Extraction of Innate Immune Genes in Dairy Cattle and the Regulation of Their Expression in Early Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Comparative Genomic Analysis
2.1.1. Genome Data Query
2.1.2. Innate Immunity Gene Family Analysis
2.1.3. Identification of Collinearity of Innate Immunity Genes
2.2. Regulation of the Expression of Innate Immunity Genes in Early Embryos of Dairy Cattle
2.2.1. RNA-Seq Data Processing
2.2.2. Short Time-Series Expression Miner Analysis
2.2.3. Innate Immunity Differentially Expressed Genes (DEGs) Analysis
2.2.4. Weighted Gene Co-Expression Network Analysis
2.2.5. Functional Enrichment and PPI Analysis
3. Results
3.1. Establishment and Collinearity Analysis of Innate Immunity Gene Families
3.2. Trends in the Expression of Innate Immunity Genes in Early Embryos in Dairy Cattle
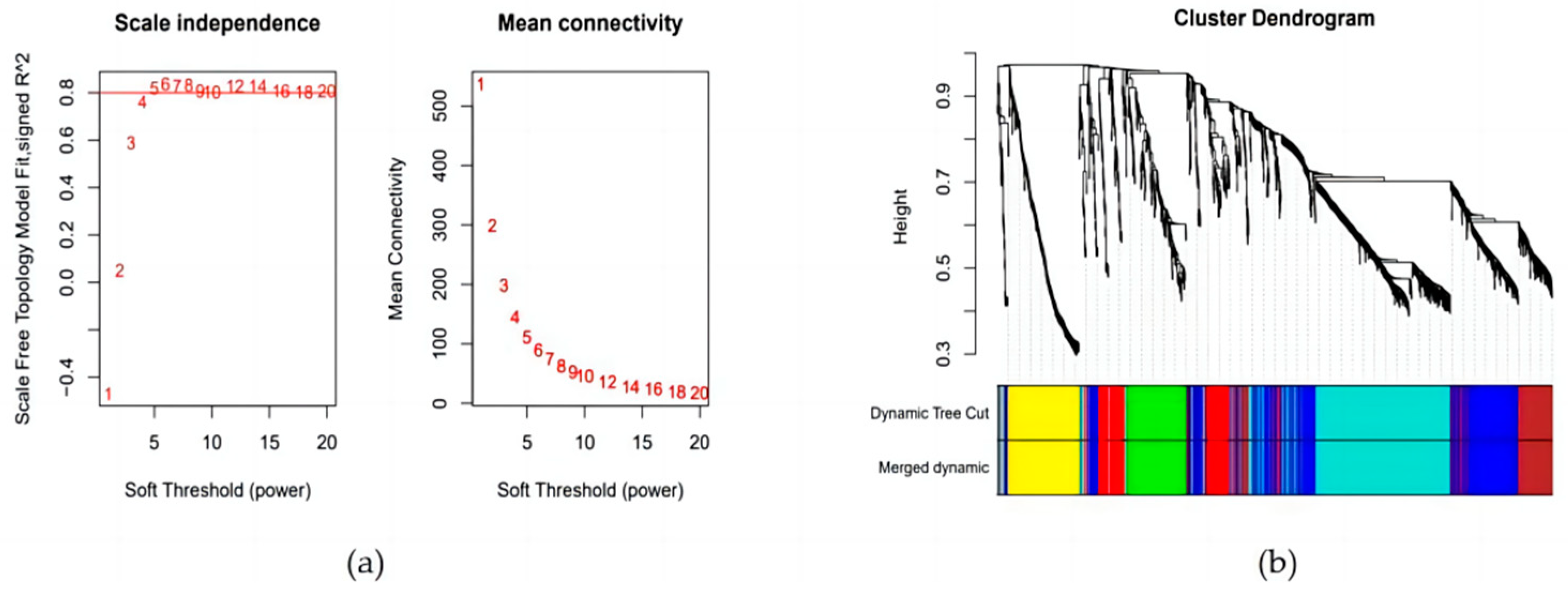
3.3. Construction of Weighted Gene Co-Expression Network and Module Detection
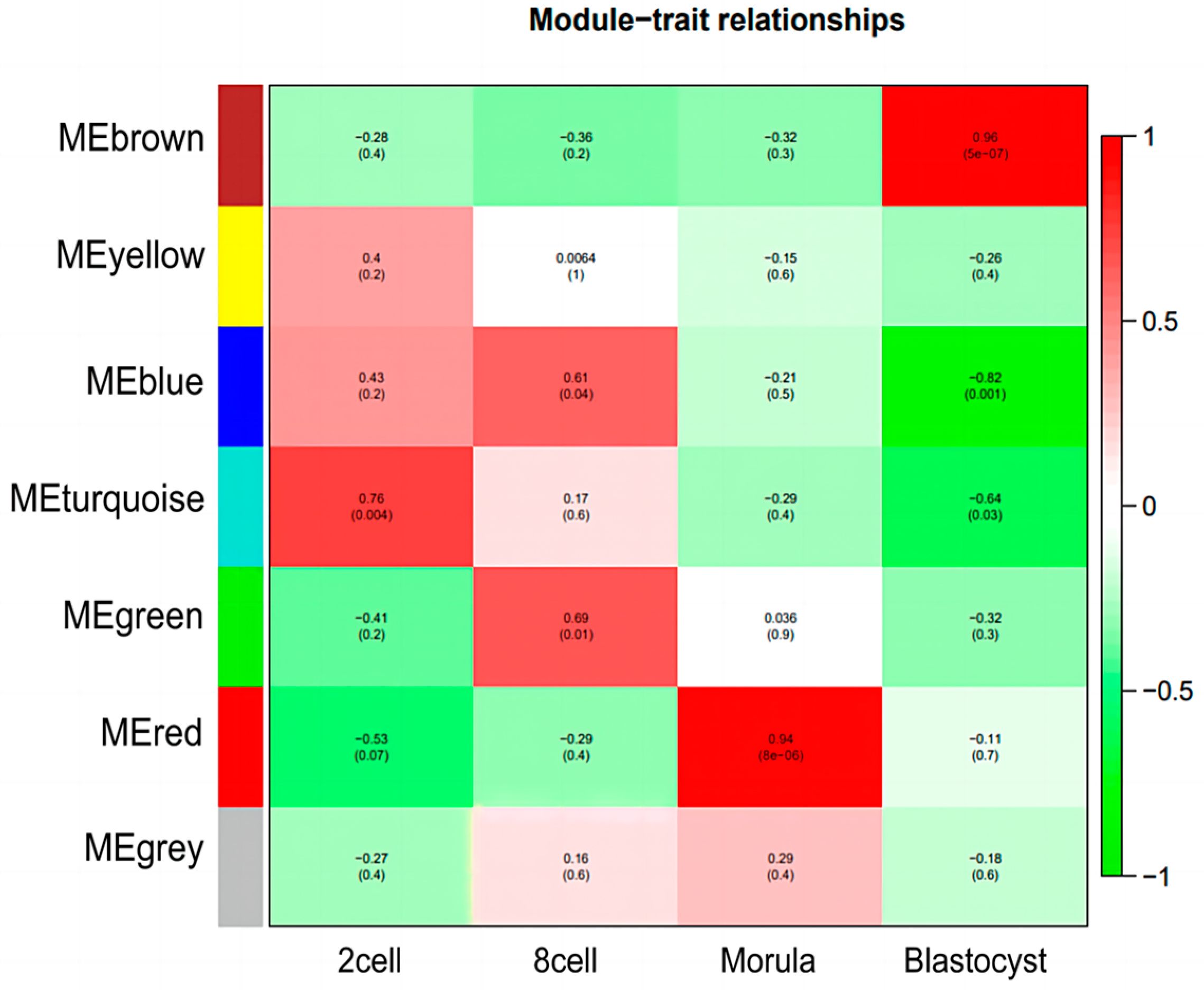
3.4. Identification of Specific Modules for Each Embryonic Stage of Development
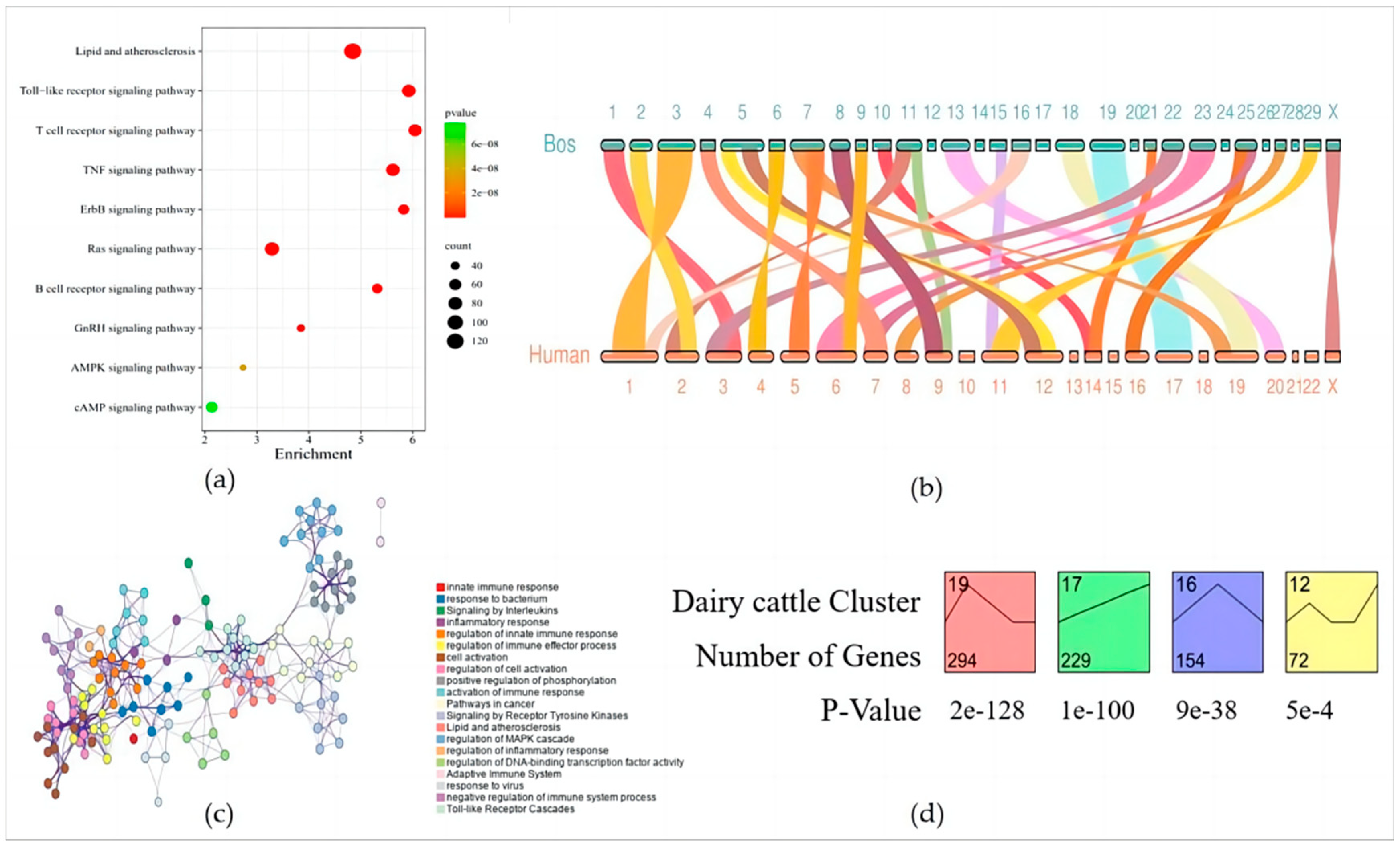
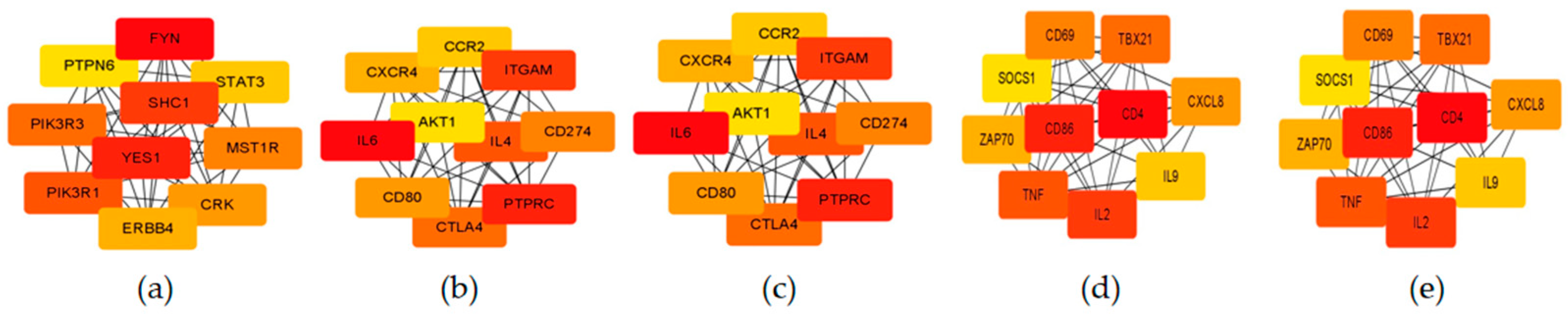
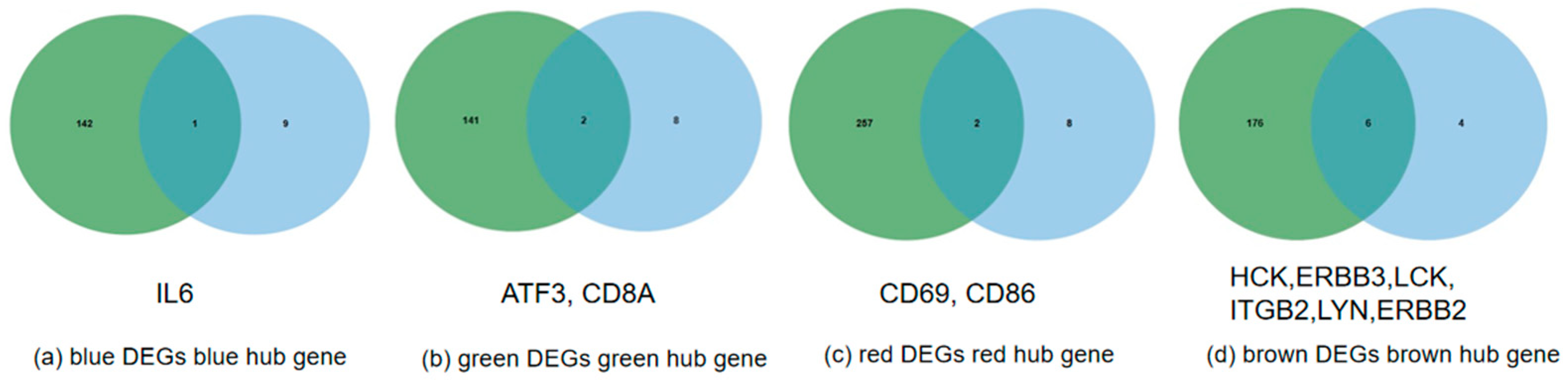
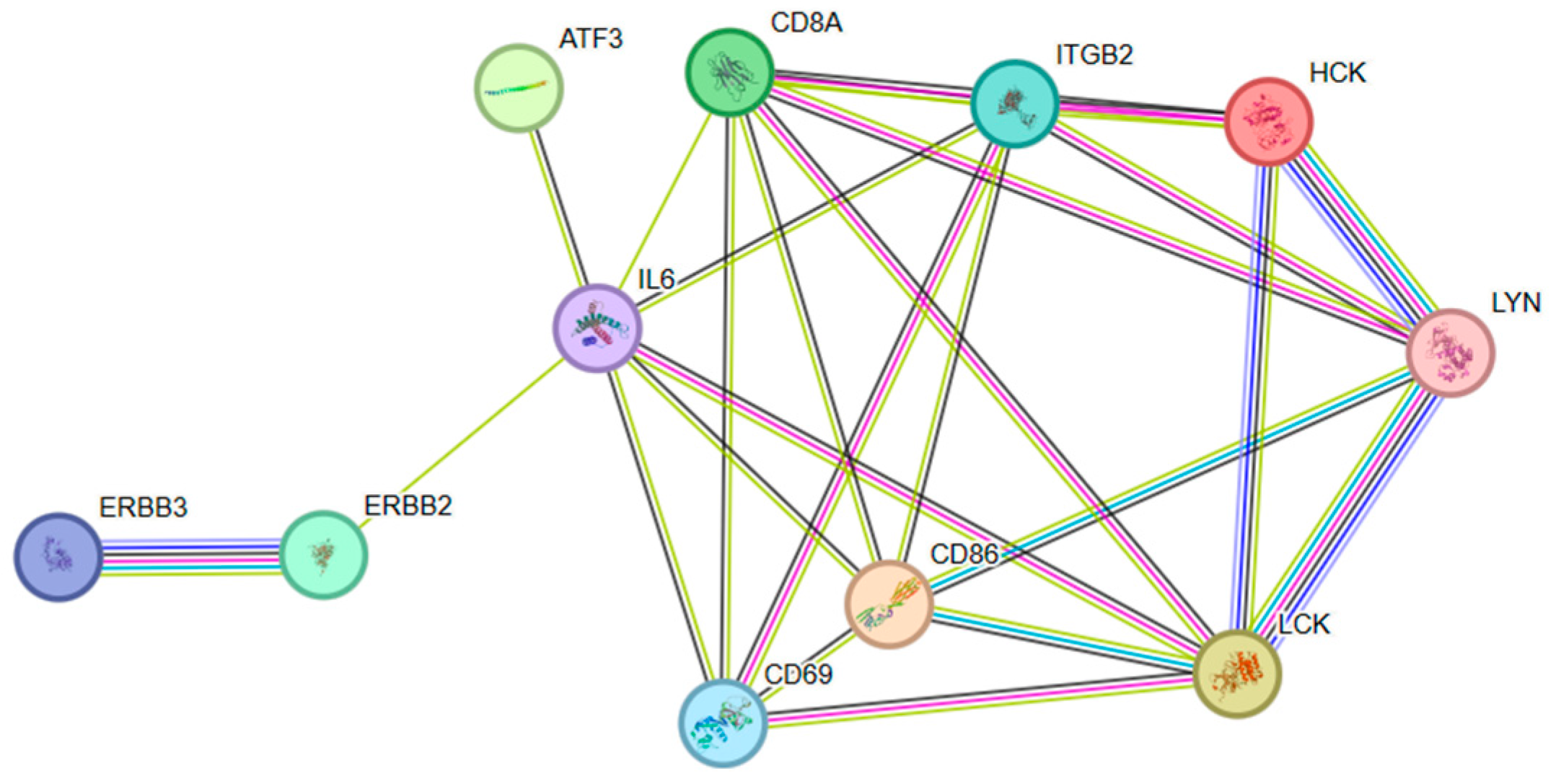
3.5. Functional Enrichment of Stage-Specific Modules and Candidate Genes for Each Embryonic Stage of Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bickhart, D.; McClure, J.; Schnabel, R.; Rosen, B.; Medrano, J.; Smith, T. Symposium review: Advances in sequencing technology herald a new frontier in cattle genomics and genome-enabled selection. J. Dairy Sci. 2020, 103, 5278–5290. [Google Scholar] [CrossRef]
- Aoki, F.; Worrad, D.M.; Schultz, R.M. Regulation of transcriptional activity during the first and second cell cycles in the preim-plantation mouse embryo. Dev. Biol. 1997, 181, 296–307. [Google Scholar] [CrossRef]
- Lee, M.T.; Bonneau, A.R.; Giraldez, A.J. Zygotic genome activation during the maternal-to-zygotic transition. Annu. Rev. Cell Dev. Biol. 2014, 30, 581–613. [Google Scholar] [CrossRef]
