Exploring Adiponectin in Autosomal Dominant Kidney Disease: Insight and Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection
2.2. ELISA
2.3. Western Blotting
2.4. SNPs Analysis by Sanger Sequencing
2.5. Statistical Analysis
3. Results
3.1. Biochemical and Clinical Parameters of ADPKD Patients Compared to Controls
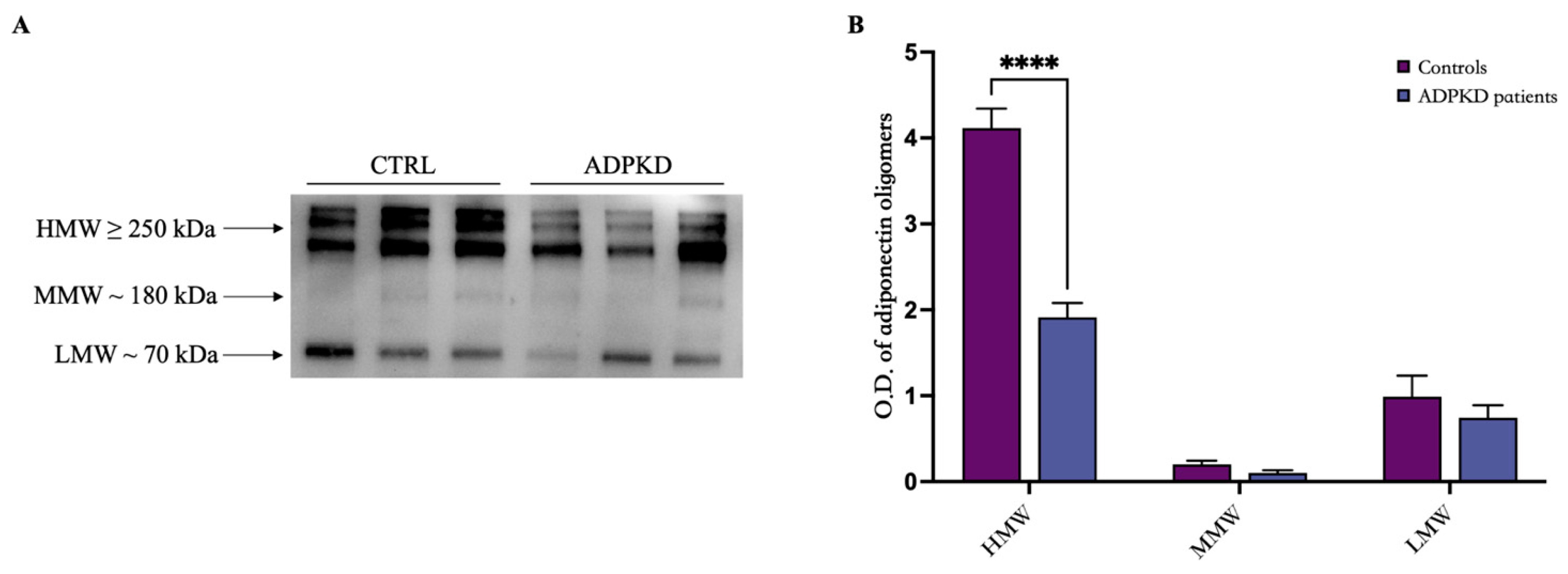
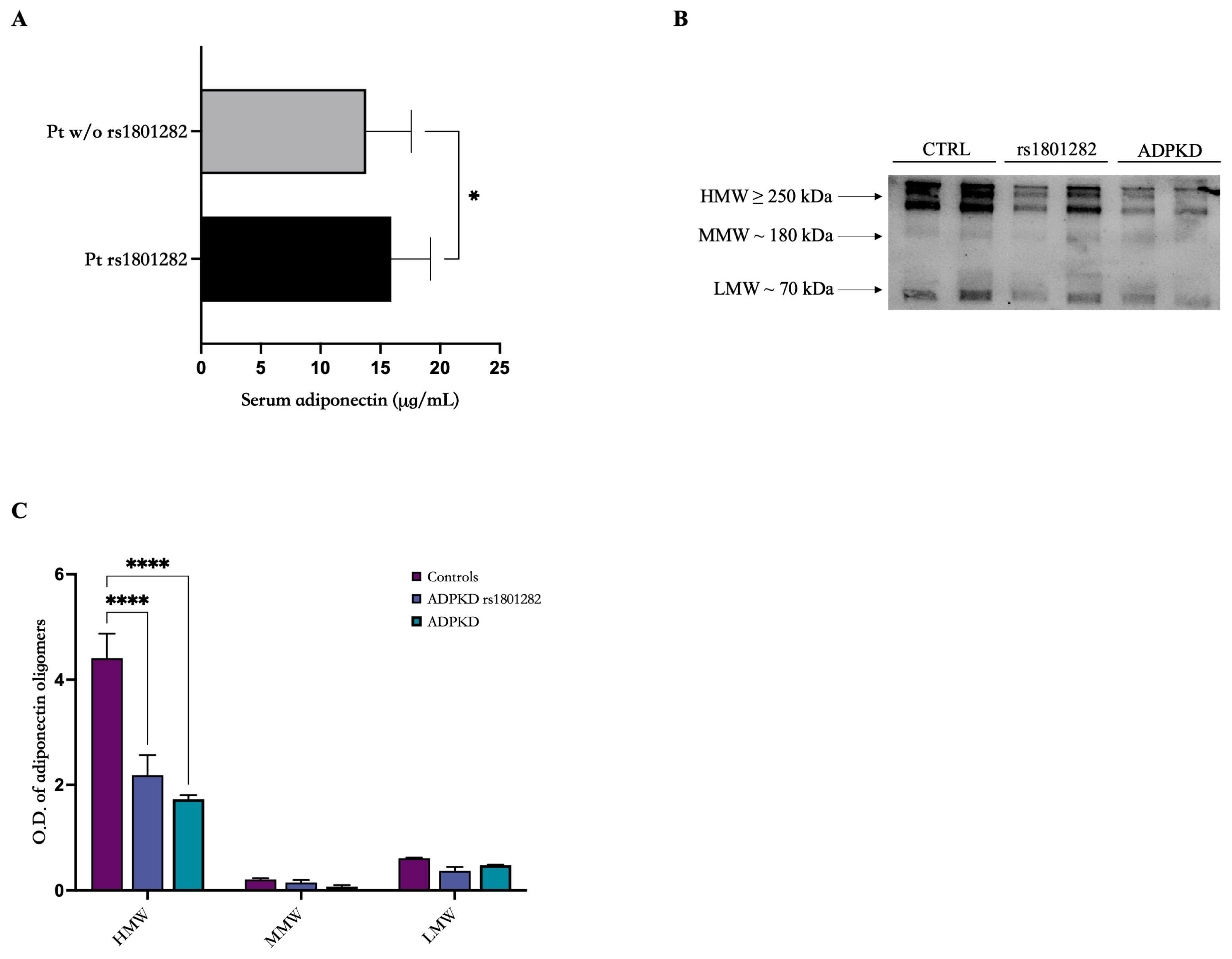
3.2. Adiponectin Evaluation and Oligomerization State
3.3. SNPs Analysis in PPARγ, ADIPOQ, ADIPOR1 and ADIPOR2 Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Satariano, M.; Ghose, S.; Raina, R. The Pathophysiology of Inherited Renal Cystic Diseases. Genes 2024, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.C.; Torres, V.E. Polycystic kidney disease. Annu. Rev. Med. 2009, 60, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, I.; Buyukkaba, M.; Cinar, A.; Tunc, M.; Cebeci, E.; Gursu, M.; Kazancioglu, R. Endothelial Dysfunction and Atherosclerosis in Patients With Autosomal Dominant Polycystic Kidney Disease. Cureus 2021, 13, e13561. [Google Scholar] [CrossRef] [PubMed]
- Azurmendi, P.J.; Fraga, A.R.; Galan, F.M.; Kotliar, C.; Arrizurieta, E.E.; Valdez, M.G.; Forcada, P.J.; Stefan, J.S.; Martin, R.S. Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol. Dial. Transplant. 2009, 24, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Pandita, S.; Khullar, D.; Saxena, R.; Verma, I.C. Autosomal Dominant Polycystic Kidney Disease: Presence of Hypomorphic Alleles in PKD1 Gene. Indian J. Nephrol. 2018, 28, 482–484. [Google Scholar] [CrossRef]
- Riccio, E.; Imbriaco, M.; Daniele, A.; Iaccarino, G.; Pisani, A. The Case|A patient with autosomal dominant polycystic kidney disease with an atypical kidney magnetic resonance image. Kidney Int. 2023, 104, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Jha, R.K.; Keerti, A. Chronic Kidney Disease: Its Relationship With Obesity. Cureu 2022, 14, e30535. [Google Scholar] [CrossRef] [PubMed]
