Biodiversity of Demersal Fish Communities in the Cosmonaut Sea Revealed by DNA Barcoding Analyses
Highlights
- Knowledge regarding the fish biodiversity of the Cosmonaut Sea, East Antarctica, has been updated and expanded.
- Indicative signals of cryptic species were detected in both Notolepis coatsorum and Bathylagus antarcticus by DNA barcoding analyses.
- Taxonomic composition and occurrence record of demersal ichthyofauna revealed by our study can help inform the ecological baseline of the Cosmonaut Sea, East Antarctica.
- DNA barcoding was further demonstrated to be a very efficient and sound method for the discrimination and classification of Antarctic fishes, even for cryptic species.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Morphological Identification
2.2. DNA Extraction, Amplification and Sequencing
2.3. Species Discrimination, Identification and Occurrence Comparison
2.4. Genetic Divergence and Phylogenetic Analysis
3. Results
3.1. Sequence Information
3.2. Morphological and Molecular Species Identification and Occurrence Comparison
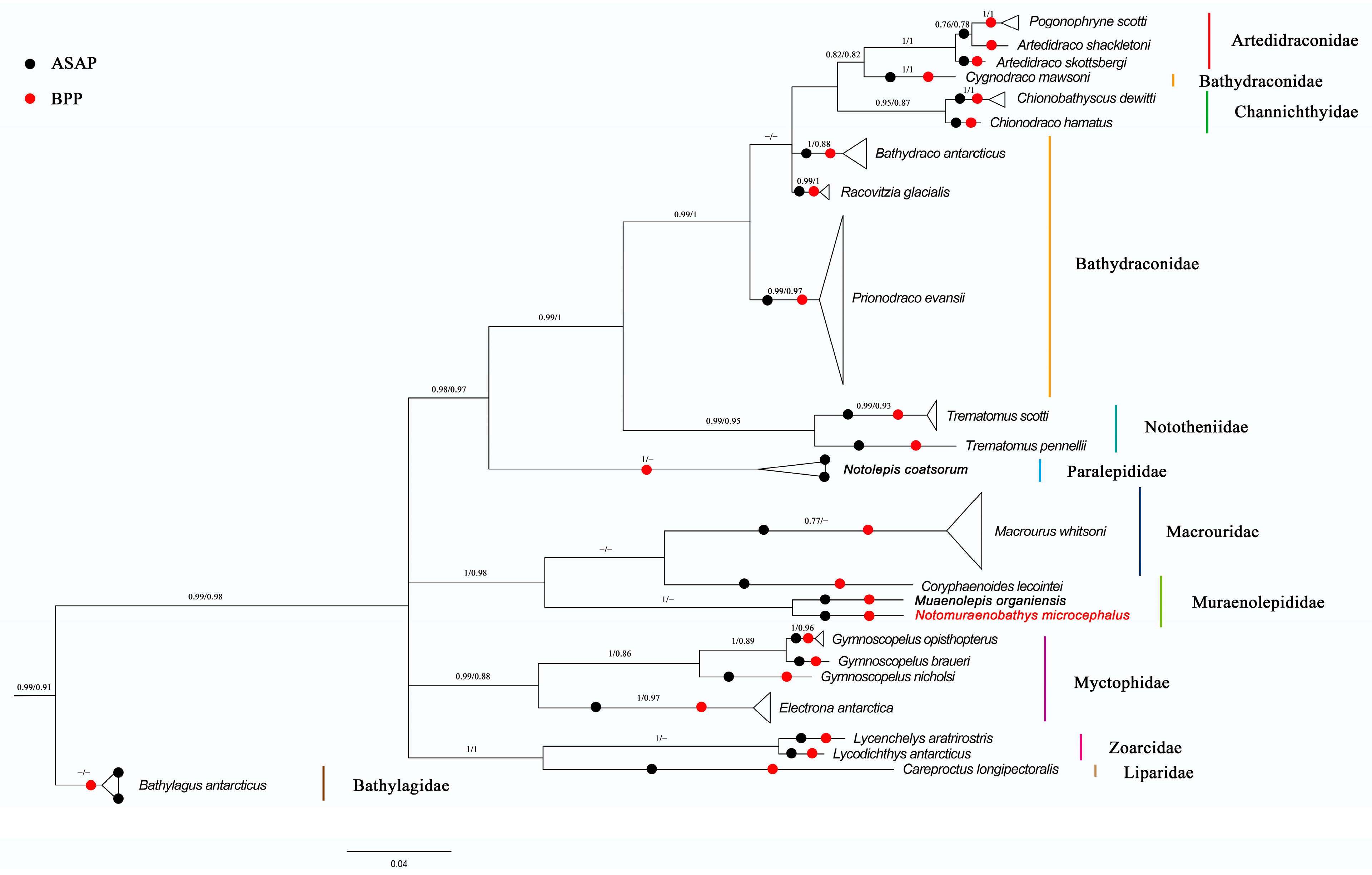
3.3. Genetic Divergence Pattern and Phylogenetic Analysis
4. Discussion
4.1. DNA Barcoding Demonstrated to Be an Effective Way to Discriminate Antarctic Fish Species
4.2. Demersal Fish Communities of the Cosmonaut Sea
4.3. Comparison of Our Results and Fish Occurrence Records in the Cosmonaut Sea
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orsi, A.H.; Whitworth, T.; Nowlin, W.D. On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep-Sea Res. Part I Oceanogr. Res. Pap. 1995, 42, 641–673. [Google Scholar] [CrossRef]
- Mintenbeck, K.; Barrera-Oro, E.R.; Brey, T.; Jacob, U.; Knust, R.; Mark, F.C.; Moreira, E.; Strobel, A.; Arntz, W.E. Impact of Climate Change on Fishes in Complex Antarctic Ecosystems. In Advance in Ecological Research; Elsevier Science & Technology: London, UK, 2012; Volume 46, pp. 351–426. [Google Scholar]
- Matschiner, M.; Colombo, M.; Damerau, M.; Ceballos, S.; Hanel, R.; Salzburger, W. The Adaptive Radiation of Notothenioid Fishes in the Waters of Antarctica. In Extremophile Fishes; Springer International Publishing AG: Cham, Switzerland, 2015; pp. 35–57. [Google Scholar]
- Dayton, P.K. Polar benthos. In Polar Oceanography, Part B: Chemistry, Biology, and Geology; Smith, W.O., Ed.; Academic Press: Boston, MA, USA, 1990; pp. 631–685. [Google Scholar]
- Mintenbeck, K. Impacts of Climate Change on the Southern Ocean. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; Phillips, B.F., Pérez Ramírez, M., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2017; pp. 663–701. [Google Scholar]