- Deng, M.; Liu, Z.; Ren, C.; Zhang, G.; Pang, J.; Zhang, Y.; Wang, F.; Wan, Y. Long noncoding RNAs exchange during zygotic genome activation in goat†. Biol. Reprod. 2018, 99, 707–717. [Google Scholar] [CrossRef]
- Abe, K.-I.; Funaya, S.; Tsukioka, D.; Kawamura, M.; Suzuki, Y.; Suzuki, M.G.; Schultz, R.M.; Aoki, F. Minor zygotic gene activation is essential for mouse preimplantation development. Proc. Natl. Acad. Sci. USA 2018, 115, E6780–E6788. [Google Scholar] [CrossRef]
- Deng, M.; Chen, B.; Yang, Y.; Wan, Y.; Liu, Z.; Fu, J.; Wang, F. Characterization of transcriptional activity during ZGA in mammalian SCNT embryo. Biol. Reprod. 2021, 105, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Masud, S.; Torraca, V.; Meijer, A.H. Modeling Infectious Diseases in the Context of a Developing Immune System. Curr. Top. Dev. Biol. 2017, 124, 277–329. [Google Scholar] [PubMed]
- Liu, G.; Zhang, H.; Zhao, C.; Zhang, H. Evolutionary History of the Toll-Like Receptor Gene Family across Vertebrates. Genome Biol. Evol. 2020, 12, 3615–3634. [Google Scholar] [CrossRef] [PubMed]
- Sardiello, M.; Cairo, S.; Fontanella, B.; Ballabio, A.; Meroni, G. Genomic analysis of the TRIM family reveals two groups of genes with distinct evolutionary properties. BMC Evol. Biol. 2008, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Shirahige, K.; Ohsugi, M.; Nakai, K. DBTMEE: A database of transcriptome in mouse early embryos. Nucleic Acids Res. 2014, 43, D771–D776. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.H.; Yang, Y.; Venkataraman, S.; Richardson, L.; Stevenson, P.; Burton, N.; Baldock, R.A.; Davidson, D.R. EMAGE: A spatial database of gene expression patterns during mouse embryo development. Nucleic Acids Res. 2006, 1, 637–641. [Google Scholar] [CrossRef]
