Redirect Tropism of Fowl Adenovirus 4 Vector by Modifying Fiber2 with Variable Domain of Heavy-Chain Antibody
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, Plasmids, and Oligonucleotides
2.2. Preparation of Primary NK Cells
2.3. Construction of Adenoviral Plasmids
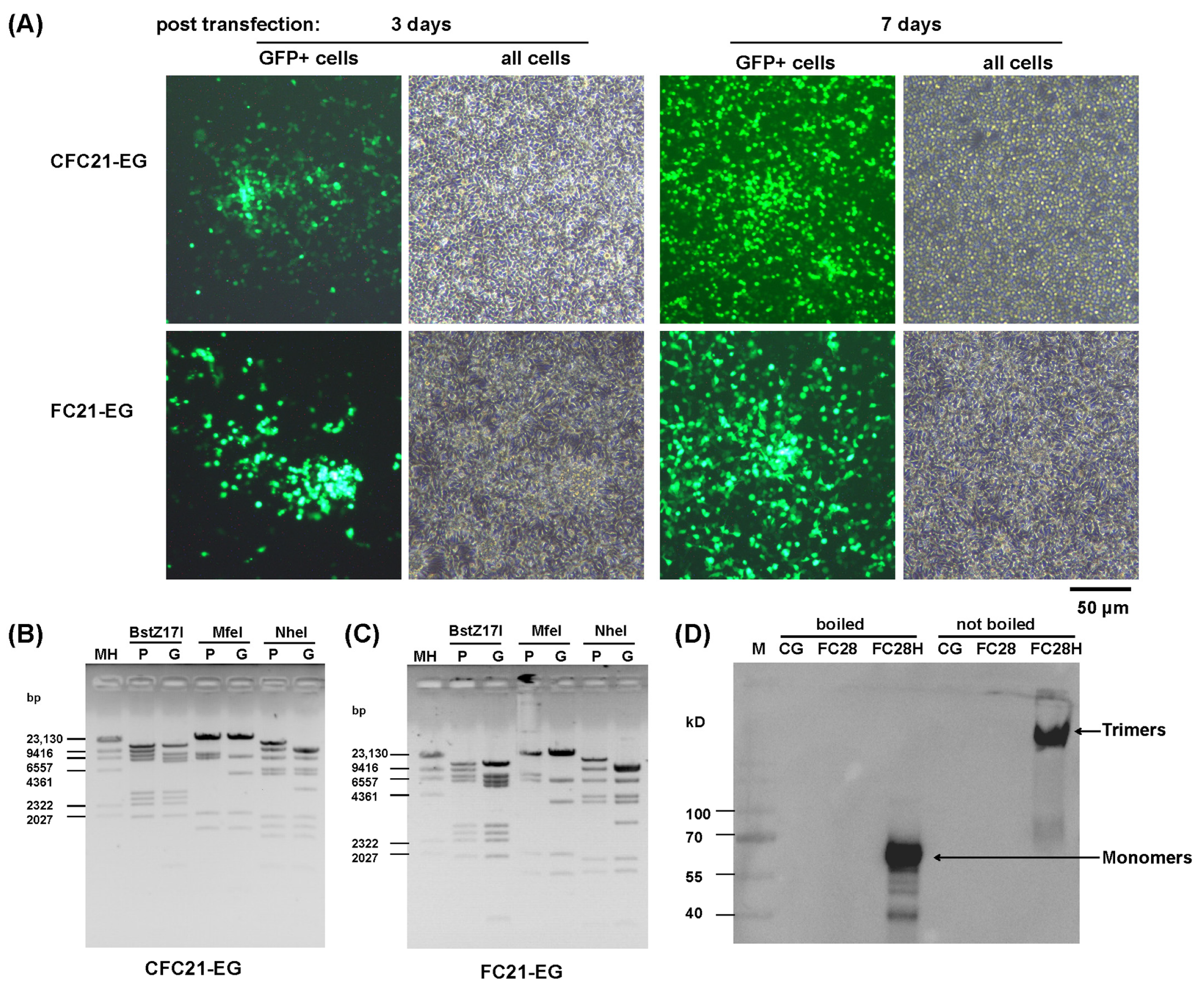
2.4. Virus Rescue, Purification, Titration, and Identification
2.5. Western Blot
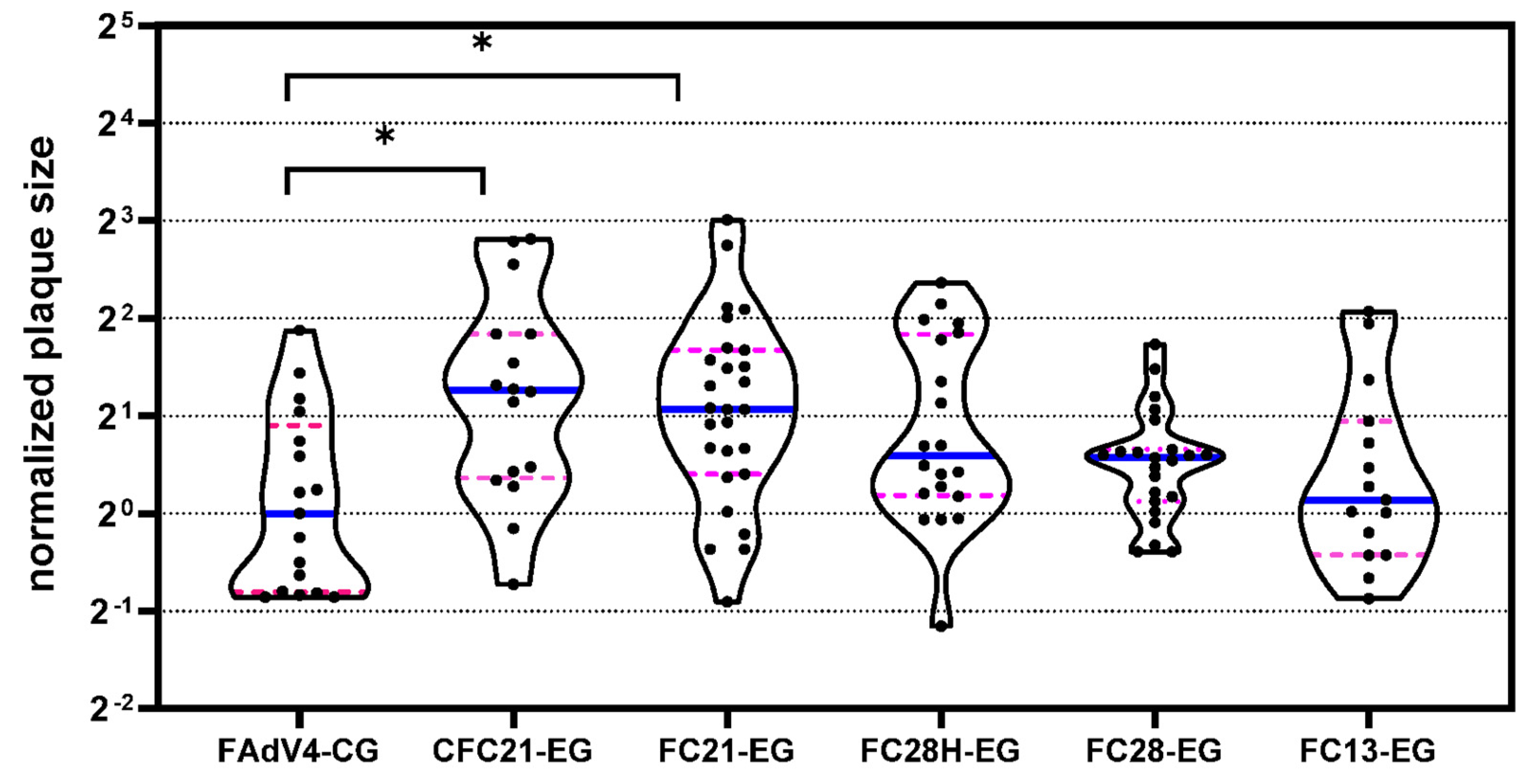
2.6. Plaque-Forming Assay
2.7. Preparation of Lentivirus Vector
2.8. Lentivirus Vector Transduction and Immunofluorescence Assay
2.9. Gene Transduction Assay
2.10. Statistical Analysis
3. Results
3.1. Construction of Recombinant Adenoviruses with VHH Displayed on the C-Terminal of Fiber
3.2. Propagation of Recombinant Adenoviruses in Packaging Cells
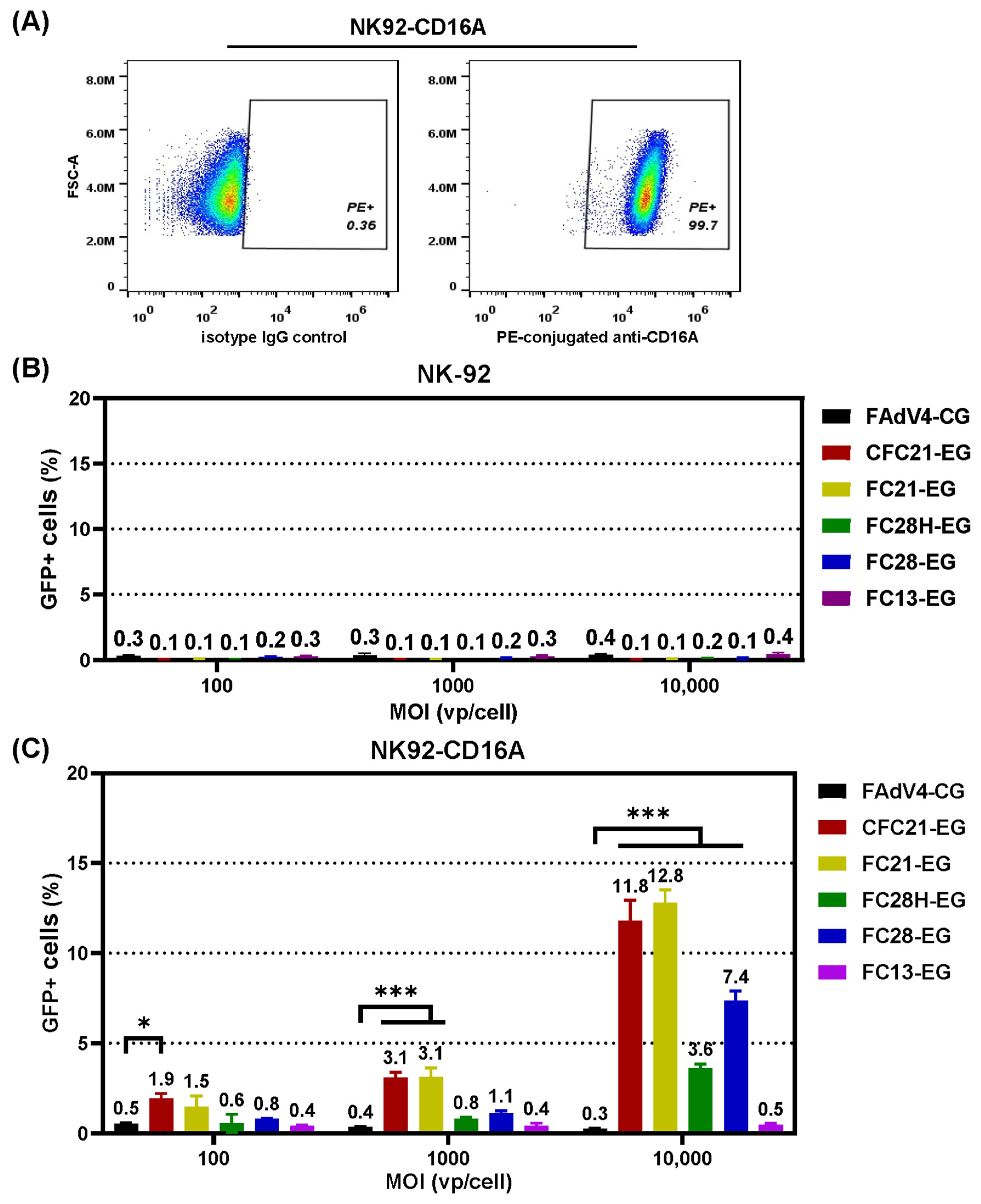
3.3. Transduction of 293 Cells or CD16A-Expressing 293 Cells
3.4. Transduction of CD16A-Expressing Suspension Cells
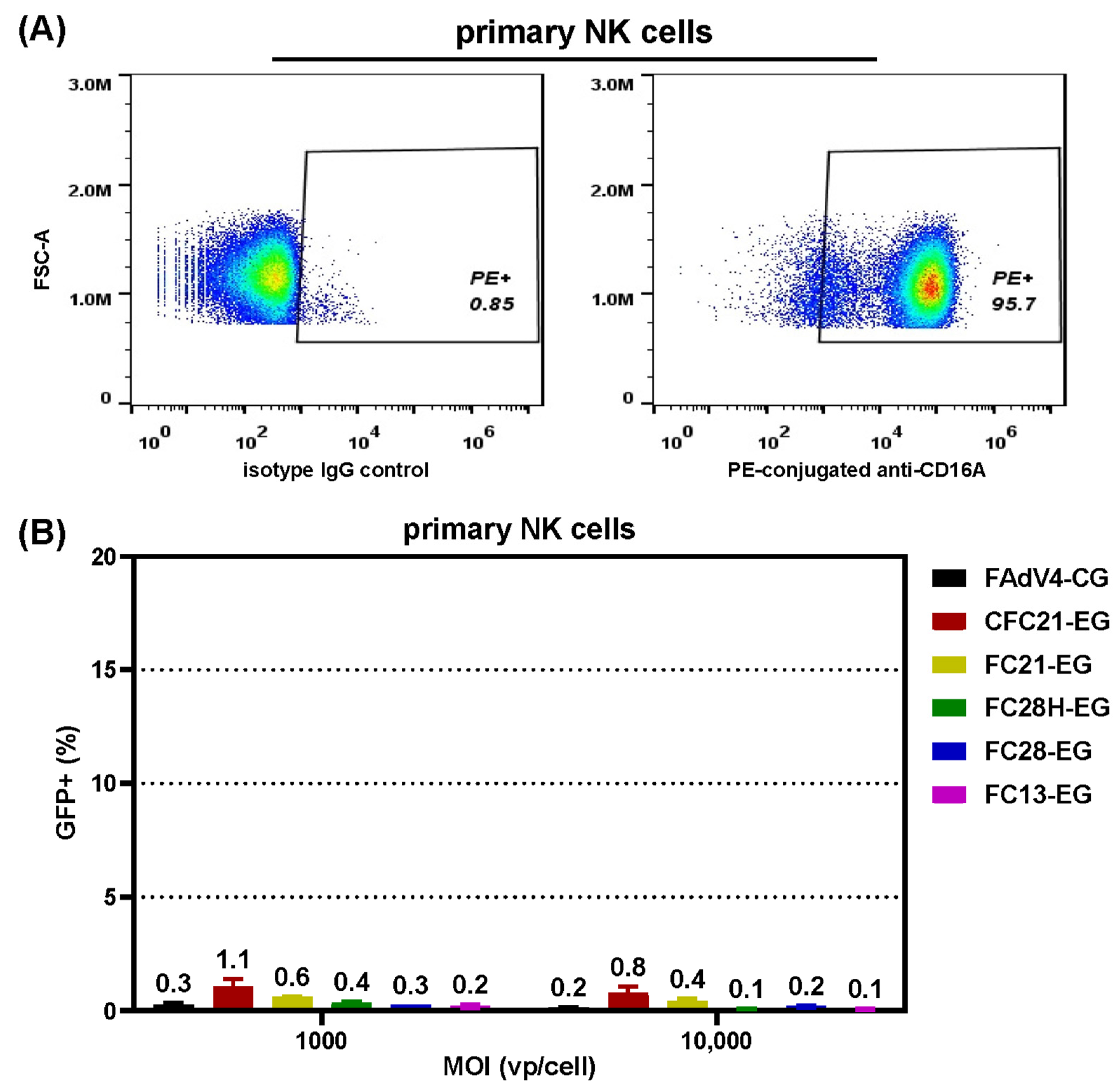
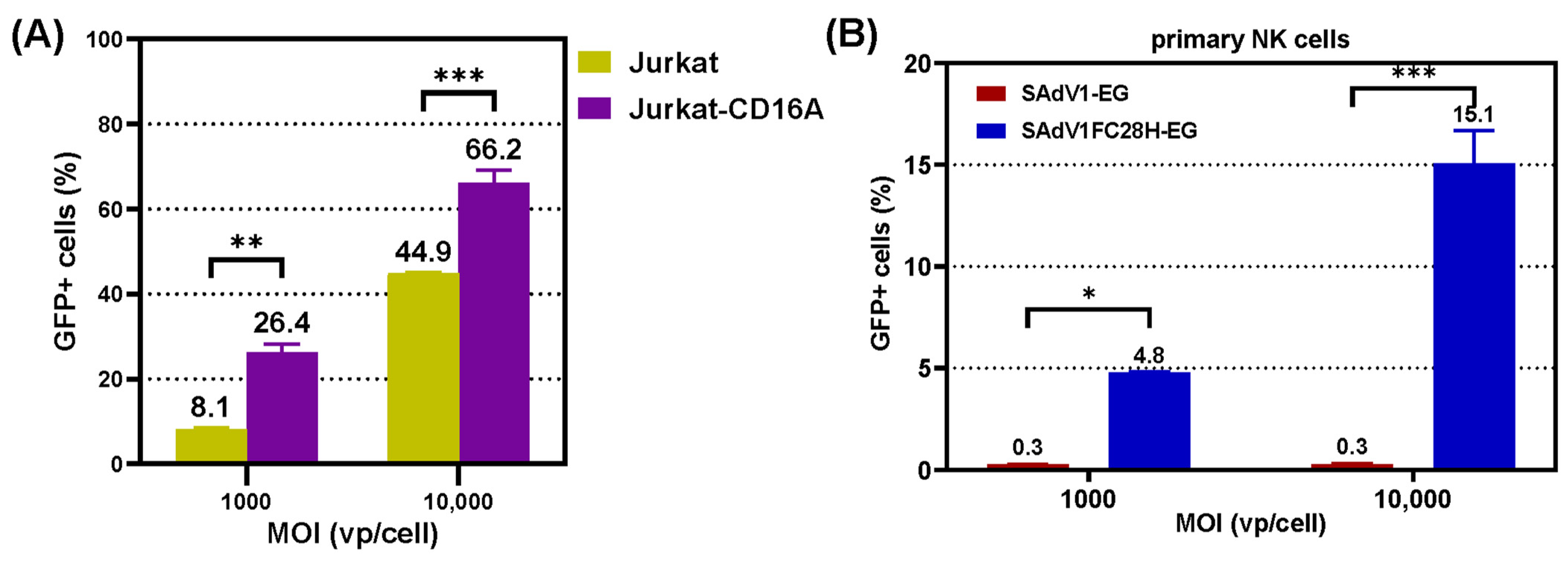
3.5. Transduction of Primary NK Cells
3.6. Transduction Ability of VHH-Modified SAdV-1 Vector
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.D.; Byrne, B.J.; Corti, M. Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines. Viruses 2023, 15, 2378. [Google Scholar] [CrossRef] [PubMed]
- Besson, S.; Vragniau, C.; Vassal-Stermann, E.; Dagher, M.C.; Fender, P. The Adenovirus Dodecahedron: Beyond the Platonic Story. Viruses 2020, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Nemerow, G.R.; Stewart, P.L.; Reddy, V.S. Structure of human adenovirus. Curr. Opin. Virol. 2012, 2, 115–121. [Google Scholar] [CrossRef]
