The First Identification of Homomorphic XY Sex Chromosomes by Integrating Cytogenetic and Transcriptomic Approaches in Plestiodon elegans (Scincidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chromosome Preparation and Staining
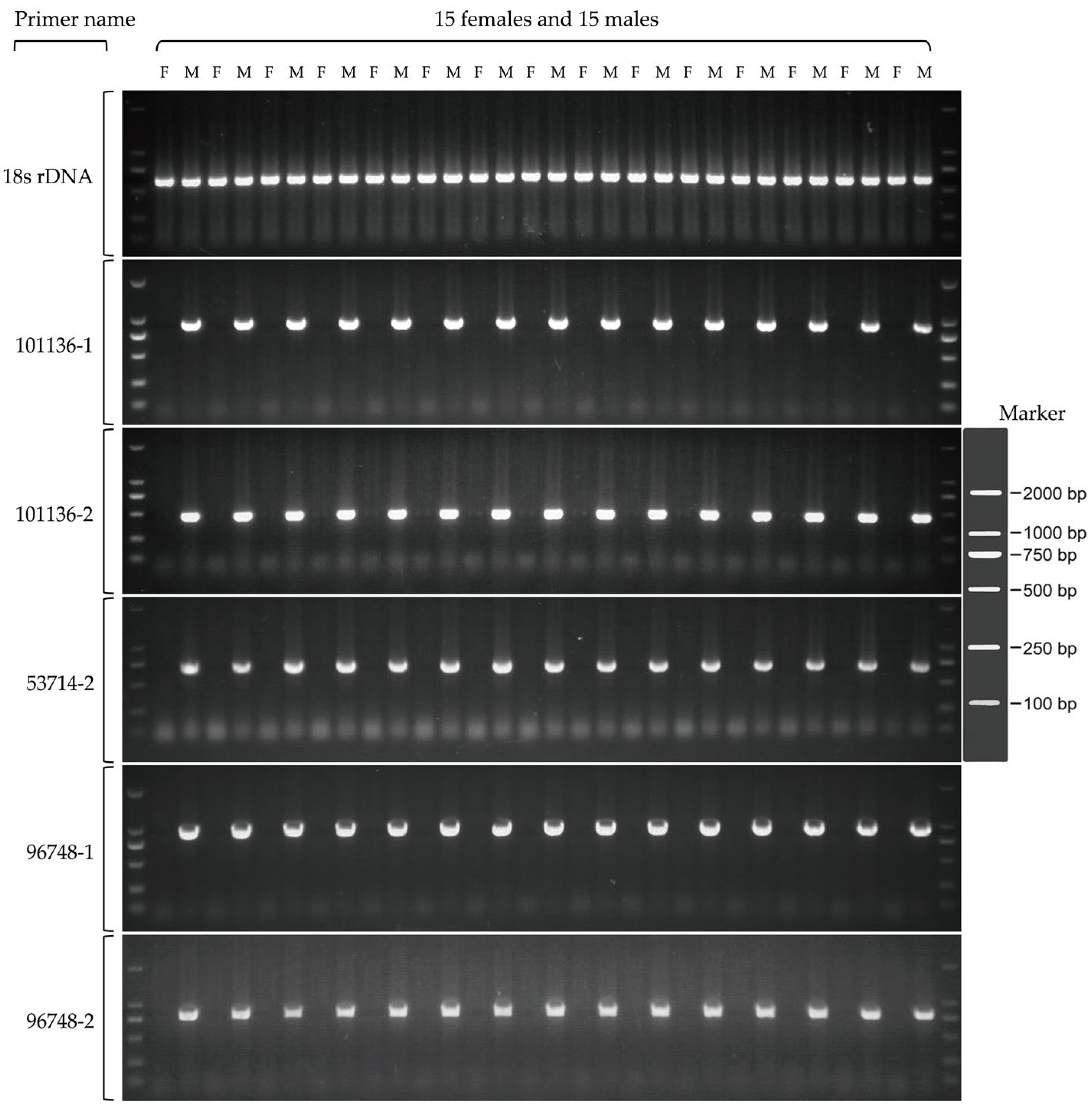
2.3. Sex Quantification of Genes
2.4. RNA Sequencing and Identification of Sex Markers
3. Results
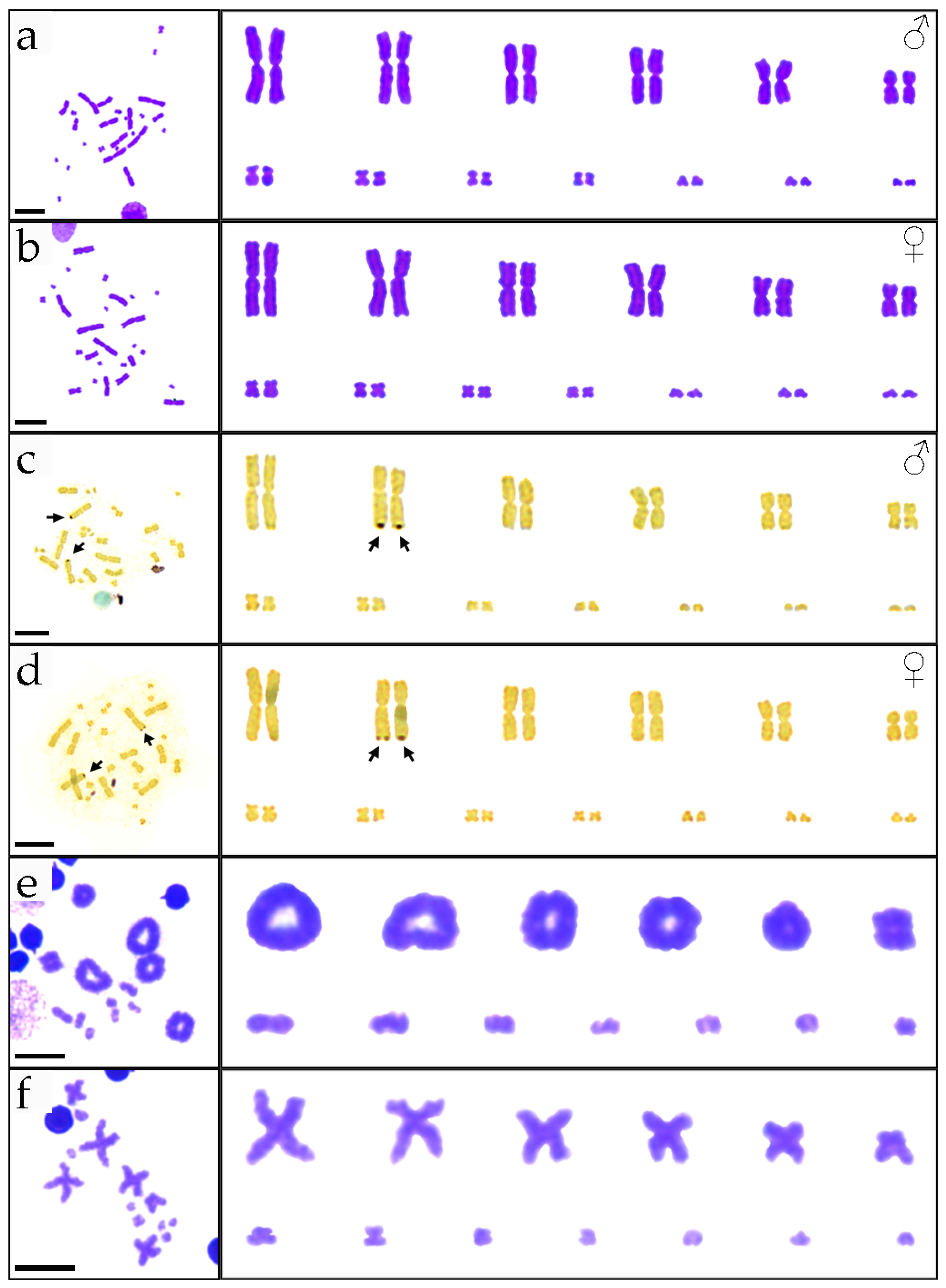
3.1. Karyotypes of P. elegans
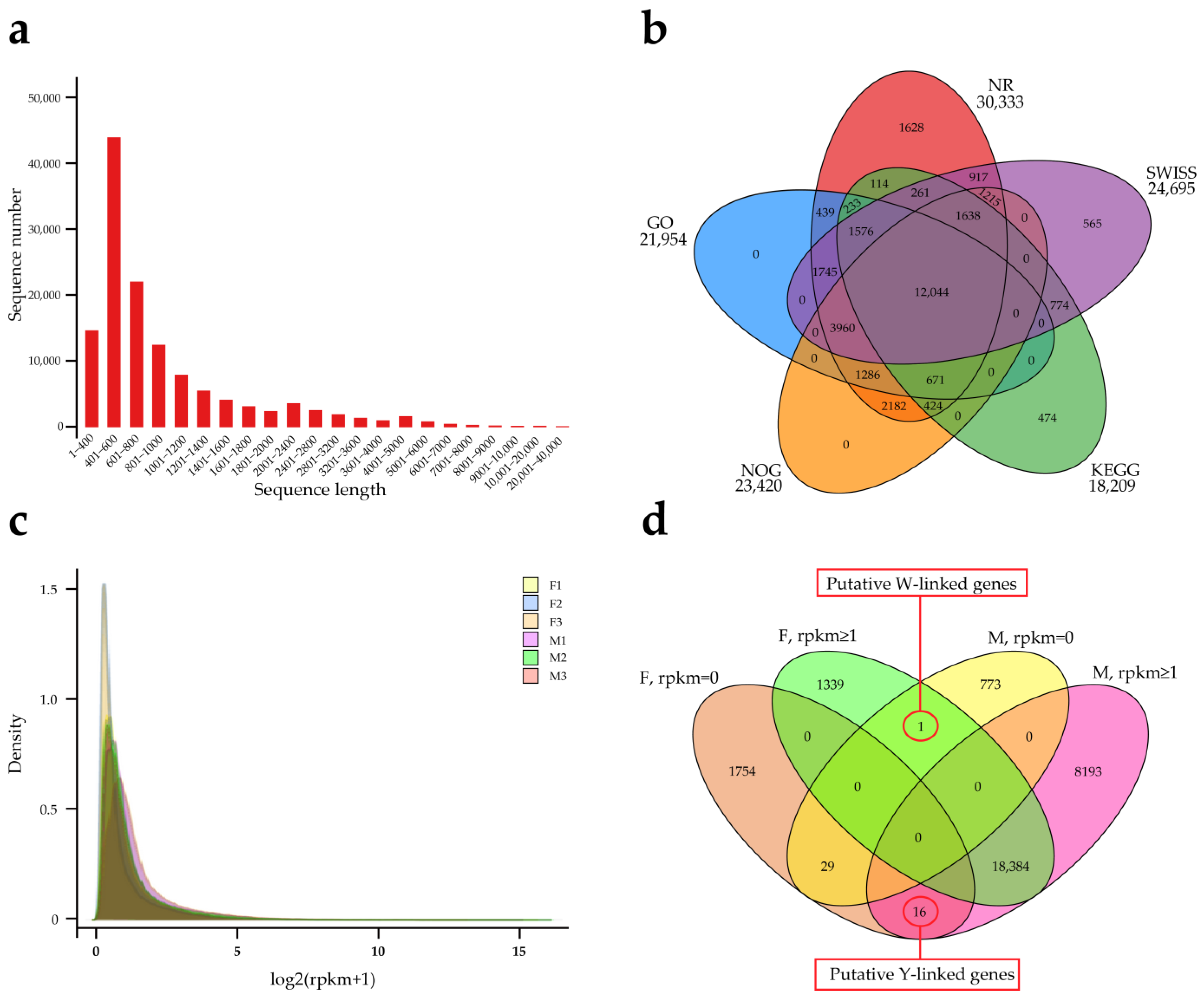
3.2. The X-Linked Genes of P. elegans
3.3. Transcriptome Analysis of Gonads and Development of Sex Markers in P. elegans
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Juárez, A.; Moreno-Mendoza, N. Mechanisms related to sexual determination by temperature in reptiles. J. Therm. Biol. 2019, 85, 102400. [Google Scholar] [CrossRef]
- Thépot, D. Sex chromosomes and master sex-determining genes in turtles and other reptiles. Genes 2021, 12, 1822. [Google Scholar] [CrossRef]
- Kostmann, A.; Kratochvíl, L.; Rovatsos, M. Poorly differentiated XX/XY sex chromosomes are widely shared across skink radiation. Proc. R. Soc. B 2021, 288, 20202139. [Google Scholar] [CrossRef]
- Singchat, W.; Sillapaprayoon, S.; Muangmai, N.; Baicharoen, S.; Indananda, C.; Duengkae, P.; Peyachoknagul, S.; O’Connor, R.E.; Griffin, D.K.; Srikulnath, K. Do sex chromosomes of snakes, monitor lizards, and iguanian lizards result from multiple fission of an “ancestral amniote super-sex chromosome”? Chromosome Res. 2020, 28, 209–228. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Guarino, F.M.; Odierna, G. Lizards as model organisms of sex chromosome evolution: What we really know from a systematic distribution of available data? Genes 2021, 12, 1341. [Google Scholar] [CrossRef]
- Uetz, P. The reptile database turns 20. Herpetol. Rev. 2016, 47, 330–334. [Google Scholar]
- Patawang, I.; Tanomtong, A.; Jumrusthanasan, S.; Khongcharoensuk, H.; Kaewsri, S.; Pinthong, K. Cytogenetic of skink (Reptilia, Scincidae) from Thailand: II: Chromosome analyses of stripe tree skink (Lipinia vittigera). Cytologia 2017, 82, 83–90. [Google Scholar] [CrossRef]
