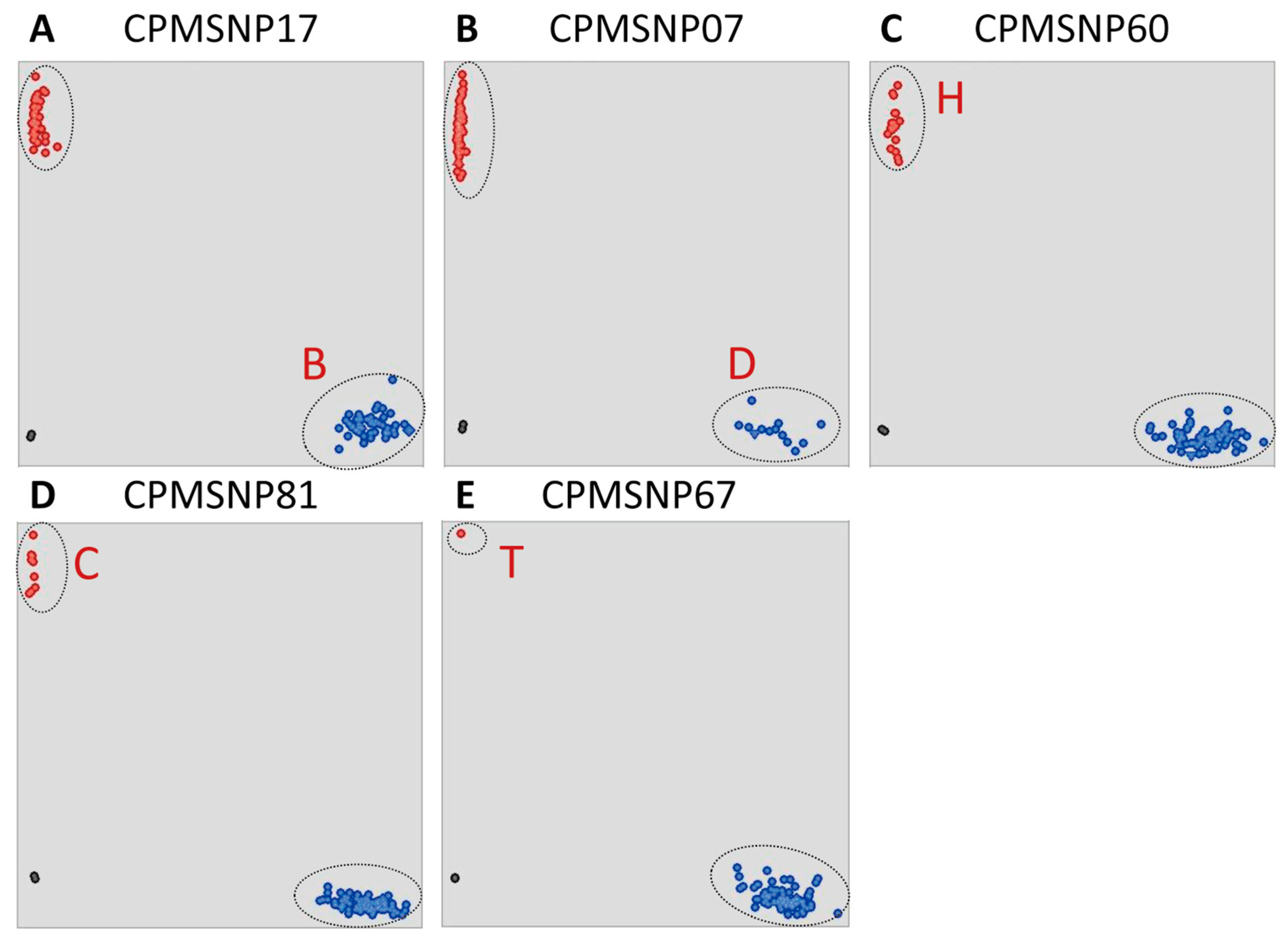
3.3. Development and Validation of Maize Chloroplast Markers
3.4. Selection of Maize Chloroplast-Specific Loci
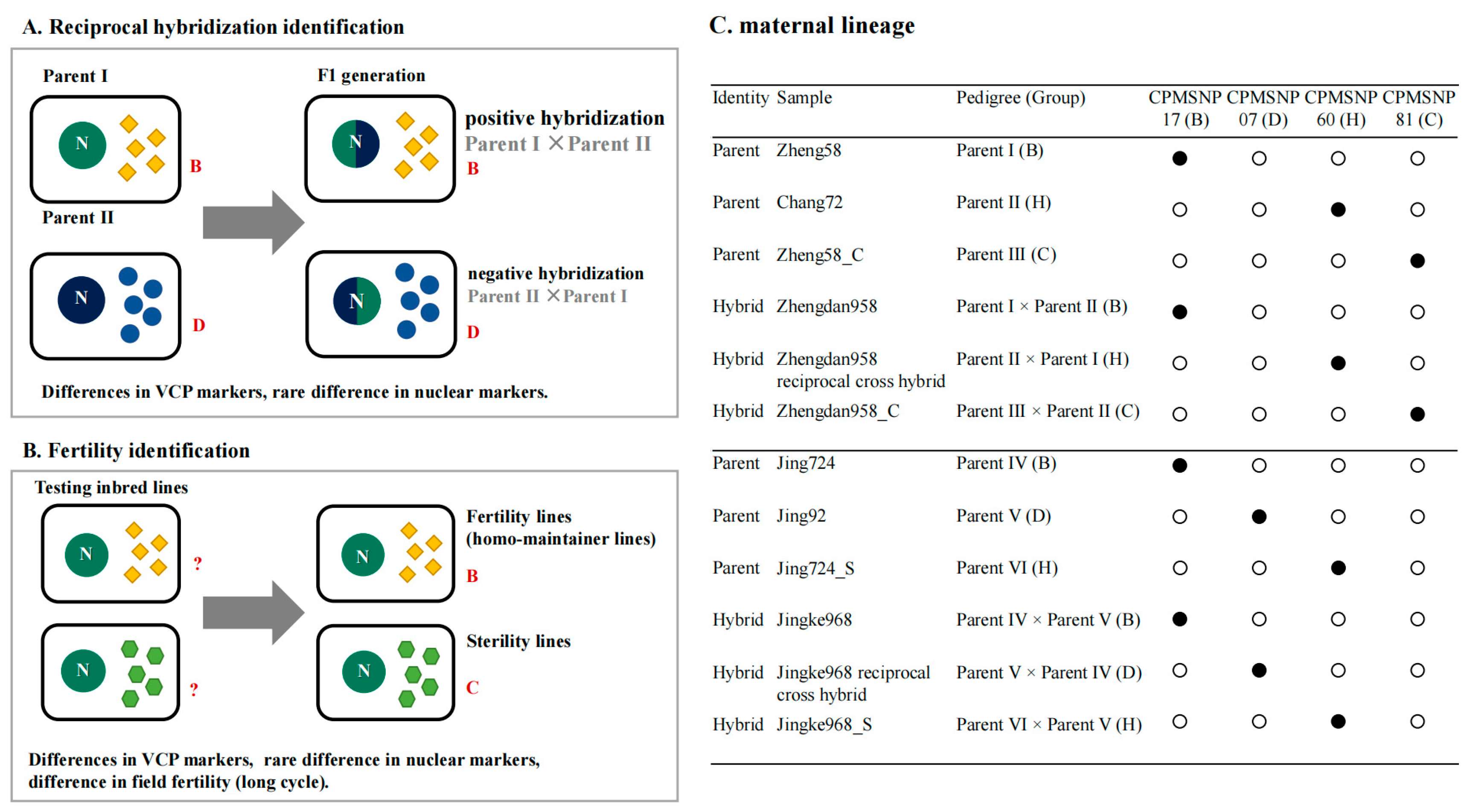
3.5. VCP Validation Using a Maternal Lineage Tracing Experiment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, F.; Ahsan, M.; Saeed, N.A.; Ahmed, M.; Ali, Q.; Kanwal, N.; Tehseen, M.M.; Ijaz, U.; Bibi, I.; Niazi, N.K. Establishment and Optimization of Callus-to-Plant Regeneration System using Mature and Immature Embryos of Maize (Zea mays). Int. J. Agric. Biol. 2016, 18, 230. [Google Scholar]
- D’Amato, R.; De Feudis, M.; Guiducci, M.; Businelli, D. Zea mays L. Grain: Increase in Nutraceutical and Antioxidant Properties Due to Se Fortification in Low and High Water Regimes. J. Agric. Food. Chem. 2019, 67, 7050–7059. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ma, L.; Jian, S.; He, Y.; Yuan, G.; Ge, F.; Chen, Z.; Zou, C.; Pan, G.; Thomas, L.; et al. Population genomic analysis reveals key genetic variations and driving force for embryonic callus induction capability in maize. J. Integr. Agric. 2023, in press. [CrossRef]
- Van Inghelandt, D.; Albrecht, E.M.; Claude, L.; Benjamin, S. Population structure and genetic diversity in a commercial maize breeding program assessed with SSR and SNP markers. Theor. Appl. Genet. 2010, 120, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tian, H.; Zhao, J.; Yi, H.; Wang, L.; Song, W. Development and characterization of a core set of SSR markers for fingerprinting analysis of Chinese maize varieties. Maydica 2011, 56, 7–17. [Google Scholar]
- Huang, J.; Yang, X.; Zhang, C.; Yin, X.; Liu, S.; Li, X. Development of Chloroplast Microsatellite Markers and Analysis of Chloroplast Diversity in Chinese Jujube (Ziziphus jujuba Mill.) and Wild Jujube (Ziziphus acidojujuba Mill.). PLoS ONE 2015, 10, e0134519. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, Y.; Wang, R.; Fan, Y.; Yi, H.; Jiang, B.; Wang, L.; Ren, J.; Xu, L.; Zhang, Y.; et al. Screening of 200 Core SNPs and the Construction of a Systematic SNP-DNA Standard Fingerprint Database with More Than 20,000 Maize Varieties. Agriculture 2021, 11, 597. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Ma, X.; Zhou, G.; Ruan, H.; Cui, H.; Pang, J.; Siffat Ullah, K.; Zong, N.; Wang, R.; et al. Genome-wide association study and metabolic pathway prediction of barrenness in maize as a response to high planting density. J. Integr. Agric. 2022, 21, 3514–3523. [Google Scholar] [CrossRef]
