Factors Influencing Mortality in Children with Central Nervous System Tumors: A Cohort Study on Clinical Characteristics and Genetic Markers
Abstract
:1. Introduction
2. Methods
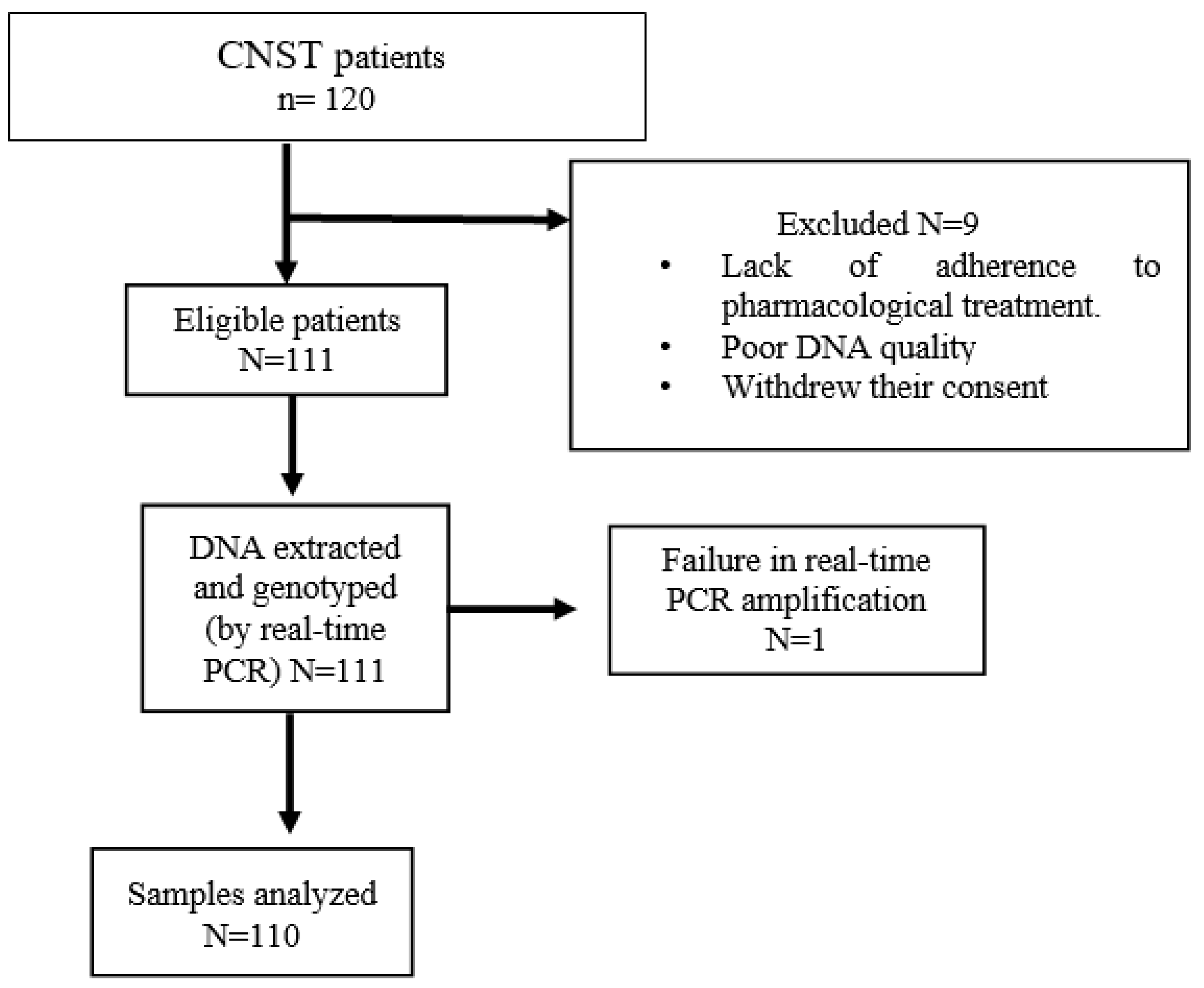
2.1. Study Subjects
2.2. Data Collection
2.3. Follow-Up (Follow-Up in Months since Initial Hospital Visit or Diagnosis)
2.4. Selection of SNP
2.5. Genotyping
2.6. Data Analysis
3. Results
3.1. Clinical and Demographic Characteristics of the Study Population
3.2. Gene Frequencies in the Study Population
3.3. Univariate Analysis of Allelic Variants in the ABCB1 and ABCG2 Genes Associated with Mortality
3.4. Multivariate Analysis to Estimate the Contributions of Clinical and Genetic Factors to Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, D.A.; King, J.B.; Lupo, P.J.; Durbin, E.B.; Tai, E.; Mills, K.; Van Dyne, E.; Buchanan Lunsford, N.; Henley, S.J.; Wilson, R.J. Counts, incidence rates, and trends of pediatric cancer in the United States, 2003–2019. J. Natl. Cancer Inst. 2023, 115, 1337–1354. [Google Scholar] [CrossRef]
- Rivera-Luna, R.; Zapata-Tarres, M.; Shalkow-Klincovstein, J.; Velasco-Hidalgo, L.; Olaya-Vargas, A.; Finkelstein-Mizrahi, N.; Cárdenas-Cardós, R.; Aguilar-Ortiz, M.R. The burden of childhood cancer in Mexico: Implications for low- and middle-income countries. Pediatr. Blood Cancer 2017, 64, e26366. [Google Scholar] [CrossRef]
- Udaka, Y.; Packer, R. Pediatric Brain Tumors. Neurol. Clin. 2018, 36, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer potential of furanocoumarins: Mechanistic and therapeutic aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef]
- Hlaváč, V.; Václavíková, R.; Brynychová, V.; Koževnikovová, R.; Kopečková, K.; Vrána, D.; Gatěk, J.; Souček, P. Role of Genetic Variation in ABC Transporters in Breast Cancer Prognosis and Therapy Response. Int. J. Mol. Sci. 2020, 21, 9556. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Zhou, Z.; Zhou, J.; Chen, S.Q. Pharmacogenomics of Drug Metabolizing Enzymes and Transporters: Relevance to Precision Medicine. Genom. Proteom. Bioinform. 2016, 14, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Au, A.; Aziz Baba, A.; Goh, A.S.; Wahid Fadilah, S.A.; Teh, A.; Rosline, H.; Ankathil, R. Association of genotypes and haplotypes of multidrug transporter genes ABCB1 and ABCG2 with clinical response to imatinib mesylate in chronic myeloid leukemia patients. Biomed. Pharmacother. 2014, 68, 343–349. [Google Scholar] [CrossRef]
- Zawadzka, I.; Jeleń, A.; Pietrzak, J.; Żebrowska-Nawrocka, M.; Michalska, K.; Szmajda-Krygier, D.; Mirowski, M.; Łochowski, M.; Kozak, J.; Balcerczak, E. The impact of ABCB1 gene polymorphism and its expression on non-small cell lung cancer development, progression and therapy—Preliminary report. Sci. Rep. 2020, 10, 6188. [Google Scholar] [CrossRef]