- Kostmann, A. Evolution of Sex Determination in Skinks and Related Lineages. Ph.D. Thesis, Charles University, Prague, Czech Republic, 2021. [Google Scholar]
- Gordon, D.H.; Haacke, W.D.; Jacobsen, N.H.G. Chromosomal studies of relationships in Gekkonidae, Chamaeleonidae and Scincidae in South Africa. J. Herpetol. Assoc. Afr. 1987, 36, 77. [Google Scholar] [CrossRef]
- Caputo, V.; Odierna, G.; Aprea, G. Karyological comparison of Sphenops sepsoides, Chalcides chalcides, and C. ocellatus (Reptilia: Scincidae): Taxonomic implications. Copeia 1993, 1993, 1180–1184. [Google Scholar] [CrossRef]
- Bouffet-Halle, A.; Yang, W.; Gardner, M.G.; Whiting, M.J.; Wapstra, E.; Uller, T.; While, G.M. Characterisation and cross-amplification of sex-specific genetic markers in Australasian Egerniinae lizards and their implications for understanding the evolution of sex determination and social complexity. Aust. J. Zool. 2022, 69, 33–40. [Google Scholar] [CrossRef]
- Patawang, I.; Chuaynkern, Y.; Supanuam, P.; Maneechot, N.; Pinthong, K.; Tanomtong, A. Cytogenetics of the skinks (Reptilia, Scincidae) from Thailand; IV: Newly investigated karyotypic features of Lygosoma quadrupes and Scincella melanosticta. Caryologia 2018, 71, 29–34. [Google Scholar] [CrossRef]
- Hardy, L.M.; Raymond, L.R.; Harris, S. The karyotype of Plestiodon anthracinus (Baird, 1850) (Sauria: Scincidae): A step toward solving an enigma. Southeast. Nat. 2017, 16, 326–330. [Google Scholar] [CrossRef]
- Du, W.G.; Yan, S.J.; Ji, X. Selected body temperature, thermal tolerance and thermal dependence of food assimilation and locomotor performance in adult blue-tailed skinks, Eumeces elegans. J. Therm. Biol. 2000, 25, 197–202. [Google Scholar] [CrossRef]
- Du, W.G.; Lu, Y.W.; Shu, L.; Bao, Y.X. Thermal dependence of food assimilation and locomotor performance in juvenile blue-tailed skinks, Eumeces elegans. Anim. Biol. 2007, 57, 29–38. [Google Scholar] [CrossRef]
- Yongzhang, L.; Yongpu, Z. Comparison of karyotypes of different geographical populations of Eumeces elegans. Chin. J. Zool. 2003, 38, 19–24. [Google Scholar]