- Marek, A.; Nolte, V.; Schachner, A.; Berger, E.; Schlotterer, C.; Hess, M. Two fiber genes of nearly equal lengths are a common and distinctive feature of Fowl adenovirus C members. Vet. Microbiol. 2012, 156, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Cuzange, A.; Ruigrok, R.W.; Chroboczek, J.; Jacrot, B. The avian adenovirus penton: Two fibres and one base. J. Mol. Biol. 1995, 252, 379–385. [Google Scholar] [CrossRef]
- Nemerow, G.; Flint, J. Lessons learned from adenovirus (1970–2019). FEBS Lett. 2019, 593, 3395–3418. [Google Scholar] [CrossRef]
- Arnberg, N. Adenovirus receptors: Implications for targeting of viral vectors. Trends Pharmacol. Sci. 2012, 33, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Seiradake, E.; Cusack, S. Crystal structure of enteric adenovirus serotype 41 short fiber head. J. Virol. 2005, 79, 14088–14094. [Google Scholar] [CrossRef] [PubMed]
- El Bakkouri, M.; Seiradake, E.; Cusack, S.; Ruigrok, R.W.; Schoehn, G. Structure of the C-terminal head domain of the fowl adenovirus type 1 short fibre. Virology 2008, 378, 169–176. [Google Scholar] [CrossRef]
- Barry, M.A.; Rubin, J.D.; Lu, S.C. Retargeting adenoviruses for therapeutic applications and vaccines. FEBS Lett. 2020, 594, 1918–1946. [Google Scholar] [CrossRef] [PubMed]
- Hajeri, P.B.; Sharma, N.S.; Yamamoto, M. Oncolytic Adenoviruses: Strategies for Improved Targeting and Specificity. Cancers 2020, 12, 1504. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Tomko, R.P.; Xu, R.; Philipson, L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 1997, 94, 3352–3356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.Y.; Liu, Y.; Persson, J.; Beyer, I.; Moller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.B.; et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011, 17, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.; Dmitriev, I.; Curiel, D. Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Ther. 1999, 6, 1336–1339. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Chekhonin, V.P. Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX. Virus Res. 2018, 257, 40–51. [Google Scholar] [CrossRef]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef]
- Morrison, C. Nanobody approval gives domain antibodies a boost. Nat. Rev. Drug Discov. 2019, 18, 485–487. [Google Scholar] [CrossRef]
- Poulin, K.L.; Lanthier, R.M.; Smith, A.C.; Christou, C.; Risco Quiroz, M.; Powell, K.L.; O’Meara, R.W.; Kothary, R.; Lorimer, I.A.; Parks, R.J. Retargeting of adenovirus vectors through genetic fusion of a single-chain or single-domain antibody to capsid protein IX. J. Virol. 2010, 84, 10074–10086. [Google Scholar] [CrossRef]
- Lee, M.; Lu, Z.H.; Shoemaker, C.B.; Tremblay, J.M.; St Croix, B.; Seaman, S.; Gonzalez-Pastor, R.; Kashentseva, E.A.; Dmitriev, I.P.; Curiel, D.T. Advanced genetic engineering to achieve in vivo targeting of adenovirus utilizing camelid single domain antibody. J. Control Release 2021, 334, 106–113. [Google Scholar] [CrossRef]
- Yan, B.; Zou, X.; Liu, X.; Zhao, J.; Zhang, W.; Guo, X.; Wang, M.; Lv, Y.; Lu, Z. User-Friendly Reverse Genetics System for Modification of the Right End of Fowl Adenovirus 4 Genome. Viruses 2020, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zou, X.; Guo, X.; Yang, C.; Hung, T.; Lu, Z. CELO Fiber1 Knob Is a Promising Candidate to Modify the Tropism of Adenoviral Vectors. Genes 2022, 13, 2316. [Google Scholar] [CrossRef]
- Guo, X.; Sun, Y.; Chen, J.; Zou, X.; Hou, W.; Tan, W.; Hung, T.; Lu, Z. Restriction-Assembly: A Solution to Construct Novel Adenovirus Vector. Viruses 2022, 14, 546. [Google Scholar] [CrossRef]