- Serrano, E.; Shenoy, P.; Cantarin, M.P. Adipose tissue metabolic changes in chronic kidney disease. Immunometabolism 2023, 5, e00023. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Sun, Y.; Xun, K.; Wang, C.; Zhao, H.; Bi, H.; Chen, X.; Wang, Y. Adiponectin, an unlocking adipocytokine. Cardiovasc. Ther. 2009, 27, 59–75. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Polito, R.; Monaco, M.L.; Cacciatore, F.; Scioli, M.; Ferrara, N.; Daniele, A.; Nigro, E. Adiponectin Expression and Genotypes in Italian People with Severe Obesity Undergone a Hypocaloric Diet and Physical Exercise Program. Nutrients 2019, 11, 2195. [Google Scholar] [CrossRef]
- Hassun, L.A.; Ruggeri, M.L.R.; de Souza, S.A.; Rossato, A.M.; Chmieleski, G.S.; de Carvalho, L.S.; Riccetto, A.G.L.; Degasperi, G.R. Adipokines from adipose tissue and common variable immunodeficiency: Is there any association? Scand. J. Immunol. 2023, 98, e13257. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Jiang, T.-X.; Hu, S.-X.; Shi, Y.-H. Association between serum adiponectin concentrations and chronic obstructive pulmonary disease: A meta-analysis. Biosci. Rep. 2020, 40, BSR20192234. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Nasri, P.; Nasri, H. Adiponectin and chronic kidney disease; a review on recent findings. J. Nephropharmacol. 2015, 4, 63–68. [Google Scholar]
- Vahdat, S. The complex effects of adipokines in the patients with kidney disease. J. Res. Med. Sci. 2018, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Przybyciński, J.; Dziedziejko, V.; Puchałowicz, K.; Domański, L.; Pawlik, A. Adiponectin in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 9375. [Google Scholar] [CrossRef]