- Hu, B.; Zheng, L.; Long, C.; Song, M.; Li, T.; Yang, L.; Zuo, Y. EmExplorer: A database for exploring time activation of gene expression in mammalian embryos. Open Biol. 2019, 9, 190054. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; An, J.; Peng, Y.; Kong, S.; Liu, Q.; Yang, M.; He, Q.; Song, S.; Chen, Y.; Chen, W.; et al. DevOmics: An integrated multi-omics database of human and mouse early embryo. Brief Bioinform. 2021, 22, bbab208. [Google Scholar] [CrossRef]
- Deschamps, M.; Laval, G.; Fagny, M.; Itan, Y.; Abel, L.; Casanova, J.-L.; Patin, E.; Quintana-Murci, L. Genomic Signatures of Selective Pressures and Introgression from Archaic Hominins at Human Innate Immunity Genes. Am. J. Hum. Genet. 2016, 98, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic fea-tures. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Kimbrell, D.A.; Beutler, B. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2001, 2, 256–267. [Google Scholar] [CrossRef]
- Keefe, D.; Kumar, M.; Kalmbach, K. Oocyte competency is the key to embryo potential. Fertil. Steril. 2015, 103, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ma, P.; Zhu, W.; Schultz, R.M. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev. Biol. 2008, 316, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P.; Franke, V.; Schultz, R.M. Sculpting the Transcriptome During the Oocyte-to-Embryo Transition in Mouse. Curr. Top. Dev. Biol. 2015, 113, 305–349. [Google Scholar]
- Graf, A.; Krebs, S.; Heininen-Brown, M.; Zakhartchenko, V.; Blum, H.; Wolf, E. Genome activation in bovine embryos: Review of the literature and new insights from RNA sequencing experiments. Anim. Reprod. Sci. 2014, 149, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dixon, M.; Zucchelli, M.; Hambiliki, F.; Levkov, L.; Hovatta, O.; Kere, J. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS ONE 2008, 3, e2755. [Google Scholar] [CrossRef]
- Wu, X. Maternal depletion of NLRP5 blocks early embryogenesis in rhesus macaque monkeys (Macaca mulatta). Hum. Reprod. 2008, 24, 415–424. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A., Jr. Innate immunity: The virtues of a nonclonal system of recognition. Cell 1997, 91, 295–298. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spätzle/toll/cactus controls the potent antifungal response in drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Aboussahoud, W.S.; Smith, H.; Stevens, A.; Wangsaputra, I.; Hunter, H.R.; Kimber, S.J.; Seif, M.W.; Brison, D.R. The expression and activity of Toll-like receptors in the preimplantation human embryo suggest a new role for innate immunity. Hum. Reprod. 2021, 36, 2661–2675. [Google Scholar] [CrossRef]
- Miller, B.A.; Brewer, A.; Nanni, P.; Lim, J.J.; Callanan, J.J.; Grossmann, J.; Kunz, L.; de Almeida, A.M.; Meade, K.G.; Chapwanya, A. Char-acterization of circulating plasma proteins in dairy cows with cytological endometritis. J. Proteom. 2019, 205, 103421. [Google Scholar] [CrossRef]
- Coulombe, P.; Meloche, S. Atypical mitogen-activated protein kinases: Structure, regulation and functions. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2007, 1773, 1376–1387. [Google Scholar] [CrossRef]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Yang, S.R.; Schultheis, A.M.; Yu, H.; Mandelker, D.; Ladanyi, M.; Büttner, R. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin. Cancer Biol. 2022, 84, 184–198. [Google Scholar] [CrossRef]
- Bora, P.; Gahurova, L.; Mašek, T.; Hauserova, A.; Potěšil, D.; Jansova, D.; Susor, A.; Zdráhal, Z.; Ajduk, A.; Pospíšek, M.; et al. p38-MAPK-mediated translation regulation during early blastocyst development is required for primitive endoderm differen-tiation in mice. Commun Biol. 2021, 4, 788. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A. Directing Transition from Innate to acquired immunity: Defining a role for IL-6. J. Immunol. 2005, 175, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Schäfer-Somi, S. Cytokines during early pregnancy of mammals: A review. Anim. Reprod. Sci. 2002, 75, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Kimber, S.J. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction 2005, 130, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Seshagiri, P.B.; Vani, V.; Madhulika, P. Cytokines and Blastocyst Hatching. Am. J. Reprod. Immunol. 2015, 75, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Interleukin 6 and its receptor: Ten years later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef]