- Griffin, B.D.; Nagy, E. Coding potential and transcript analysis of fowl adenovirus 4: Insight into upstream ORFs as common sequence features in adenoviral transcripts. J. Gen. Virol. 2011, 92, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Rong, Y.; Guo, X.; Hou, W.; Yan, B.; Hung, T.; Lu, Z. Fiber1, but not fiber2, is the essential fiber gene for fowl adenovirus 4 (FAdV-4). J. Gen. Virol. 2021, 102, 001559. [Google Scholar] [CrossRef] [PubMed]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Frangsmyr, L.; Frank, M.; Blaum, B.S.; Chai, W.; Podgorski, I.I.; Harrach, B.; Benko, M.; et al. Polysialic acid is a cellular receptor for human adenovirus 52. Proc. Natl. Acad. Sci. USA 2018, 115, E4264–E4273. [Google Scholar] [CrossRef]
- Kovacs, G.M.; Harrach, B.; Zakhartchouk, A.N.; Davison, A.J. Complete genome sequence of simian adenovirus 1: An Old World monkey adenovirus with two fiber genes. J. Gen. Virol. 2005, 86, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Karvouni, M.; Vidal-Manrique, M.; Lundqvist, A.; Alici, E. Engineered NK Cells against Cancer and Their Potential Applications Beyond. Front. Immunol. 2022, 13, 825979. [Google Scholar] [CrossRef] [PubMed]
- Behar, G.; Siberil, S.; Groulet, A.; Chames, P.; Pugniere, M.; Boix, C.; Sautes-Fridman, C.; Teillaud, J.L.; Baty, D. Isolation and characterization of anti-FcgammaRIII (CD16) llama single-domain antibodies that activate natural killer cells. Protein Eng. Des. Sel. 2008, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zou, X.; Zhang, W.; Guo, X.; Wang, M.; Lv, Y.; Hung, T.; Lu, Z. No Genus-Specific Gene Is Essential for the Replication of Fowl Adenovirus 4 in Chicken LMH Cells. Microbiol. Spectr. 2022, 10, e0047022. [Google Scholar] [CrossRef]