- Song, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Oh, K.H.; Ahn, C.; Wan Kim, S.; Bae, E.H. High serum adiponectin as a biomarker of renal dysfunction: Results from the KNOW-CKD study. Sci. Rep. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Shoji, T.; Shinohara, K.; Hatsuda, S.; Kimoto, E.; Fukumoto, S.; Emoto, M.; Tahara, H.; Koyama, H.; Ishimura, E.; Miki, T.; et al. Altered relationship between body fat and plasma adiponectin in end-stage renal disease. Metabolism 2005, 54, 330–334. [Google Scholar] [CrossRef]
- Menon, V.; Li, L.; Wang, X.; Greene, T.; Balakrishnan, V.; Madero, M.; Pereira, A.A.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; et al. Adiponectin and mortality in patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 2599–2606. [Google Scholar] [CrossRef]
- Siitonen, N.; Pulkkinen, L.; Lindström, J.; Kolehmainen, M.; Eriksson, J.G.; Venojärvi, M.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Uusitupa, M. Association of ADIPOQ gene variants with body weight, type 2 diabetes and serum adiponectin concentrations: The Finnish Diabetes Prevention Study. BMC Med. Genet. 2011, 12, 5. [Google Scholar] [CrossRef]
- Hivert, M.-F.; Manning, A.K.; McAteer, J.B.; Florez, J.C.; Dupuis, J.; Fox, C.S.; O’Donnell, C.J.; Cupples, L.A.; Meig, J.B. Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: The Framingham Offspring Study. Diabetes 2008, 57, 3353–3359. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, A.; Ierodiakonou, D.; Awofala, A.A.; Petrou, M.; Kales, S.N.; Christiani, D.C.; Mantzoros, C.S.; Christophi, C.A. Variants in ADIPOQ gene are linked to adiponectin levels and lung function in young males independent of obesity. PLoS ONE 2020, 15, e0225662. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, H.; Kuang, J.; Hou, X.; Feng, Y.; Su, Z. Genetic variations in ADIPOQ gene are associated with chronic obstructive pulmonary disease. PLoS ONE 2012, 7, e50848. [Google Scholar] [CrossRef]
- Chen, H.-H.; Huang, Y.-L.; Chen, M.-C.; Wu, C.-Y.; Lin, Y.-C.; Shiue, H.-S.; Hsu, S.-L.; Hsueh, Y.-M. Chronic Kidney Disease: Interaction of Adiponectin Gene Polymorphisms and Diabetes. Int. J. Mol. Sci. 2023, 24, 8128. [Google Scholar] [CrossRef]