- Zhang, Y.; Iaffaldano, B.; Zhuang, X.; Cardina, J.; Cornish, K. Chloroplast genome resources and molecular markers differentiate rubber dandelion species from weedy relatives. BMC Plant Biol. 2017, 17, 34. [Google Scholar] [CrossRef]
- Jansen, R.K.; Raubeson, L.A.; Boore, J.L.; DePamphilis, C.W.; Chumley, T.W.; Haberle, R.C.; Wyman, S.K.; Alverson, A.J.; Peery, R.; Herman, S.J.; et al. Methods for obtaining and analyzing whole chloroplast genome sequences. Methods Enzymol. 2005, 395, 348–384. [Google Scholar] [PubMed]
- Birky, C.W. The inheritance of genes in mitochondria and chloroplasts: Laws, mechanisms, and models. Annu. Rev. Genet. 2001, 35, 125–148. [Google Scholar] [CrossRef]
- Birky, C.W. Uniparental inheritance of mitochondrial and chloroplast genes: Mechanisms and evolution. Proc. Natl. Acad. Sci. 1995, 92, 11331–11338. [Google Scholar] [CrossRef]
- Xu, D.; Abe, J.; Gai, J.; Shimamoto, Y. Diversity of chloroplast DNA SSRs in wild and cultivated soybeans: Evidence for multiple origins of cultivated soybean. Theor. Appl. Genet. 2002, 105, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, T.; Duan, D.; Yang, J.; Feng, L.; Zhao, G. Comparative Analysis of the Complete Chloroplast Genomes of Five Quercus Species. Front. Plant Sci. 2016, 7, 959. [Google Scholar] [CrossRef]
- Kahraman, K.; Lucas, S.J. Comparison of different annotation tools for characterization of the complete chloroplast genome of Corylus avellana cv Tombul. BMC Genom. 2019, 20, 874. [Google Scholar] [CrossRef]
- Budar, F.; Roux, F. The role of organelle genomes in plant adaptation: Time to get to work. Plant Signal. Behav. 2011, 6, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zhu, Z.; Li, Z.; Zhan, Q.; Feng, Q.; Zhou, C.; Zhao, Q.; Zhao, Y.; Peng, X.; Dai, B.; et al. Cytoplasmic and nuclear genome variations of rice hybrids and their parents inform the trajectory and strategy of hybrid rice breeding. Mol. Plant 2021, 14, 2056–2071. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Lv, J.; Xu, J.; Zhu, S.; Li, M.; Chen, N. Development of Chloroplast Genomic Resources for Oryza Species Discrimination. Front. Plant Sci. 2017, 8, 1854. [Google Scholar] [CrossRef]