- Wang, C.; Tang, X.; Xin, Y.; Yue, F.; Yan, X.; Liu, B.; An, B.; Wang, X.; Chen, Q. Identification of sex chromosomes by means of comparative genomic hybridization in a lizard. Eremias multiocellata. Zool. Sci. 2015, 32, 151–156. [Google Scholar] [CrossRef]
- Chooseangjaew, S.; Tanyaros, S.; Maneechot, N.; Buasriyot, P.; Getlekha, N.; Tanomtong, A. Chromosomal characteristics of the tropical oyster, Crassostrea belcheri Sowerby, 1871 (Ostreoida, Ostreidae) by conventional and Ag-NOR banding techniques. Cytologia 2017, 82, 3–8. [Google Scholar] [CrossRef]
- Howell, W.M.; Black, D.A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experientia 1980, 36, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.V.; Yadav, S.R.; Lekhak, M.M. Karyomorphological study in Ledebouria botryoides (Asparagaceae). Cytologia 2023, 88, 15–19. [Google Scholar] [CrossRef]
- Rovatsos, M.; Kratochvíl, L. Molecular sexing applicable in 4000 species of lizards and snakes? From dream to real possibility. Methods Ecol. Evol. 2017, 8, 902–906. [Google Scholar] [CrossRef]
- Rovatsos, M.; Farkačová, K.; Altmanová, M.; Johnson Pokorná, M.J.; Kratochvíl, L. The rise and fall of differentiated sex chromosomes in geckos. Mol. Ecol. 2019, 28, 3042–3052. [Google Scholar] [CrossRef]
- Pensabene, E.; Kratochvíl, L.; Rovatsos, M. Independent evolution of sex chromosomes in eublepharid geckos, a lineage with environmental and genotypic sex determination. Life 2020, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Vukić, J.; Altmanová, M.; Pokorná, M.J.; Moravec, J.; Kratochvíl, L. Conservation of sex chromosomes in lacertid lizards. Mol. Ecol. 2016, 25, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Pokorná, M.; Altmanová, M.; Kratochvíl, L. Cretaceous park of sex determination: Sex chromosomes are conserved across iguanas. Biol. Lett. 2014, 10, 20131093. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Altmanová, M.; Pokorná, M.; Kratochvíl, L. Conserved sex chromosomes across adaptively radiated Anolis lizards. Evolution 2014, 68, 2079–2085. [Google Scholar] [CrossRef]