- Loscocco, F.; Visani, G.; Ruzzo, A.; Bagaloni, I.; Fuligni, F.; Galimberti, S.; Di Paolo, A.; Stagno, F.; Pregno, P.; Annunziata, M.; et al. Relevance of ABCB1, ABCG2, and ABCC2 Gene Polymorphisms in Chronic Myeloid Leukemia Patients Treated with Nilotinib. Front. Oncol. 2021, 11, 672287. [Google Scholar] [CrossRef]
- Qu, J.; Zhou, B.T.; Yin, J.Y.; Xu, X.J.; Zhao, Y.C.; Lei, G.H.; Tang, Q.; Zhou, H.H.; Liu, Z.Q. ABCC2 polymorphisms and haplotype are associated with drug resistance in Chinese epileptic patients. CNS Neurosci. Ther. 2012, 18, 647–651. [Google Scholar] [CrossRef]
- Wu, T.; Kang, H.; Zhuang, D.; Ma, Y.; Lin, Z.; Suolitiken, D.; Chen, B.; Xu, X. The role of ABCB1 polymorphism as a prognostic marker for primary central nervous system lymphoma. Ann. Hematol. 2019, 98, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, R.; Chauhan, L.; Alonzo, T.A.; Wang, Y.C.; Elmasry, A.; Loken, M.R.; Pollard, J.; Aplenc, R.; Raimondi, S.; Hirsch, B.A.; et al. ABCB1 SNP predicts outcome in patients with acute myeloid leukemia treated with Gemtuzumab ozogamicin: A report from Children’s Oncology Group AAML0531 Trial. Blood Cancer J. 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Singh, R.R.; Cho-Vega, J.H.; Drakos, E.; Davuluri, Y.; Khokhar, F.A.; Fayad, L.; Medeiros, L.J.; Vega, F. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod. Pathol. 2009, 22, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R. Protocols of Seguro Popular Whichare Based on Children’s Oncology Group Guidelines, 1st ed.; Editores de Textos Mexicanos, S.A de C.V.: Ciudad de México, Mexico, 2010. [Google Scholar]
- Orlandi, A.; Paolino, M.C.; Striano, P.; Parisi, P. Clinical reappraisal of the influence of drug-transporter polymorphisms in epilepsy. Expert Opin. Drug Metab. Toxicol. 2018, 14, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Soranzo, N.; Cavalleri, G.L.; Weale, M.E.; Wood, N.W.; Depondt, C.; Marguerie, R.; Sisodiya, S.M.; Goldstein, D.B. Identifying candidate causal variants responsible for altered activity of the ABCB1 multidrug resistance gene. Genome Res. 2004, 14, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, F.; Sunder-Plassmann, R.; Stogmann, E.; Gleiss, A.; Dal-Bianco, A.; Zimprich, A.; Plumer, S.; Baumgartner, C.; Mannhalter, C. Association of an ABCB1 gene haplotype with pharmacoresistance in temporal lobe epilepsy. Neurology 2004, 63, 1087–1089. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, X.; Du, F.; Shi, Y.; Sun, J.; Jia, J.; Liu, C.; Xiao, Y.; Yu, J.; Zhang, X.; et al. Association between polymorphisms of ABCB1 and prognosis in esophageal squamous cell carcinoma patients treated with taxane. J. Gene Med. 2022, 24, e3434. [Google Scholar] [CrossRef]