- Koehl, U.; Brehm, C.; Huenecke, S.; Zimmermann, S.Y.; Kloess, S.; Bremm, M.; Ullrich, E.; Soerensen, J.; Quaiser, A.; Erben, S.; et al. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front. Oncol. 2013, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Wu, Y.; Xie, S.; Ma, J.; Yue, J.; Lv, R.; Tian, Z.; Fang, F.; Xiao, W. Anti-Tumor Activity of Expanded PBMC-Derived NK Cells by Feeder-Free Protocol in Ovarian Cancer. Cancers 2021, 13, 5866. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Wivel, N.A.; Palladino, J.L.; Tao, L.; Wilson, J.M. Construction of adenoviral vectors. Methods Mol. Biol. 2000, 135, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kutner, R.H.; Zhang, X.Y.; Reiser, J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 2009, 4, 495–505. [Google Scholar] [CrossRef]
- Papanikolopoulou, K.; Forge, V.; Goeltz, P.; Mitraki, A. Formation of highly stable chimeric trimers by fusion of an adenovirus fiber shaft fragment with the foldon domain of bacteriophage t4 fibritin. J. Biol. Chem. 2004, 279, 8991–8998. [Google Scholar] [CrossRef]
- Boudko, S.P.; Londer, Y.Y.; Letarov, A.V.; Sernova, N.V.; Engel, J.; Mesyanzhinov, V.V. Domain organization, folding and stability of bacteriophage T4 fibritin, a segmented coiled-coil protein. Eur. J. Biochem. 2002, 269, 833–841. [Google Scholar] [CrossRef] [PubMed]
- van Erp, E.A.; Kaliberova, L.N.; Kaliberov, S.A.; Curiel, D.T. Retargeted oncolytic adenovirus displaying a single variable domain of camelid heavy-chain-only antibody in a fiber protein. Mol. Ther. Oncolytics 2015, 2, 15001. [Google Scholar] [CrossRef] [PubMed]