- Zha, D.; Wu, X.; Gao, P. Adiponectin and Its Receptors in Diabetic Kidney Disease: Molecular Mechanisms and Clinical Potential. Endocrinology 2017, 158, 2022–2034. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, C.; Lei, Y.; Xu, S.; Zhao, D.; Lu, X.-Y. Role of the adipose PPARŒ≥-adiponectin axis in susceptibility to stress. Mol. Psychiatry 2017, 22, 1056–1068. [Google Scholar] [CrossRef]
- Nigro, E.; Amicone, M.; D’arco, D.; Sellitti, G.; De Marco, O.; Guarino, M.; Riccio, E.; Pisani, A.; Daniele, A. Molecular Diagnosis and Identification of Novel Pathogenic Variants in a Large Cohort of Italian Patients Affected by Polycystic Kidney Diseases. Genes 2023, 14, 1236. [Google Scholar] [CrossRef] [PubMed]
- Becic, T.; Studenik, C.; Hoffmann, G. Exercise Increases Adiponectin and Reduces Leptin Levels in Prediabetic and Diabetic Individuals: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2018, 6, 97. [Google Scholar] [CrossRef]
- Signoriello, E.; Lus, G.; Polito, R.; Casertano, S.; Scudiero, O.; Coletta, M.; Monaco, M.L.; Rossi, F.; Nigro, E.; Daniele, A. Adiponectin profile at baseline is correlated to progression and severity of multiple sclerosis. Eur. J. Neurol. 2019, 26, 348–355. [Google Scholar] [CrossRef]
- Christou, G.A.; Kiortsis, D.N. The role of adiponectin in renal physiology and development of albuminuria. J. Endocrinol. 2014, 221, R49–R61. [Google Scholar] [CrossRef]
- Kim, H.Y.; Bae, E.H.; Ma, S.K.; Chae, D.W.; Choi, K.H.; Kim, Y.-S.; Hwang, Y.-H.; Ahn, C.; Kim, S.W. Association of serum adiponectin level with albuminuria in chronic kidney disease patients. Clin. Exp. Nephrol. 2016, 20, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sweiss, N.; Sharma, K. Adiponectin effects on the kidney. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Carrero, J.J.; Lindholm, B.; Stenvinkel, P. The complex role of adiponectin in chronic kidney disease. Biochimie 2012, 94, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Howlader, M.; Sultana, I.; Akter, F.; Murad Hossain, M. Adiponectin gene polymorphisms associated with diabetes mellitus: A descriptive review. Heliyon 2021, 7, e07851. [Google Scholar] [CrossRef] [PubMed]