- Jacobs, S.S.; Dome, J.S.; Gai, J.; Gross, A.M.; Postell, E.; Hinds, P.S.; Davenport, L.; van den Anker, J.N.; Mowbray, C. Pharmacogenetic and clinical predictors of ondansetron failure in a diverse pediatric oncology population. Support. Care Cancer 2022, 30, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Olarte Carrillo, I.; García Laguna, A.I.; De la Cruz Rosas, A.; Ramos Peñafiel, C.O.; Collazo Jaloma, J.; Martínez Tovar, A. High expression levels and the C3435T SNP of the ABCB1 gene are associated with lower survival in adult patients with acute myeloblastic leukemia in Mexico City. BMC Med. Genom. 2021, 14, 251. [Google Scholar] [CrossRef]
- Sun, X.; Li, A.; Geng, X.; Liu, Y.; Zhao, H.; Zhao, Y. Effect of ABCB1 polymorphism on the clinical outcome of osteosarcoma patients after receiving chemotherapy. Pak. J. Med. Sci. 2014, 30, 886–890. [Google Scholar]
- Drain, S.; Catherwood, M.A.; Orr, N.; Galligan, L.; Rea, I.M.; Hodkinson, C.; Drake, M.B.; Kettle, P.J.; Morris, T.C.; Alexander, H.D. ABCB1 (MDR1) rs1045642 is associated with increased overall survival in plasma cell myeloma. Leuk. Lymphoma 2009, 50, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Zmorzynski, S.; Wojcierowska-Litwin, M.; Popek-Marciniec, S.; Szudy-Szczyrek, A.; Styk, W.; Chocholska, S.; Filip, A.A. The Relationship of ABCB1/MDR1 and CYP1A1 Variants with the Risk of Disease Development and Shortening of Overall Survival in Patients with Multiple Myeloma. J. Clin. Med. 2021, 10, 5276. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Saeed, M.E.M.; Munder, M.; Spuller, A.; Greten, H.J.; Efferth, T. Effect of ABC transporter expression and mutational status on survival rates of cancer patients. Biomed. Pharmacother. 2020, 131, 110718. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Xu, M.; Liu, Y.; Chen, Y.; Feng, J.; Wang, X.; Liang, S.; Li, D.; Yang, X. Influence of the ABCB1 polymorphisms on the response to Taxane-containing chemotherapy: A systematic review and meta-analysis. Cancer Chemother. Pharmacol. 2018, 81, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Im, S.A.; Keam, B.; Ham, H.S.; Lee, K.H.; Kim, T.Y.; Kim, Y.J.; Oh, D.Y.; Kim, J.H.; Han, W.; et al. ABCB1 polymorphism as prognostic factor in breast cancer patients treated with docetaxel and doxorubicin neoadjuvant chemotherapy. Cancer Sci. 2015, 106, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Seike, M.; Noro, R.; Kunugi, S.; Kubota, K.; Gemma, A. Prognostic significance of ABCB1 in stage I lung adenocarcinoma. Oncol. Lett. 2017, 14, 313–321. [Google Scholar] [CrossRef]
- Kerr, I.D.; Haider, A.J.; Gelissen, I.C. The ABCG family of membrane-associated transporters: You do not have to be big to be mighty. Br. J. Pharmacol. 2011, 16, 1767–1779. [Google Scholar] [CrossRef]
- Wolking, S.; Schaeffeler, E.; Lerche, H.; Schwab, M.; Nies, A.T. Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature. Clin. Pharmacokinet. 2015, 54, 709–735. [Google Scholar] [CrossRef]