- Kishimoto, T.; Akira, S.; Narazaki, M.; Taga, T. Interleukin-6 family of cytokines and gp130. Blood 1995, 86, 1243–1254. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Meng, F.; Forrester-Gauntlett, B.; Turner, P.; Henderson, H.; Oback, B. Signal Inhibition Reveals JAK/STAT3 Pathway as Critical for Bovine Inner Cell Mass Development. Biol. Reprod. 2015, 93, 132. [Google Scholar] [CrossRef]
- Wooldridge, L.K.; E Johnson, S.; Cockrum, R.R.; Ealy, A.D. Interleukin-6 requires JAK to stimulate inner cell mass expansion in bovine embryos. Reproduction 2019, 158, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Wooldridge, L.K.; Ealy, A.D. Interleukin-6 increases inner cell mass numbers in bovine embryos. BMC Dev. Biol. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Wooldridge, L.K.; Ealy, A.D. Interleukin-6 promotes primitive endoderm development in bovine blastocysts. BMC Dev. Biol. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.R.; Gomez-Lopez, N.; Robertson, S.A. Interleukin-6 in pregnancy and gestational disorders. J. Reprod. Immunol. 2012, 95, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Ortiz, W.; Xiao, Y.; Estrada-Cortes, E.; Jannaman, E.; Hansen, P. Actions of putative embryokines on development of the preimplantation bovine embryo to the blastocyst stage. J. Dairy. Sci. 2020, 103, 11930–11944. [Google Scholar] [CrossRef] [PubMed]
- Seekford, Z.K.; Wooldridge, L.K.; Dias, N.W.; Timlin, C.L.; Sales, F.; Speckhart, S.L.; Pohler, K.G.; Cockrum, R.R.; Mercadante, V.R.; Ealy, A.D. Interleukin-6 supplementation improves post-transfer embryonic and fetal development of in vitro-produced bovine embryos. Theriogenology 2021, 170, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Ding, D.; Li, Y. Regulation of Hepatic Metabolism and Cell Growth by the ATF/CREB Family of Transcription Factors. Diabetes 2021, 70, 653–664. [Google Scholar] [CrossRef]