- Zusi, C.; Rioda, M.; Maguolo, A.; Emiliani, F.; Unali, I.; Costantini, S.; Corradi, M.; Contreas, G.; Morandi, A.; Maffeis, C. IGF1 and PPARG polymorphisms are associated with reduced estimated glomerular filtration rate in a cohort of children and adolescents with type 1 diabetes. Acta Diabetol. 2023, 60, 1351–1358. [Google Scholar] [CrossRef]
- von Frankenberg, A.D.; Reis, A.; Gerchman, F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arch. Endocrinol. Metab. 2017, 61, 614–622. [Google Scholar] [CrossRef] [PubMed]
- AlSaleh, A.; Sanders, T.A.B.; O’Dell, S.D. Effect of interaction between PPARG, PPARA and ADIPOQ gene variants and dietary fatty acids on plasma lipid profile and adiponectin concentration in a large intervention study. Proc. Nutr. Soc. 2012, 71, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Riera-Guardia, N.; Rothenbacher, D. The effect of thiazolidinediones on adiponectin serum level: A meta-analysis. Diabetes Obes. Metab. 2008, 10, 367–375. [Google Scholar] [CrossRef]
- Mao, Z.; Valluru, M.K.; Ong, A.C.M. Drug repurposing in autosomal dominant polycystic kidney disease: Back to the future with pioglitazone. Clin. Kidney J. 2021, 14, 1715–1718. [Google Scholar] [CrossRef]
- Pileggi, S.; Barlera, S.; Nicolis, E.; Crociati, L.; Pietri, S.; Specchia, C.; Franzosi, M.G. Association of ADIPOQ variants and heart failure in an Italian population. Ther. Adv. Cardiovasc. Dis. 2014, 8, 89–96. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Izaola, O.; de la Fuente, B.; Primo, D.; Ovalle, H.F.; Romero, E. rs1501299 Polymorphism in the Adiponectin Gene and Their Association with Total Adiponectin Levels, Insulin Resistance and Metabolic Syndrome in Obese Subjects. Ann. Nutr. Metab. 