- Müller, P.J.; Dally, H.; Klappenecker, C.N.; Edler, L.; Jäger, B.; Gerst, M.; Spiegelhalder, B.; Tuengerthal, S.; Fischer, J.R.; Drings, P.; et al. Polymorphisms in ABCG2, ABCC3 and CNT1 genes and their possible impact on chemotherapy outcome of lung cancer patients. Int. J. Cancer 2009, 124, 1669–1674. [Google Scholar] [CrossRef]
- Zhu, W.; Deng, Y.; Zhou, X. Multiple Membrane Transporters and Some Immune Regulatory Genes are Major Genetic Factors to Gout. Open Rheumatol. J. 2018, 212, 94–113. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, M.; Shen, W.; Wang, C.; Dai, J.; Xu, L.; Jin, G.; Hu, Z.; Ma, H.; Shen, H. A potentially functional polymorphism in ABCG2 predicts clinical outcome of non-small cell lung cancer in a Chinese population. Pharmacogenomics J. 2017, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Taylor, J.A. SNPinfo: Integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009, 37, W600-5. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Ross, D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Poonkuzhali, B.; Lamba, J.; Strom, S.; Sparreboom, A.; Thummel, K.; Watkins, P.; Schuetz, E. Association of breast cancer resistance protein/ABCG2 phenotypes and novel promoter and intron 1 single nucleotide polymorphisms. Drug Metab. Dispos. 2008, 36, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Johnatty, S.E.; Beesley, J.; Gao, B.; Chen, X.; Lu, Y.; Law, M.H.; Henderson, M.J.; Russell, A.J.; Hedditch, E.L.; Emmanuel, C.; et al. ABCB1 (MDR1) polymorphisms and ovarian cancer progression and survival: A comprehensive analysis from the Ovarian Cancer Association Consortium and The Cancer Genome Atlas. Gynecol. Oncol. 2013, 131, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hornicek, F.J.; Gebhardt, M.C.; Wolfe, M.W.; Kharrazi, F.D.; Takeshita, H.; Parekh, S.G.; Zurakowski, D.; Mankin, H.J. P-glycoprotein levels predict poor outcome in patients with osteosarcoma. Clin. Orthop. Relat. Res. 2000, 373, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, B.; Li, R.; Li, W.; Chen, W.; Yu, Z.; Zhang, J. Expression levels of resistance gene resistant genes affect cervical cancer prognosis. Mol. Med. Rep. 2017, 15, 2802–2806. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Haddad, G.; Thorner, P.S.; DeBoer, G.; Lin, Y.P.; Ondrusek, N.; Yeger, H.; Ling, V. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N. Engl. J. Med. 1991, 325, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Thorner, P.S.; Haddad, G.; Ling, V. Immunohistochemical detection of P-glycoprotein: Prognostic correlation in soft tissue sarcoma of childhood. J. Clin. Oncol. 1990, 8, 689–704. [Google Scholar] [CrossRef]
- International Transporter Consortium; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 15–36. [Google Scholar]