- Hu, G.; Wu, Y.; Guo, C.; Lu, D.; Dong, N.; Chen, B.; Qiao, Y.; Zhang, Y.; Pan, Q. Haplotype Analysis of Chloroplast Genomes for Jujube Breeding. Front. Plant Sci. 2023, 13, 841767. [Google Scholar] [CrossRef] [PubMed]
- Bondar, E.I.; Putintseva, Y.; Oreshkova, N.; Krutovsky, K. Siberian larch (Larix sibirica Ledeb.) chloroplast genome and development of polymorphic chloroplast markers. BMC Bioinform. 2019, 20, 47–52. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, M.; Zhu, J.; Wang, Q.; Ge, Y.; Cheng, R. The complete chloroplast genomes of Tetrastigma hemsleyanum (Vitaceae) from different regions of China: Molecular structure, comparative analysis and development of DNA barcodes for its geographical origin discrimination. BMC Genom. 2022, 23, 620. [Google Scholar] [CrossRef]
- Ishikawa, R.; Badenoch, N.; Miyagi, K.; Medoruma, K.; Osada, T.; Onishi, M. Multi-lineages of Shiikuwasha (Citrus depressa Hayata) evaluated by using whole chloroplast genome sequences and its bio-diversity in Okinawa, Japan. Breed. Sci. 2016, 66, 490–498. [Google Scholar] [CrossRef]
- Raveendar, S.; Na, Y.; Lee, J.; Shim, D.; Ma, K.H.; Lee, S.Y.; Chung, J.W. The complete chloroplast genome of Capsicum annuum var. glabriusculum using Illumina sequencing. Molecules 2016, 20, 13080–13088. [Google Scholar]
- Beckett, J.B. Classification of male-sterile cytoplasms in maize (Zea mays L.). Crop Sci. 1971, 11, 724–727. [Google Scholar] [CrossRef]
- Tian, H.; Wang, F.; Zhao, J.; Yi, H.M.; Wang, L.; Wang, R.; Yang, Y.; Song, W. Development of maizeSNP3072, a high-throughput compatible SNP array, for DNA fingerprinting identification of Chinese maize varieties. Mol. Breed. 2015, 35, 136. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2015, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [Google Scholar] [CrossRef]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Kahlau, S.; Bock, R. OrganellarGenomeDRAW—A suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013, 41, W575–W581. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.F.; Ma, D.P.; Renfroe, W.T. Insertion/deletion mutations in the Zea chloroplast genome. Curr. Genet. 1987, 11, 614–624. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Yao, W.; Bi, Y.; Liu, L.; Chen, H.; Kang, M. Reciprocal Diallel Crosses Impact Combining Ability, Variance Estimation, and Heterotic Group Classification. Crop Sci. 2014, 54, 89–97. [Google Scholar] [CrossRef]
- Jones, R.; Schreiber, B.M.N.; Roessler, J. Kernel Sink Capacity in Maize: Genotypic and Maternal Regulation. Crop Sci. 1996, 36, 301–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, X.; Yao, W.; Piepho, H.; Kang, M. Diallel Analysis of Four Maize Traits and a Modified Heterosis Hypothesis. Crop Sci. 2016, 56, 1115–1126. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, G.; Chin, E.; Register, J.; Riley, R.; Niebur, W.; Smith, J. Identification of parents of F1 hybrids through SSR profiling of maternal and hybrid tissue. Euphytica 2002, 124, 29–34. [Google Scholar] [CrossRef]

| T. dactyloides | Z. genus | |
|---|---|---|
| Size(bp) | 140,982 | 140,440–140,810 |
| LSC length (bp) | 82,928 | 82,391–82,741 |
| IR length (bp) | 22,750 | 22,737–22,771 |
| SSC length (bp) | 12,554 | 12,527–12,546 |
| Total number of genes | 110 | 110 |
| Protein coding genes | 77 | 77 |
| tRNA | 29 | 29 |
| rRNA | 4 | 4 |
| % GC content | 38.40% | 38.40% |
| Marker | Variation Loci | AlleleFAM | AlleleHEX | Specific Type | Corresponding Alleles of cpGenome Groups | Primer_AlleleFAM | Primer_AlleleHEX | Primer_Common | Flank1 | Flank2 |
|---|---|---|---|---|---|---|---|---|---|---|
| CSNP01K | CPMSNP01 | T | G | B | T | AGCAATCTGAGTTTTTCATTTTTACTAACTTA | GCAATCTGAGTTTTTCATTTTTACTAACTTC | CTTCATTTACCAAATCCAAAAATTTGGGAA | ||
| CSNP02K | CPMSNP02 | A | G | B | A | AACAAACATAAACTAATTAGATAGAAAAGGAGT | CAAACATAAACTAATTAGATAGAAAAGGAGC | GAAAGAAAGGGAGTCTAATCCATAGAACTT | ||
| CSNP03K | CPMSNP03 | C | G | T | G | AGGATCCATTTGACCCCCAATATG | AGGATCCATTTGACCCCCAATATC | GGAAAATAAATAGGGGGTACTTCTTTTCTT | ||