- Hedley, S.J.; Auf der Maur, A.; Hohn, S.; Escher, D.; Barberis, A.; Glasgow, J.N.; Douglas, J.T.; Korokhov, N.; Curiel, D.T. An adenovirus vector with a chimeric fiber incorporating stabilized single chain antibody achieves targeted gene delivery. Gene Ther. 2006, 13, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, J.N.; Mikheeva, G.; Krasnykh, V.; Curiel, D.T. A strategy for adenovirus vector targeting with a secreted single chain antibody. PLoS ONE 2009, 4, e8355. [Google Scholar] [CrossRef] [PubMed]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Salazar Arenas, S.; Sirima, S.B.; Takoudjou Dzomo, G.R.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A review of 65 years of human adenovirus seroprevalence. Expert. Rev. Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Machado, H.B.; Herschman, H.R. The influence of innate and pre-existing immunity on adenovirus therapy. J. Cell. Biochem. 2009, 108, 778–790. [Google Scholar] [CrossRef]
- Peipp, M.; Klausz, K.; Boje, A.S.; Zeller, T.; Zielonka, S.; Kellner, C. Immunotherapeutic targeting of activating natural killer cell receptors and their ligands in cancer. Clin. Exp. Immunol. 2022, 209, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Suomalainen, M. Adenovirus entry: Stability, uncoating, and nuclear import. Mol. Microbiol. 2022, 118, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Nestic, D.; Bozinovic, K.; Pehar, I.; Wallace, R.; Parker, A.L.; Majhen, D. The Revolving Door of Adenovirus Cell Entry: Not All Pathways Are Equal. Pharmaceutics 2021, 13, 1585. [Google Scholar] [CrossRef]
- Greber, U.F.; Gomez-Gonzalez, A. Adenovirus—A blueprint for gene delivery. Curr. Opin. Virol. 2021, 48, 49–56. [Google Scholar] [CrossRef]