2016, 69, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; Izaola, O.; Primo, D.; de Luis, D.A. The effect of single-nucleotide polymorphisms at the ADIPOQ gene locus rs1501299 on metabolic parameters after 9 mo of a high-protein/low-carbohydrate versus a standard hypocaloric diet. Nutrition 2019, 65, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ntalla, I.; Dedoussis, G.; Yannakoulia, M.; Smart, M.C.; Louizou, E.; Sakka, S.D.; Papoutsakis, C.; Talmud, P.J. ADIPOQ gene polymorphism rs1501299 interacts with fibre intake to affect adiponectin concentration in children: The GENe-Diet Attica Investigation on childhood obesity. Eur. J. Nutr. 2009, 48, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Simeone, C.A.; Wilkerson, J.L.; Poss, A.M.; Banks, J.A.; Varre, J.V.; Guevara, J.L.; Hernandez, E.J.; Gorsi, B.; Atkinson, D.L.; Turapov, T.; et al. A dominant negative ADIPOQ mutation in a diabetic family with renal disease, hypoadiponectinemia, and hyperceramidemia. NPJ Genom. Med. 2022, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, C.W. Mechanisms of Adiponectin Action: Implication of Adiponectin Receptor Agonism in Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1782. [Google Scholar] [CrossRef]
- Kobayashi, H.; Otsuka, H.; Yanai, M.; Hara, M.; Hishiki, M.; Soma, M.; Abe, M. Adiponectin Receptor gene Polymorphisms are Associated with Kidney Function in Elderly Japanese Populations. J. Atheroscler. Thromb. 2019, 26, 328–339. [Google Scholar] [CrossRef]
| Parameter | ADPKD n = 92 | Controls n = 104 | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Age (years) | 42.84 (14.7) | 45.96 (16.6) | 0.169 | |
| Sex | F | 49 | 48 | 0.86 |
| M | 43 | 56 | ||
| BMI (kg/m2) | 25.81 (4.8) | 24.66 (2.5) | 0.065 | |
| Systolic pressure (mmHg) | 132.89 (16.7) | 123.66 (3.35) | 0.006 | |
| Diastolic pressure (mmHg) | 82.35 (10.3) | 83.66 (5.23) | 0.50 | |