- Whitmore, M.M.; Iparraguirre, A.; Kubelka, L.; Weninger, W.; Hai, T.; Williams, B.R.G. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J. Immunol. 2007, 179, 3622–3630. [Google Scholar] [CrossRef]
- Gilchrist, M.; Thorsson, V.; Li, B.; Rust, A.G.; Korb, M.; Roach, J.C.; Kennedy, K.; Hai, T.; Bolouri, H.; Aderem, A. Systems biology ap-proaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 2006, 441, 173–178. [Google Scholar] [CrossRef]
- Thompson, M.R.; Xu, D.; Williams, B.R. Activating transcription factor 3 contributes to Toll-like receptor-mediated macrophage survival via repression of Bax and Bak. J. Interferon Cytokine Res. 2013, 33, 682–693. [Google Scholar] [CrossRef]
- Petrelli, A.; van Wijk, F. CD8+ T cells in human autoimmune arthritis: The unusual suspects. Nat. Rev. Rheumatol. 2016, 12, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Vallejos-Vidal, E.; Reyes-López, F.E.; Sandino, A.M.; Imarai, M. Sleeping with the Enemy? The Current Knowledge of Piscine Or-thoreovirus (PRV) Immune Response Elicited to Counteract Infection. Front. Immunol. 2022, 13, 768621. [Google Scholar] [CrossRef] [PubMed]
- Testi, R.; Pulcinelli, F.; Frati, L.; Gazzaniga, P.P.; Santoni, A. CD69 is expressed on platelets and mediates platelet activation and aggregation. J. Exp. Med. 1990, 172, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, T.; Wang, X.-J.; Zhou, L.; Shi, A.-C.; Ning, Q. CD69+NK cells contribute to the murine hepatitis virus strain 3-induced murine hepatitis. Curr. Med. Sci. 2013, 33, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Waters, E.; Rowshanravan, B.; Hinze, C.; Williams, C.; Janman, D.; Fox, T.A.; Booth, C.; Pesenacker, A.M.; Halliday, N.; et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat. Immunol. 