| CSNP04K | CPMSNP04 | A | G | T | G | AAATAAATAGGGGGTACTTCTTTTCTTTCA | AAATAGGGGGTACTTCTTTTCTTTCG | CTTACAGGATCCATTTGACCCCCAA | ||
| CSNP07K | CPMSNP07 | T | G | D | T | GCAGGGGGTAGAAAGGCTGATA | CAGGGGGTAGAAAGGCTGATC | CTACATTGAATGTATAGCTGCAGCAATAAA | ||
| CSNP08K | CPMSNP08 | C | A | H | A | AATAAATAAAGGGTTTCAAAAGTCAATTTTTC | AATAAATAAAGGGTTTCAAAAGTCAATTTTTA | GGAATTCTGAAAAAAAAAAGAAAGATATTG | ||
| CSNP09K | CPMSNP09 | C | T | H | T | TCAACGTCCAATTATGAAATCCTTGG | GTTCAACGTCCAATTATGAAATCCTTGA | GTAGCAGCTATATTTCGGTTCATCCTTT | ||
| CSNP10K | CPMSNP10 | C | A | H | A | ATATTTTATAGGGTATATCCACCTGG | CCTATATTTTATAGGGTATATCCACCTGT | ACATAGACGGTCGACCCAGACATA | ||
| CSNP12K | CPMSNP12 | T | C | H | C | TTTCTTTCATTTTTTTTTTTTTTTTTTCT | GCTTTTCTTTCATTTTTTTTTTTTTTTTTTCC | TATCCAACCCTTTTTTTTTATTTAGCAGGC | ||
| CSNP16K | CPMSNP16 | A | G | B | A | ATGTAGGATATGCTTTTTATTTTTTGTTGGA | GTAGGATATGCTTTTTATTTTTTGTTGGG | CTGCAGAGTATCAAAATTATACTACTGCCT | ||
| CSNP17K | CPMSNP17 | C | T | B | C | AAATTCATTCATTTCTTTTTTGAAAATGTCC | CTAAATTCATTCATTTCTTTTTTGAAAATGTCT | GGCATCTCGCACTAAACTAAGTCATAAA | ||
| CSNP18K | CPMSNP18 | T | G | C | G | GTGCTCGTTTAGTGTTCAGACCA | GTGCTCGTTTAGTGTTCAGACCC | CTTAGTTTAGTGCGAGATGCCCACAT | ||
| CSNP19K | CPMSNP19 | A | C | D | C | AGTTGATGGTTAGGTTAATTCACGGAT | GTTGATGGTTAGGTTAATTCACGGAG | TAACCTTAAAAAGCTTAAAAAGTAGGGGAT | ||
| CSNP21K | CPMSNP21 | A | C | / | / | GGTTTTTTCCTTTTACTTTTTTTCTTTTACTAT | GGTTTTTTCCTTTTACTTTTTTTCTTTTACTAG | GAGAAAAATAATACGAGAATAGACTAGAAT | ||
| CSNP22K | CPMSNP22 | A | G | / | / | CCTTTTTTAAGCATGAAAGATTCGTAGGT | CTTTTTTAAGCATGAAAGATTCGTAGGC | CGAGAATAGACTAGAATAGATTATAGTAAA | ||
| CSNP26K | CPMSNP26 | A | G | H | G | ACTTACTTTTTTAGAATCTTTTTCAAAAAATA | ACTTACTTTTTTAGAATCTTTTTCAAAAAATG | AGCGAAACTGGATCCAAAAAAGCAGAAAT | ||
| CSNP28K | CPMSNP28 | T | G | H | G | ATTTATTCTTATTCTATTTTATTATGCCATTCA | TATTCTTATTCTATTTTATTATGCCATTCC | TCTTAAATCGGTATTCCCCCCCATTATTT | ||
| CSNP29K | CPMSNP29 | G | T | T | T | ATATTCTAAAAAGATTGGATAGCAAAGATTTC | GATATTCTAAAAAGATTGGATAGCAAAGATTTA | GCTTTATCCCGTTTCATAGAAAGGAGATA | ||
| CSNP30K | CPMSNP30 | A | G | B | A | TAGGAAATCGCGAATTAGATCATTTGTTT | GGAAATCGCGAATTAGATCATTTGTTC | GCTCGTGCTTCTCTTGTTGAGGTAA | ||