| Total cholesterol (mg/dL) | 178.48 (35.9) | 185.84 (33.1) | 0.06 | |
| Triglycerides (mg/dL) | 101.34 (67.3) | 94.06 (50.51) | 0.39 | |
| Glycemia (mg/dL) | 81.47 (17.3) | 83.96 (15.0) | 0.29 | |
| AST (U/L) | 20.66 (6.4) | 20.36 (6.5) | 0.74 | |
| ALT (U/L) | 20.04 (11.4) | 22.15 (15.9) | 0.08 | |
| WBC (×103/μL) | 6.91 (2.0) | 6.57 (1.5) | 0.30 | |
| Neutrophils % | 60.20 (10.2) | 56.91 (9.6) | 0.02 | |
| Lymphocytes %, | 28.15 (7.8) | 32.63 (8.2) | 0.00 | |
| NLR | 2.46 (1.3) | 1.95 (0.9) | 0.004 | |
| RBC (×106/μL) | 4.66 (0.7) | 4.92 (0.9) | 0.06 | |
| HGB (g/dL) | 15.27 (1.3) | 14.09 (1.3) | 0.40 | |
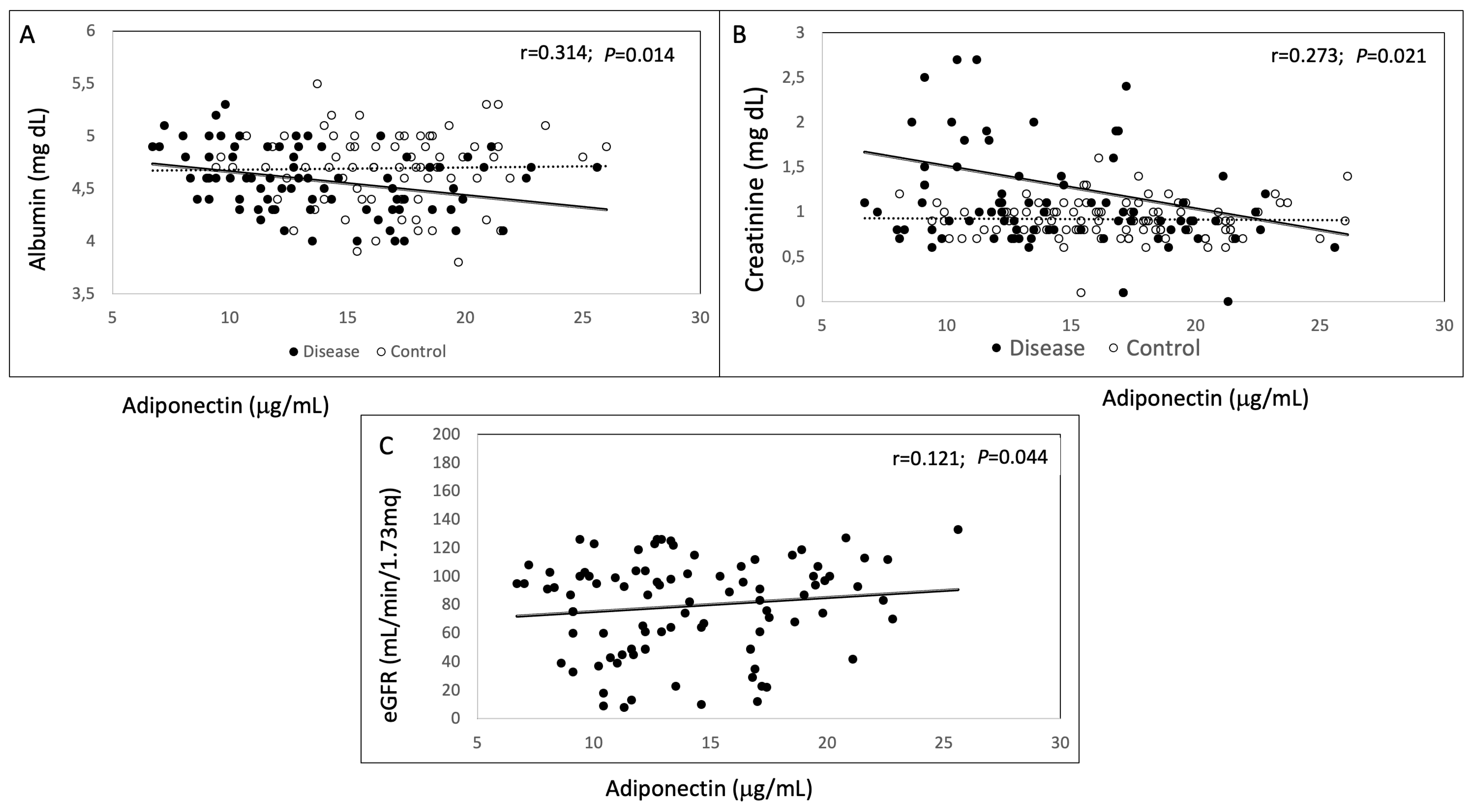
| Albumin (mg/dL) | 4.55 (0.3) | 4.70 (0.3) | 0.01 | |
| Creatinine (mg/dL) | 1.29 (1.1) | 0.91 (0.2) | 0.001 | |
| Uric Acid (mg/dL) | 5.45 (1.6) | 4.79 (1.2) | 0.005 | |
| Urea (mg/dL) | 49.89 (30.2) | 36.20 (9.5) | 0.000 | |
| EGFR (mL/min/1.73 m2) | 78.99 (33.3) | 90 (6.7) |
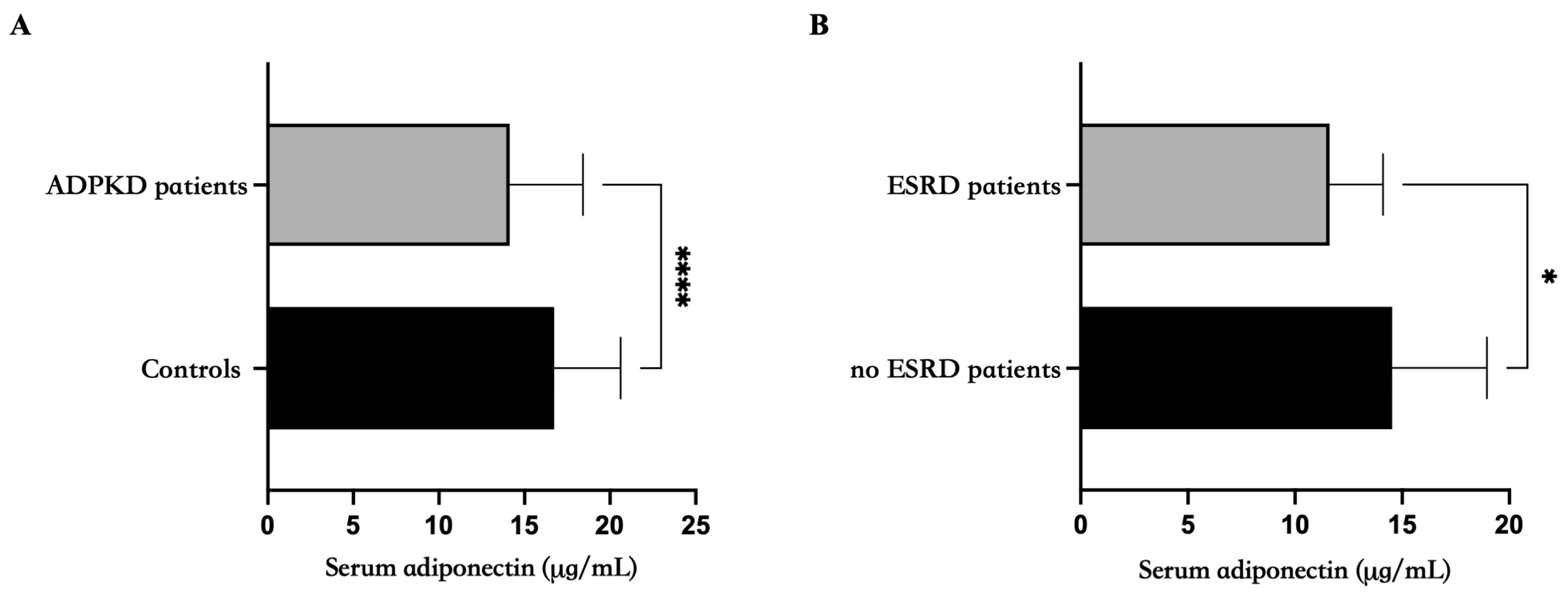
| Adjusted | Adiponectin | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | ADPKD | |||||||
| Mean | ES | Mean | ± | ES | ||||
| Age | years | 16.7 | ± | 0.402 | 14.1 | ± | 0.425 | 0.00001 |
| Body Weight | kg | 16.6 | ± | 0.414 | 14.3 | ± | 0.431 | 0.0003 |
| BMI | kg/m2 | 16.7 | ± | 0.404 | 14.2 | ± | 0.428 | 0.00004 |
| Systolic pressure | mmHG | 17.0 | ± | 0.717 | 14.3 | ± | 0.445 | 0.002 |
| Diastolic Pressure | mmHG | 17.1 | ± | 0.700 | 14.3 | ± | 0.441 | 0.0009 |
| Gene | SNP (rs Number) | n Patients | Het/Homo | Age | Adiponectin (µg/mL) (st.dev) | eGFR (mL/min/1.73 mq) (st.dev) | Systolic Blood Pressure (mm/Hg) (st.dev) | Diastolic Blood Pressure (mm/Hg) (st.dev) |
|---|---|---|---|---|---|---|---|---|
| PPARγ | c.34 C>G (rs1801282) | 14 | 14/0 | 46 (15.35) | 15.92 (3.27) | 65.21 (36.87) | 133.30 (22.65) | 83.52 (18.18) |
| ADIPOQ | c.45C>T (rs2241766) | 26 | 20/6 | 43 (14.84) | 14.11 (4.39) | 66.18 (31.85) | 136.09 18.17) | 82.97 (9.49) |