2022, 23, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Xie, E.; Tang, B.; Mu, Q.; Song, Z.; Chen, J.; Wang, F.; Min, J. Rewiring ERBB3 and ERK signaling confers resistance to FGFR1 inhibition in gastrointestinal cancer harbored an ERBB3-E928G mutation. Protein Cell 2020, 11, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, S.; Moriyoshi, M. Alteration of the endometrial EGF profile as a Potential mechanism connecting the alterations in the ovarian steroid hormone profile to embryonic loss in repeat breeders and high-producing cows. J. Reprod. Dev. 2013, 59, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Engen, J.R.; Wales, T.E.; Hochrein, J.M.; Meyn, M.A., 3rd; Banu Ozkan, S.; Bahar, I.; Smithgall, T.E. Structure and dynamic regulation of Src-family kinases. Cell. Mol. Life Sci. 2008, 65, 3058–3073. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, C.; Tian, Y.; Lu, J.; Zhang, G.; Liang, S.; Chen, D.; Liu, X.; Kuang, W.; Zhu, M. Src family kinases and pulmonary fibrosis: A review. Biomed. Pharmacother. 2020, 127, 110183. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, L.; Zhang, H.; Baruzzi, A.; Lowell, C.A.; Berton, G. The src family kinases hck and fgr regulate neutrophil responses to n-formyl-methionyl-leucyl-phenylalanine. J. Immunol. 2007, 178, 3874–3885. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.R.; O’donoghue, R.J.; Ernst, M. Hematopoietic cell kinase (HCK) as a therapeutic target in immune and cancer cells. Oncotarget 2015, 6, 15752–15771. [Google Scholar] [CrossRef] [PubMed]
- Raynor, J.L.; Chi, H. LCK senses asparagine for T cell activation. Nature 2021, 23, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Bommhardt, U.; Schraven, B.; Simeoni, L. Beyond TCR Signaling: Emerging Functions of Lck in Cancer and Immunotherapy. Int. J. Mol. Sci. 2019, 20, 3500. [Google Scholar] [CrossRef]
- Xiao, W.; Nishimoto, H.; Hong, H.; Kitaura, J.; Nunomura, S.; Maeda-Yamamoto, M.; Kawakami, Y.; Lowell, C.A.; Ra, C.; Kawakami, T. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J. Immunol. 2005, 175, 6885–6892. [Google Scholar] [CrossRef]