| CSNP31K | CPMSNP31 | T | G | H | G | TTAAGTATACATAAAGCAATTTTTTTTACTTT | TAAGTATACATAAAGCAATTTTTTTTACTTG | GTTAGCATTCTAAGGTCAAAAGTATAGTTT | ||
| CSNP33K | CPMSNP33 | T | G | H | G | ACTGACTTCTTTTACTTATTAAAATACAATTTA | ACTGACTTCTTTTACTTATTAAAATACAATTTC | CTAACAGGTCTGATTTTCGATTTTGTACTT | ||
| CSNP37K | CPMSNP37 | C | T | H | T | CAATTTTTATCAGAGGACAATATGAATATTAC | CAATTTTTATCAGAGGACAATATGAATATTAT | TATAACCCCTTGAGTGTTTTAATGGAACAT | ||
| CSNP38K | CPMSNP38 | G | A | C | A | ATTCTAAAATCATTCTTTAGAAAGCCACAC | CTAAAATCATTCTTTAGAAAGCCACAT | GGCCAAGTCAGGTTAGATCTATATCTTTA | ||
| CSNP39K | CPMSNP39 | A | C | C | C | ATGGGAACTCAAAGATATCGAAGAGTA | GGGAACTCAAAGATATCGAAGAGTC | CAACCAATCACTCTTTTATTCCATCCTTTT | ||
| CSNP40K | CPMSNP40 | A | T | B | A | CTATCAATTTTTATTTTCCATTTATTTAGTTA | CTATCAATTTTTATTTTCCATTTATTTAGTTT | GTTTCTTTATTTGTGTTTGCTCTGTTAGTT | ||
| CSNP41K | CPMSNP41 | A | T | / | / | TTATGATCTCTTCCCGAACCAAACAT | TATGATCTCTTCCCGAACCAAACAA | CGGGAGAGCCAAATGAATCGAAAGAT | ||
| CSNP44K | CPMSNP44 | A | C | C | C | GCCTATACTACTATTCTATGGATAAAGCT | CCTATACTACTATTCTATGGATAAAGCG | TCGCTCACTAATTGATCTTTACGGTGTTT | ||
| CSNP45K | CPMSNP45 | T | G | H | G | AAGCGCGGGTTTCCTTTACTAATTTT | AGCGCGGGTTTCCTTTACTAATTTG | AGAGAGAGGGTTCGCATAGAGAGAA | ||
| CSNP47K | CPMSNP47 | T | G | T | G | GAACTATTTATCCTTAAATTATTAACAAATAA | GAACTATTTATCCTTAAATTATTAACAAATAC | GCCAAGAGATTGGCATTTTCATTTGATCAT | ||
| CSNP48K | CPMSNP48 | T | A | C | A | ATCCTCGTCCGATTAATCCACTTTTA | ATCCTCGTCCGATTAATCCACTTTTT | CCTTCAATTCATTGTTTTCGAGATCTTTTA | ||
| CSNP49K | CPMSNP49 | T | G | H | G | AGTGAATCTTAAACCCATTGATAAAAGA | AGTGAATCTTAAACCCATTGATAAAAGC | TTTATTCCCTAACCATAGTTGTTATCCTTT | ||
| CSNP52K | CPMSNP52 | C | T | H | T | CCAAAAGGATAATCCTAGAATCCCG | CCCAAAAGGATAATCCTAGAATCCCA | ATCGGCACTTCTCCAAACCCAGAAA | ||
| CSNP56K | CPMSNP56 | A | C | / | / | CCTATTTTAATATATATTAATCATCCTATTTT | CCTATTTTAATATATATTAATCATCCTATTTG | ACTTACTACTAATTGGATTAGAACCTAATT | ||
| CSNP57K | CPMSNP57 | G | A | C | A | TATTTAGTACTTGTTTATAGACTCGAC | CCTTATTTAGTACTTGTTTATAGACTCGAT | AATGCTTTTATCTCTATTCTATGGCGCAAT | ||
| CSNP58K | CPMSNP58 | A | T | B | A | CAACAAGGTCAATTATGTTCATTGCATAAA | CAACAAGGTCAATTATGTTCATTGCATAAT | GCGCCAATGCTTTTCAAGGGAACTT | ||