- López-Aguilar, E.; Sepúlveda-Vildósola, A.C.; Rivera-Márquez, H.; Cerecedo-Díaz, F.; Valdés-Sánchez, M.; Delgado-Huerta, S.; Wanzke-del Angel, V.; Ramón-García, G.; Rodríguez-Jiménez, H.; Hernández-Contreras, I.; et al. Preirradiation ifosfamide, carboplatin and etoposide (ICE) for the treatment of high-grade astrocytomas in children. Childs Nerv. Syst. 2003, 19, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, A.; Hamoda, A.; Zaher, A.; Khorshed, E.; Elwakeel, M.; Hassanein, O.; Sidhom, I. Outcome and toxicity of ifosfamide, carboplatin, and etoposide versus gemcitabine and vinorelbine regimen for pediatric patients with relapsed or refractory Hodgkin’s lymphoma. Front. Oncol. 2023, 27, 1153128. [Google Scholar] [CrossRef] [PubMed]
- Torres Espíndola, L.M.; Rojo-Serrato, D.; Álvaro-Heredia, A.; Castillejos López, M.J.; de Uña-Flores, A.; Pérez-García, M.; Zapata-Tarres, M.; Cárdenas-Cardos, R.; Granados, J.; Chávez-Pacheco, J.L.; et al. Analysis of CYP450 gene allelic variants can predict ifosfamide toxicity in Mexican paediatric patients. Biomarkers 2020, 25, 331–340. [Google Scholar] [CrossRef] [PubMed]
| Gen | SNP | Location SNP | Protein | Protein Level |
|---|---|---|---|---|
| ABCB1/MDR1 | rs1045642 | 3435C > T, Exon26 | Ile1145Ile | Reduced |
| rs2032582 | 2677C > A, Exon 21 | Ala893Ser | Reduced | |
| rs1128503 | 1236C > T, Exon 12 | Gly412Gly | Reduced | |
| rs6949448 | A > G, intron | - | altered mRNA | |
| ABCC1/MRP1 | rs12921623 | 5540C > G, Intron | - | altered mRNA |
| rs12921748 | 5522G > A, Intron | - | altered mRNA | |
| rs35605 | c.1684T > C, Exon14 | Leu562Leu | Reduced | |
| rs2230671 | 4002G > A, Exon 28 | Ser1334Ser | Reduced | |
| ABCC2/MRP2 | rs3740066 | 3972C > T, Exon 28 | Ile1324Ile | Reduced |
| rs2756109 | G1658T, Intron7 | - | altered mRNA | |
| ABCC4/MRP4 | rs1059751 | 4976T > C, 3′ URT | - | Reduced |
| rs4148551 | 311A > G, 3′ URT | - | Reduced | |
| rs3742106 | 38T > G, 3′ URT | - | Reduced | |
| ABCG2 | rs3114020 | −15622C > T, Promotor | - | Reduced |
| rs2231142 | C421A | Gln141Lys | Reduced |
| Characteristic | N (%) |
|---|---|
| Sex | |
| Male | 62 (55.9) |
| Female | 49 (44.1) |
| Age (years) | |
| Median | 12 |
| Tumor type | |
| Medulloblastoma | 31 (27.9) |
| Astrocytoma | 27 (24.3) |
| Ependymoma | 20 (18.0) |
| Germinoma | 8 (7.2) |
| Glioma | 6 (5.4) |
| Glioblastoma | 5 (4.5) |
| Neuroectodermic tumor | 4 (3.6) |
| Pineoblastoma | 2 (1.8) |
| Neuroglial | 2 (1.8) |
| Rhabdomyosarcoma | 2 (1.8) |
| Teratoma | 1 (1.7) |
| Atypical rhabdoid teratoid | 1 (1.7) |
| Plexus carcinoma | 1 (1.7) |
| Astroblastoma | 1 (1.7) |
| Grade | |
| High | 71 (64) |
| Low | 40 (36) |
| Status | |
| Alive | 75 (67.6) |
| Deceased | 36 (32.4) |
| Comorbidity | |
| Present | 8 (7.2) |