- Caputo, V.; Odierna, G.; Aprea, G. A chromosomal study of Eumeces and Scincus, primitive members of the Scincidae (Reptilia, squamata). Ital. J. Zool. 1994, 61, 155–162. [Google Scholar]
- Saunders, P.A.; Ferre-Ortega, C.; Hill, P.; Simakov, O.; Ezaz, T.; Burridge, C.P.; Wapstra, E. Using a handful of transcriptomes to detect sex-linked markers in a lizard with homomorphic sex chromosomes. BioRxiv 2023. [Google Scholar] [CrossRef]
- Zeng, C.; Fukunaga, T.; Hamada, M. Identification and analysis of ribosome-associated lncRNAs using ribosome profiling data. BMC Genom. 2018, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D.S.B.; Holleley, C.E.; Hill, L.K.; O’Meally, D.; Deakin, J.E.; Georges, A. Identification of Y chromosome markers in the eastern three-lined skink (Bassiana duperreyi) using in silico whole genome subtraction. BMC Genom. 2020, 21, 667. [Google Scholar] [CrossRef]
- Cornejo-Páramo, P.; Dissanayake, D.S.B.; Lira-Noriega, A.; Martínez-Pacheco, M.L.; Acosta, A.; Ramírez-Suástegui, C.; Méndez-De-La-Cruz, F.R.; Székely, T.; Urrutia, A.O.; Georges, A.; et al. Viviparous reptile regarded to have temperature-dependent sex determination has old XY chromosomes. Genome Biol. Evol. 2020, 12, 924–930. [Google Scholar] [CrossRef]
- Lamatsch, D.K.; Adolfsson, S.; Senior, A.M.; Christiansen, G.; Pichler, M.; Ozaki, Y.; Smeds, L.; Schartl, M.; Nakagawa, S. A transcriptome derived female-specific marker from the invasive western mosquitofish (Gambusia affinis). PLoS ONE 2015, 10, e0118214. [Google Scholar]
- Small, C.M.; Carney, G.E.; Mo, Q.; Vannucci, M.; Jones, A.G. A microarray analysis of sex-and gonad-biased gene expression in the zebrafish: Evidence for masculinization of the transcriptome. BMC Genom. 2009, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Trabzuni, D.; Ramasamy, A.; Imran, S.; Walker, R.; Smith, C.; Weale, M.E.; Hardy, J.; Ryten, M.; North American Brain Expression Consortium. Widespread sex differences in gene expression and splicing in the adult human brain. Nat. Commun. 