| CSNP59K | CPMSNP59 | G | A | C | A | ATTCAACAAGAAAAAAAATTTCGACAAATTCC | ATTCAACAAGAAAAAAAATTTCGACAAATTCT | GCGAAGTAGTAGGATTGGTTCTCATAATT | ||
| CSNP60K | CPMSNP60 | A | T | H | T | GATTCAAAATATCAAAGGGGAAGAACTTTA | CAAAATATCAAAGGGGAAGAACTTTT | GCAACCCAAACCCTAATCTTTATTTTACAA | ||
| CSNP62K | CPMSNP62 | T | G | / | / | AAAGAAATACCTCTTTCAGAATACCCTTTA | GAAATACCTCTTTCAGAATACCCTTTC | CAACTGGGTATTCTATTCCACTTCTACTT | ||
| CSNP64K | CPMSNP64 | A | G | / | / | AATTAGCATATTTCTTTTCTTCCTTTAGAAATA | AATTAGCATATTTCTTTTCTTCCTTTAGAAATG | ATTTTGTTAAAAAGGAAAAGGGCTTTCTTT | ||
| CSNP65K | CPMSNP65 | G | A | H | A | CGATTTCTGTATCGATCATGATATACG | ATCGATTTCTGTATCGATCATGATATACA | GATATGCGTTTGAAATAGATGTGCGAGTT | ||
| CSNP66K | CPMSNP66 | C | T | H | T | TATTTGTTTTGTCAAAGATTACTATTTATTC | CTTATTTGTTTTGTCAAAGATTACTATTTATTT | GGAAGTCCAAAAGACAGACCCGAAT | ||
| CSNP67K | CPMSNP67 | A | C | T | C | AGTTGAACTTAATTCAAAAAGTAAAGCAATTCT | GTTGAACTTAATTCAAAAAGTAAAGCAATTCG | CGGGGACACATTTCTTGTGAGCAAA | ||
| CSNP69K | CPMSNP69 | T | A | B | T | CCCCTCAAAAAGGGAACTATTCCTA | CCCCTCAAAAAGGGAACTATTCCTT | CCACTTTTGTTGGGGTTCAAAAAACGAAT | ||
| CSNP72K | CPMSNP72 | T | C | C | C | TCATATACTAAAAAAGAATTCAAAAAGGGGA | CATATACTAAAAAAGAATTCAAAAAGGGGG | GAGATAGAATTCTTCGTGACATGACGAAA | ||
| CSNP73K | CPMSNP73 | T | C | T | C | ATTTCAAAAATTTTGTATTCTATTGGATTGGAT | TCAAAAATTTTGTATTCTATTGGATTGGAC | TTTGTTGTAATTCTTCGAATTCTCGAACAA | ||
| CSNP74K | CPMSNP74 | T | G | B | T | TGTATTCTATTGGATTGGATTTGTTCGAT | GTATTCTATTGGATTGGATTTGTTCGAG | TCTAAAGATTTTGTTGTAATTCTTCGAATT | ||
| CSNP79K | CPMSNP79 | G | T | H | T | GTATTTCTATTTTCTATAGCATAAAACCCG | AAGTATTTCTATTTTCTATAGCATAAAACCCT | GGATTTCTTGTAAATTTATCTCAAACCTAA | ||
| CSNP81K | CPMSNP81 | G | A | C | A | AGGCGTGGGCGAATTAGAGTC | CAGGCGTGGGCGAATTAGAGTT | GTCTTTGTTTATGCTTCGGATTGGAACAA | ||
| CSNP83K | CPMSNP83 | T | A | B | T | TAGTAGATTTTGTCTCACGTATATGCTTA | AGTAGATTTTGTCTCACGTATATGCTTT | CATGTTTTCCCTTTTCTTTAAATTTAGGAT | ||
| CSNP85K | CPMSNP85 | G | T | / | / | TTTTCTTTTTTAAGTTTAAGAAAGTCAAAATC | TTTCTTTTTTAAGTTTAAGAAAGTCAAAATA | CACATCAATATATAATAGAAAAAGTTAGGT | ||
| CSNP86K | CPMSNP86 | A | C | T | C | TTGAATCCTGCAATGGAGCTTCCA | GAATCCTGCAATGGAGCTTCCC | GCAGCCGGGTTAATAAAACTGAGAAAATT | ||