| Absent | 103 (92.8) |
| Symptom | N | % |
|---|---|---|
| Headache | 78 | 70.3 |
| Threw up | 76 | 68.5 |
| Gait disturbance | 66 | 59.5 |
| Nausea | 45 | 40.5 |
| Ataxia | 44 | 39.6 |
| Decreased muscle strength | 36 | 32.4 |
| Visual disturbance | 35 | 31.5 |
| Weightless | 22 | 19.8 |
| Irritability | 18 | 16.2 |
| Behavior changes | 15 | 13.5 |
| Paresthesia | 15 | 13.5 |
| Convulsive crisis | 14 | 12.6 |
| Language alteration | 11 | 9.9 |
| Papilledema | 11 | 9.9 |
| Decreased sensitivity | 8 | 7.2 |
| Cognitive changes | 8 | 7.2 |
| Hemiplegia | 7 | 6.3 |
| Dyskinesias | 6 | 5.4 |
| Lymphadenopathy | 4 | 3.6 |
| Gene | Genotypic Frequency N = (%) | Allelic Frequency |
|---|---|---|
| ABCB1 | ||
| rs1045642 | N = 110 | |
| CC | 31 (30) | C = 0.52 |
| CT | 52 (50) | |
| TT | 27(20) | T = 0.48 |
| rs2032582 | N = 110 | |
| CC | 37 (34) | C = 0.56 |
| CA | 49 (44) | |
| AA | 24(22) | A = 0.44 |
| rs1128503 | N = 110 | |
| CC | 25 (23) | C = 0.50 |
| CT | 59 (54) | |
| TT | 26 (23) | T = 0.50 |
| rs6949448 | N = 110 | |
| CC | 37 (34) | C = 0.57 |
| CT | 51(46) | |
| TT | 22 (20) | T = 0.43 |
| ABCC1 | ||
| rs12921623 | N = 110 | |
| GG | 34 (30) | G = 0.56 |
| GC | 55 (50) | |
| CC | 21 (20) | C = 0.44 |
| rs12921748 | N = 108 | |
| GG | 33 (30) | G = 0.56 |
| GA | 54 (50) | |
| AA | 21(20) | A = 0.44 |
| rs35605 | N = 110 | |
| CC | 68 (62) | C = 0.78 |
| CT | 36 (33) | |
| TT | 6 (5) | T = 0.22 |
| ABCC2 | ||
| rs2756109 | N = 107 | |
| TT | 24 (22) | T = 0.51 |
| TG | 62 (58) | |
| GG | 21 (20) | G = 0.49 |
| rs3740066 | N = 110 | |
| CC | 47 (43) | C = 0.65 |
| TC | 50 (45) | |
| TT | 13 (12) | T = 0.35 |
| ABCC4 | N = 110 | |
| rs1059751 | ||
| TT | 44 (40) | T = 0.67 |
| TC | 59 (54) | |
| CC | 7 (6) | C = 0.33 |
| rs4148551 | N = 110 | |
| TT | 26 (24) | T = 0.48 |
| CT | 54 (49) | |
| CC | 30 (27) | C = 0.52 |
| rs3742106 | N = 110 | |
| AA | 32 (29) | A = 0.52 |
| AC | 50 (45) | |
| CC | 28 (26) | C = 0.48 |
| ABCG2 | N = 105 | |
| rs3114020 | ||
| CC | 43 (41) | C = 0.66 |
| CT | 54 (51) | |
| TT | 8 (8) | T = 0.34 |
| rs2231142 | N = 105 | |
| GG | 52 (50) | G = 0.70 |
| GT | 44 (42) | |
| TT | 9 (8) | T = 0.3 |
| rs2230671 | N = 110 | |
| GG | 49 (45) | G = 0.64 |
| AG | 43 (39) | |
| AA | 18 (16) | A = 0.36 |
| Gene | Codominant | Dominant | Recessive | |||||
|---|---|---|---|---|---|---|---|---|
| ABCB1 | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p value |
| CC vs. TT | CC vs. TC | TT + TC vs. CC | TT vs. TC + CC | |||||
| rs1045642 | 0.853 (0.331–2.203) | 0.743 | 1.456 (0.543–3.901) | 0.455 | 1.009 (0.441–2.310) | 0.983 | 2.433 (1.098–5.392) | 0.029 * |
| Sex | 0.466 (0.177–1.226) | 0.122 | 0.474 (0.192–1.171 | 0.106 | 0.591 (0.295–1.184) | 0.138 | 0.409 (0.186–0.896) | 0.026 |
| Age | 0.785 (0.674–0.913) | 0.002 | 0.931 (0.838–1.033) | 0.179 | 0.956 (0.891–1.026) | 0.214 | 0.875 (0.786–0.974) | 0.014 |