- Shayakhmetov, D.M.; Eberly, A.M.; Li, Z.Y.; Lieber, A. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J. Virol. 2005, 79, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Sakurai, F.; Kawabata, K.; Okada, N.; Fujita, T.; Yamamoto, A.; Hayakawa, T.; Mizuguchi, H. Interaction of penton base Arg-Gly-Asp motifs with integrins is crucial for adenovirus serotype 35 vector transduction in human hematopoietic cells. Gene Ther. 2007, 14, 1525–1533. [Google Scholar] [CrossRef] [PubMed]



| Fragment | Primer Name | Sequence | Template | Product (bp) | Annotation |
|---|---|---|---|---|---|
| EF1α-GFP | 2207F2CF1KEGf | cgtcctttcg ttacagatct tcct | pKFAV4S-F2CF1K-EG | 2126 | |
| 2207F2CF1KEGr | cggtggatcg gatatcttat ctaga | ||||
| BamHI foldon | 2304XCCfoldon-f | gtcaacaaca ctctggaagt gaaacc | NaeI-linearized pMD-FAV4FsF2cfC21 | 167 | BamHI |
| 2304XCCfoldon-r | ggagcttcgg ggatgtagcc gggtgtggag acgctcccc | NaeI | |||
| FAV4F2FC28xH | 2307F2FC28HxHf | ggccacggcc gtctactact gcaatg | pMD-FAV4FSF-C28H | 110 | EagI |
| 2307F2FC28HxHr | ggcctctaga ttatgaggag acggtgacct gggt | XbaI | |||
| SAV1F2C28Frag1 | 2307SAV1F2C28H1 | gccattaact gtaagcaacg ggacccta | pKSAV1H5E4p-AscI | 498 | AvrII |
| 2307SAV1F2C28H2 | gggatgtagc cgtcaggcga tgtaacttga ggagaa | ||||
| SAV1F2C28Frag2 | 2307SAV1F2C28H3 | gttacatcgc ctgacggcta catccccgaa gctcct | pMD-FAV4FSF-C28H | 558 | XmnI |
| 2307SAV1F2C28H4 | ggatgtcaaa tggttgtctt gaaacagttc ttagtggtga tgatgatggt gtgag | ||||
| WPREFrag | 1309WPRE-F1 | cctttccggg actttcgctt t | cell genomic DNA | 176 | |
| 1309WPRE-R1 | gcagaatcca ggtggcaaca | ||||
| 1309WPRE-probe | actcatcgcc gcctgccttg cc | ||||
| RNasePFrag | 1309RNaseP-F1 | agatttggac ctgcgagcg | cell genomic DNA | 65 | |