| CSNP91K | CPMSNP91 | A | C | / | / | ATACTGAAAGATACTGAAAGATACTTAAATTCT | CTGAAAGATACTGAAAGATACTTAAATTCG | CCACATTAGACAAAATGAACTAAAGAAGAA | ||
| CSNP92K | CPMSNP92 | C | T | B | C | CTTGCAATAGGACTTACAACCTCC | CTTGCAATAGGACTTACAACCTCT | CCCATTTATATGGGAATTTTGGATAAGATT | ||
| CSNP93K | CPMSNP93 | T | A | B | T | CCAATTTCACCATGGCGGCTAATTTA | CCAATTTCACCATGGCGGCTAATTTT | CCCAGTCTCGACGATTCACGATAAA | ||
| CSNP95K | CPMSNP95 | A | G | / | / | AAAAGATATGGAATACAATACAAAAAAGGATCT | AGATATGGAATACAATACAAAAAAGGATCC | GAATAGGGATAAAGGAAGGAAAGAATAAAT | ||
| CSNP96K | CPMSNP96 | A | G | H | G | AAAAGATCCTATTTTAACGAATCACACGTA | AGATCCTATTTTAACGAATCACACGTG | TACCATTAACTTTTTGTGTACTAGCAATAT | ||
| CIDP01K | CPMIDP01 | ACTGTATACACGGATACAGAATCCGCTATATCCGTTTGTGAAATAAAGGCTAAATCCCCTCCCCTCAACTCCATATCTAAATA | - | H | I | TTTTATTAAAACTTTTTCCTTACCGCTTTTA | CTTTTTATTAAAACTTTTTCCTTACCGCTTTTT | ATGCAAGTCCACTTTCAATATATCTCTGTA | CCCTCCCCTCAACTCCATATCTAAA | |
| CIDP02K | CPMIDP02 | - | TCTTT | H | D | CAAGTTTGAAAGATTGTACTGCTCTTTC | GCAAGTTTGAAAGATTGTACTGCTCTTTT | ATTAGGAGGGGTTCTTTTGTGCAGAAAAA | ||
| CIDP04K | CPMIDP04 | - | ATGAACTTCTAATG | / | / | AGAATTTAGGAACATTAGAAGTTCATCATTAA | GAATTTAGGAACATTAGAAGTTCATCATTAG | TCTAAAATACAAAATGCATTTCATTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yang, Y.; Tian, H.; Yi, H.; Xu, L.; Lv, Y.; Ge, J.; Zhao, Y.; Wang, L.; Zhou, S.; et al. A Scalable and Robust Chloroplast Genotyping Solution: Development and Application of SNP and InDel Markers in the Maize Chloroplast Genome. Genes 2024, 15, 293. https://doi.org/10.3390/genes15030293
Wang R, Yang Y, Tian H, Yi H, Xu L, Lv Y, Ge J, Zhao Y, Wang L, Zhou S, et al. A Scalable and Robust Chloroplast Genotyping Solution: Development and Application of SNP and InDel Markers in the Maize Chloroplast Genome. Genes. 2024; 15(3):293. https://doi.org/10.3390/genes15030293
Chicago/Turabian StyleWang, Rui, Yang Yang, Hongli Tian, Hongmei Yi, Liwen Xu, Yuanda Lv, Jianrong Ge, Yikun Zhao, Lu Wang, Shiliang Zhou, and et al. 2024. "A Scalable and Robust Chloroplast Genotyping Solution: Development and Application of SNP and InDel Markers in the Maize Chloroplast Genome" Genes 15, no. 3: 293. https://doi.org/10.3390/genes15030293