2013, 4, 2771. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wiens, J.J. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenetics Evol. 2016, 94, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Ezaz, T.; Sarre, S.D.; O’Meally, D.; Marshall Graves, J.A.; Georges, A. Sex chromosome evolution in lizards: Independent origins and rapid transitions. Cytogenet. Genome Res. 2010, 127, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.I.; Sarre, S.D.; Gleeson, D.; Georges, A.; Ezaz, T. Did lizards follow unique pathways in sex chromosome evolution? Genes 2018, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.N.; Zhu, W.R. First karyological analysis of Plestiodon capito (Squamata: Scincidae). Cytologia, 2024; accepted. [Google Scholar]
- Guo, C.; Dong, Y.W. A comparative study on the karyotypes and Ag-stained NORs of two species of wild skinks from Huang Shan. Hereditas 1988, 10, 17–19. [Google Scholar]
- Ferguson-Smith, M. The evolution of sex chromosomes and sex determination in vertebrates and the key role of DMRT1. Sex. Dev. 2006, 1, 2–11. [Google Scholar] [CrossRef]
- Literman, R.; Radhakrishnan, S.; Tamplin, J.; Burke, R.; Dresser, C.; Valenzuela, N. Development of sexing primers in Glyptemys insculpta and Apalone spinifera turtles uncovers an XX/XY sex-determining system in the critically-endangered bog turtle Glyptemys muhlenbergii. Conserv. Genet. Resour. 2017, 9, 651–658. [Google Scholar] [CrossRef]
- Sulandart, S.R.I.; Zein, M.S.A. Application of two molecular sexing methods for Indonesian bird species: Implication for captive breeding programs in Indonesia. HAYATI J. Biosci. 2012, 19, 183–190. [Google Scholar] [CrossRef]
- Whiteley, S.L.; Weisbecker, V.; Georges, A.; Gauthier, A.R.G.; Whitehead, D.L.; Holleley, C.E. Developmental asynchrony and antagonism of sex determination pathways in a lizard with temperature-induced sex reversal. Sci. Rep. 2018, 8, 14892. [Google Scholar] [CrossRef]
- Gershon, E.; Dekel, N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef]