- Mabragaña, E.; Delpiani, S.M.; Rosso, J.J.; González-Castro, M.; Deli Antoni, M.; Hanner, R.; Díaz De Astarloa, J.M. Barcoding Antarctic Fishes: Species Discrimination and Contribution to Elucidate Ontogenetic Changes in Nototheniidae. In DNA Barcoding in Marine Perspectives; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 213–242. [Google Scholar]
- Andriashev, A.P. Andriashev, A.P. A general review of the Antarctic bottom fish fauna. In Fifth Congress of European Ichthyologists; Swedish Museum of Natural History: Stockholm, Sweden, 1987. [Google Scholar]
- Eastman, J.T. Antarctic Fish Biology: Evolution in a Unique Environment; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Eastman, J.T. The nature of the diversity of Antarctic fishes. Polar Biol. 2005, 28, 93–107. [Google Scholar] [CrossRef]
- Rogers, A.D.; Frinault, B.; Barnes, D.; Bindoff, N.L.; Downie, R.; Ducklow, H.W.; Friedlaender, A.S.; Hart, T.; Hill, S.L.; Hofmann, E.E.; et al. Antarctic futures: An assessment of climate-driven changes in ecosystem structure, function, and service provisioning in the Southern Ocean. Annu. Rev. Mar. Sci. 2020, 12, 87–120. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, H.E.; Burrows, M.T.; Pecl, G.T.; Robinson, L.M.; Poloczanska, E.S. Are fish outside their usual ranges early indicators of climate-driven range shifts? Glob. Change Biol. 2017, 23, 2047–2057. [Google Scholar] [CrossRef]
- Li, H.; Cao, S.; Li, Y.; Song, P.; Zhang, R.; Wang, R.; Liu, S.; Miao, X.; Lin, L. Molecular assessment of demersal fish diversity in Prydz Bay using DNA taxonomy. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2022, 202, 105140. [Google Scholar] [CrossRef]
- Smith, P.J.; Steinke, D.; Mcveagh, S.M.; Stewart, A.L.; Struthers, C.D.; Roberts, C.D. Molecular analysis of Southern Ocean skates (Bathyraja) reveals a new species of Antarctic skate. J. Fish Biol. 2008, 73, 1170–1182. [Google Scholar] [CrossRef]
- Balushkin, A.V.; Korolkova, E.D. New species of plunderfish Pogonophryne favosa sp. n. (Artedidraconidae, Notothenioidei, Perciformes) from the Cosmonauts Sea (Antarctica) with description in artedidraconids of unusual anatomical structures-convexitas superaxillaris. J. Ichthyol. 2013, 53, 562–574. [Google Scholar] [CrossRef]
- Smith, P.J.; Steinke, D.; McMillan, P.J.; Stewart, A.L.; McVeagh, S.M.; Diaz De Astarloa, J.M.; Welsford, D.; Ward, R.D. DNA barcoding highlights a cryptic species of grenadier Macrourus in the Southern Ocean. J. Fish Biol. 2011, 78, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, H.; Dettai, A.; Heindler, F.M.; Collins, M.A.; Duhamel, G.; Hautecoeur, M.; Steinke, D.; Volckaert, F.A.M.; Van de Putte, A.P. Diversity of mesopelagic fishes in the Southern Ocean-a phylogeographic perspective using DNA barcoding. Front. Ecol. Evol. 2018, 6, 120. [Google Scholar] [CrossRef]