| 1309RNaseP-R1 | gagcggctgt ctccacaagt | ||||
| 1309RNaseP-probe | ttctgacctg aaggctctgc gcg |
| Virus Name | Short Name | Yield from Six 15 cm Dishes (×1012 vp) | Physical Titer (×1011 vp/mL) | Infectivity Titer (×109 IU/mL) | Particle-to-IU Ratio |
|---|---|---|---|---|---|
| FAdV4-CG | - | 5.3 | 35 | 8.4 | 420 |
| FAdV4CFC21-EG | CFC21-EG | 1.3 | 8.4 | 1.4 | 600 |
| FAdV4FC21-EG | FC21-EG | 2.4 | 16 | 8.5 | 190 |
| FAdV4FC28H-EG | FC28H-EG | 4.0 | 20 | 3.5 | 570 |
| FAdV4FC28-EG | FC28-EG | 1.1 | 10 | 3.0 | 330 |
| FAdV4FC13-EG | FC13-EG | 1.1 | 6.8 | 6.5 | 110 |
| SAdV1-EG | - | 0.28 | 5.1 | 19 | 30 |
| SAdV1FC28H-EG | SFC28H-EG | 0.52 | 5.8 | 1.5 | 390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zou, X.; Guo, X.; Zhang, Z.; Wang, M.; Hung, T.; Lu, Z. Redirect Tropism of Fowl Adenovirus 4 Vector by Modifying Fiber2 with Variable Domain of Heavy-Chain Antibody. Genes 2024, 15, 467. https://doi.org/10.3390/genes15040467
Wang Y, Zou X, Guo X, Zhang Z, Wang M, Hung T, Lu Z. Redirect Tropism of Fowl Adenovirus 4 Vector by Modifying Fiber2 with Variable Domain of Heavy-Chain Antibody. Genes. 2024; 15(4):467. https://doi.org/10.3390/genes15040467
Chicago/Turabian StyleWang, Yongjin, Xiaohui Zou, Xiaojuan Guo, Zhichao Zhang, Min Wang, Tao Hung, and Zhuozhuang Lu. 2024. "Redirect Tropism of Fowl Adenovirus 4 Vector by Modifying Fiber2 with Variable Domain of Heavy-Chain Antibody" Genes 15, no. 4: 467. https://doi.org/10.3390/genes15040467
APA StyleWang, Y., Zou, X., Guo, X., Zhang, Z., Wang, M., Hung, T., & Lu, Z. (2024). Redirect Tropism of Fowl Adenovirus 4 Vector by Modifying Fiber2 with Variable Domain of Heavy-Chain Antibody. Genes, 15(4), 467. https://doi.org/10.3390/genes15040467