| ICE scheme | 9.810 (2.74–35.06) | <0.001 * | 0.274 (0.110–0.682) | 0.005 | 6.807 (2.87–16.103) | <0.001 * | 6.903 (2.915–16.544) | 0.038 * |
| Radiotherapy | 0.092 (0.019–0.441) | 0.003 | NC | 0.098 (0.026–0.367) | 0.001 | 0.116 (0.027–0.501) | <0.001 | |
| ABCG2 | CC vs. TT | CC vs. TC | TT + TC vs. CC | TT vs. TC + CC | ||||
| rs3114020 | 2.752 (0.951–7.964) | 0.062 | 5.35 (1.83–15.39) | 0.002 * | 4.421 (1.747–11.185) | 0.002 * | 0.807 (0.365–1.785) | 0.597 |
| Sex | 0.132 (0.036–0.488) | 0.002 | 0.467 (0.178–1.223) | 0.121 | 0.454 (0.219–0.942) | 0.034 | 0.516 (0.243–1.094) | 0.084 |
| Age | 0.835 (0.732–0.954) | 0.008 | 0.979 (0.895–1.071) | 0.645 | 0.952 (0.88–1.029) | 0.215 | 0.939 (0.870–1.014) | 0.110 |
| ICE scheme | 6.351 (1.831–22.02) | 0.004 * | 9.571 (2.856–32.07) | <0.001 * | 6.592 (2.669–16.280) | <0.001 * | 5.798 (2.411–13.940) | <0.001 * |
| Radiotherapy | 0.588 (0.076–4.535) | 0.610 | NC | 0.262 (0.048–1.419) | 0.120 | 0.113 (0.025–0.520) | 0.005 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Espíndola, L.M.; Pérez-De Marcos, J.C.; Castillejos-López, M.; Velasco-Hidalgo, L.; Cárdenas-Cardós, R.; De Uña-Flores, A.; Salinas-Lara, C.; Caballero-Salazar, S.; Fernández-Plata, R.; Aquíno-Gálvez, A. Factors Influencing Mortality in Children with Central Nervous System Tumors: A Cohort Study on Clinical Characteristics and Genetic Markers. Genes 2024, 15, 473. https://doi.org/10.3390/genes15040473
Torres-Espíndola LM, Pérez-De Marcos JC, Castillejos-López M, Velasco-Hidalgo L, Cárdenas-Cardós R, De Uña-Flores A, Salinas-Lara C, Caballero-Salazar S, Fernández-Plata R, Aquíno-Gálvez A. Factors Influencing Mortality in Children with Central Nervous System Tumors: A Cohort Study on Clinical Characteristics and Genetic Markers. Genes. 2024; 15(4):473. https://doi.org/10.3390/genes15040473
Chicago/Turabian StyleTorres-Espíndola, Luz María, Juan Carlos Pérez-De Marcos, Manuel Castillejos-López, Liliana Velasco-Hidalgo, Rocío Cárdenas-Cardós, Armando De Uña-Flores, Citlaltepetl Salinas-Lara, Silvia Caballero-Salazar, Rosario Fernández-Plata, and Arnoldo Aquíno-Gálvez. 2024. "Factors Influencing Mortality in Children with Central Nervous System Tumors: A Cohort Study on Clinical Characteristics and Genetic Markers" Genes 15, no. 4: 473. https://doi.org/10.3390/genes15040473
APA StyleTorres-Espíndola, L. M., Pérez-De Marcos, J. C., Castillejos-López, M., Velasco-Hidalgo, L., Cárdenas-Cardós, R., De Uña-Flores, A., Salinas-Lara, C., Caballero-Salazar, S., Fernández-Plata, R., & Aquíno-Gálvez, A. (2024). Factors Influencing Mortality in Children with Central Nervous System Tumors: A Cohort Study on Clinical Characteristics and Genetic Markers. Genes, 15(4), 473. https://doi.org/10.3390/genes15040473