- Fiorini, M.; Piovani, G.; Schumacher, R.F.; Magri, C.; Bertini, V.; Mazzolari, E.; Notarangelo, L.; Notarangelo, L.D.; Barlati, S. ITGB2 mutation combined with deleted ring 21 chromosome in a child with leukocyte adhesion deficiency. J. Allergy Clin. Immunol. 2009, 124, 1356–1358. [Google Scholar] [CrossRef]
- Hoijman, E.; Häkkinen, H.-M.; Tolosa-Ramon, Q.; Jiménez-Delgado, S.; Wyatt, C.; Miret-Cuesta, M.; Irimia, M.; Callan-Jones, A.; Wieser, S.; Ruprecht, V. Cooperative epithelial phagocytosis enables error correction in the early embryo. Nature 2021, 590, 618–623. [Google Scholar] [CrossRef] [PubMed]

| Property | Value |
|---|---|
| Number of species | 2 |
| Number of genes | 2850 |
| Number of genes in orthogroups | 2834 |
| Number of unassigned genes | 16 |
| Percentage of genes in orthogroups | 99.4 |
| Percentage of unassigned genes | 0.6 |
| Number of orthogroups | 1177 |
| Number of species-specific orthogroups | 13 |
| Number of genes in species-specific orthogroups | 66 |
| Percentage of genes in species-specific orthogroups | 2.3 |
| Number of orthogroups with all species present | 1164 |
| Number of single-copy orthogroups | 984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, L.; Zhang, W. Extraction of Innate Immune Genes in Dairy Cattle and the Regulation of Their Expression in Early Embryos. Genes 2024, 15, 372. https://doi.org/10.3390/genes15030372
Wang X, Guo L, Zhang W. Extraction of Innate Immune Genes in Dairy Cattle and the Regulation of Their Expression in Early Embryos. Genes. 2024; 15(3):372. https://doi.org/10.3390/genes15030372
Chicago/Turabian StyleWang, Xue, Lili Guo, and Wenguang Zhang. 2024. "Extraction of Innate Immune Genes in Dairy Cattle and the Regulation of Their Expression in Early Embryos" Genes 15, no. 3: 372. https://doi.org/10.3390/genes15030372
APA StyleWang, X., Guo, L., & Zhang, W. (2024). Extraction of Innate Immune Genes in Dairy Cattle and the Regulation of Their Expression in Early Embryos. Genes, 15(3), 372. https://doi.org/10.3390/genes15030372