| c.268G>A (rs62625753) | 4 | 4/0 | 58 (18.6) | 14.25 (5.00) | 50.25 (37.73) | 136.67 (5.77) | 88.33 (7.63) | |
| c.214+62G>T (rs1501299) | 29 | 24/5 | 43.7(16.28) | 14.5 (4.42) | 78.3 (35.15) | 134.0 (18.11) | 84.4 (10.21) | |
| ADIPOR1 | c. 94-8T>G (rs2275737) | 23 | 17/6 | 44 (12.92) | 13.25 (3.86) | 64.34 (31.36) | 134.14 (13.36) | 82.14 (8.16) |
| c. 94-12>G (rs2275738) | 25 | 18/7 | 44 (12.92) | 13.25 (3.86) | 64.34 (31.36) | 134.14 (13.36) | 82.14 (8.16) | |
| ADIPOR2 | c.795G>A (rs16928751) | 18 | 16/2 | 44 (13.60) | 14.11 (3.97) | 67.80 (38.35) | 133.53 (13.89) | 84.12 (10.64) |
| c.1718C>T (rs1044471) | 23 | 17/6 | 41 (12.65) | 12.98 (3.56) | 68.99 (33.83) | 132.32 (13.50) | 80.89 (8.39) | |
| c.1642C>T (rs12342) | 16 | 13/3 | 45 (14.47) | 14.57 (4.39) | 68.86 (34.50) | 134.25 (15.83) | 82.97 (9.53) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigro, E.; Mallardo, M.; Amicone, M.; D’Arco, D.; Riccio, E.; Marra, M.; Pasanisi, F.; Pisani, A.; Daniele, A. Exploring Adiponectin in Autosomal Dominant Kidney Disease: Insight and Implications. Genes 2024, 15, 484. https://doi.org/10.3390/genes15040484
Nigro E, Mallardo M, Amicone M, D’Arco D, Riccio E, Marra M, Pasanisi F, Pisani A, Daniele A. Exploring Adiponectin in Autosomal Dominant Kidney Disease: Insight and Implications. Genes. 2024; 15(4):484. https://doi.org/10.3390/genes15040484
Chicago/Turabian StyleNigro, Ersilia, Marta Mallardo, Maria Amicone, Daniela D’Arco, Eleonora Riccio, Maurizio Marra, Fabrizio Pasanisi, Antonio Pisani, and Aurora Daniele. 2024. "Exploring Adiponectin in Autosomal Dominant Kidney Disease: Insight and Implications" Genes 15, no. 4: 484. https://doi.org/10.3390/genes15040484
APA StyleNigro, E., Mallardo, M., Amicone, M., D’Arco, D., Riccio, E., Marra, M., Pasanisi, F., Pisani, A., & Daniele, A. (2024). Exploring Adiponectin in Autosomal Dominant Kidney Disease: Insight and Implications. Genes, 15(4), 484. https://doi.org/10.3390/genes15040484