- Chen, K.; Li, S.; Xiang, J.; Sagi, A.; Li, F. Transcriptome analysis reveals the endocrine regulation on the expression of iag in Litopenaeus vannamei. J. Mar. Sci. Eng. 2021, 9, 677. [Google Scholar] [CrossRef]
- Seufert, W.; Jentsch, S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 1990, 9, 543–550. [Google Scholar] [CrossRef]
- Wing, S.S.; Bédard, N.; Morales, C.; Hingamp, P.; Trasler, J. A novel rat homolog of the Saccharomyces cerevisiae ubiquitin-conjugating enzymes UBC4 and UBC5 with distinct biochemical features is induced during spermatogenesis. Mol. Cell. Biol. 2005, 16, 4064–4072. [Google Scholar] [CrossRef]
- Yokota, N.; Harada, Y.; Sawada, H. Identification of testis-specific ubiquitin-conjugating enzyme in the ascidian Ciona intestinalis. Mol. Reprod. Dev. 2010, 77, 640–647. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, T.; Li, J.; Liu, S.; Yu, X.; Tang, M.; Dong, J.; Liu, J.; Bu, X.; Xia, X.; et al. The First Identification of Homomorphic XY Sex Chromosomes by Integrating Cytogenetic and Transcriptomic Approaches in Plestiodon elegans (Scincidae). Genes 2024, 15, 664. https://doi.org/10.3390/genes15060664
Xu W, Li T, Li J, Liu S, Yu X, Tang M, Dong J, Liu J, Bu X, Xia X, et al. The First Identification of Homomorphic XY Sex Chromosomes by Integrating Cytogenetic and Transcriptomic Approaches in Plestiodon elegans (Scincidae). Genes. 2024; 15(6):664. https://doi.org/10.3390/genes15060664
Chicago/Turabian StyleXu, Wannan, Taiyue Li, Jiahui Li, Siqi Liu, Xing Yu, Min Tang, Jingxiu Dong, Jianjun Liu, Xingjiang Bu, Xingquan Xia, and et al. 2024. "The First Identification of Homomorphic XY Sex Chromosomes by Integrating Cytogenetic and Transcriptomic Approaches in Plestiodon elegans (Scincidae)" Genes 15, no. 6: 664. https://doi.org/10.3390/genes15060664
APA StyleXu, W., Li, T., Li, J., Liu, S., Yu, X., Tang, M., Dong, J., Liu, J., Bu, X., Xia, X., Zhou, H., & Nie, L. (2024). The First Identification of Homomorphic XY Sex Chromosomes by Integrating Cytogenetic and Transcriptomic Approaches in Plestiodon elegans (Scincidae). Genes, 15(6), 664. https://doi.org/10.3390/genes15060664