- Duhamel, G.; Hulley, P.; Causse, R.; Koubbi, P.; Vacchi, M.; Pruvost, P.; Vigetta, S.; Irisson, J.; Mormede, S.; Belchier, M. Biogeographic patterns of fish. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research: Cambridge, UK, 2014. [Google Scholar]
- Xiong, X.; Yao, L.; Ying, X.; Lu, L.; Guardone, L.; Armani, A.; Guidi, A.; Xiong, X. Multiple fish species identified from China’s roasted Xue Yu fillet products using DNA and mini-DNA barcoding: Implications on human health and marine sustainability. Food Control 2018, 88, 123–130. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Van Damme, K.; Huang, D.; Li, Y.; Wang, L.; Ning, J.; Du, F. Assessment of fish diversity in the South China Sea using DNA taxonomy. Fish. Res. 2021, 233, 105771. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Gon, O.; Heemstra, P.C. Fishes of the Southern Ocean; JLB Smith Institute of Ichthyology: Grahamstown, South Africa, 1990. [Google Scholar]
- Murphy, K.R.; Kalmanek, E.A.; Cheng, C.H.C. Diversity and biogeography of larval and juvenile notothenioid fishes in McMurdo Sound, Antarctica. Polar Biol. 2017, 40, 161–176. [Google Scholar] [CrossRef]
- Eastman, J.T.; Eakin, R.R. Decomplicating and identifying species in the radiation of the Antarctic fish genus Pogonophryne (Artedidraconidae). Polar Biol. 2022, 45, 825–832. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Song, P.; Zhang, R.; Wang, L.; Lin, L. Fish diversity and molecular taxonomy in the Prydz Bay during the 29th CHINARE. Acta Oceanol. Sin. 2018, 37, 15–20. [Google Scholar] [CrossRef]
- Koubbi, P.; Duhamel, G.; Hecq, J.; Beans, C.; Loots, C.; Pruvost, P.; Tavernier, E.; Vacchi, M.; Vallet, C. Ichthyoplankton in the neritic and coastal zone of Antarctica and Subantarctic islands: A review. J. Mar. Syst. 2009, 78, 547–556. [Google Scholar] [CrossRef]
- Smith, P.J.; Steinke, D.; Dettai, A.; McMillan, P.; Welsford, D.; Stewart, A.; Ward, R.D. DNA barcodes and species identifications in Ross Sea and Southern Ocean fishes. Polar Biol. 2012, 35, 1297–1310. [Google Scholar] [CrossRef]
- Rock, J.; Costa, F.O.; Walker, D.I.; North, A.W.; Hutchinson, W.F.; Carvalho, G.R. DNA barcodes of fish of the Scotia Sea, Antarctica indicate priority groups for taxonomic and systematics focus. Antarct. Sci. 2008, 20, 253–262. [Google Scholar] [CrossRef]
- Lautredou, A.C.; Bonillo, C.; Denys, G.; Cruaud, C.; Ozouf-Costaz, C.; Lecointre, G.; Dettai, A. Molecular taxonomy and identification within the Antarctic genus Trematomus (Notothenioidei, Teleostei): How valuable is barcoding with COI? Polar Sci. 2010, 4, 333–352. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, W.; Wang, R.; Chen, Z. Diatom based reconstruction of climate evolution through the Last Glacial Maximum to Holocene in the Cosmonaut Sea, East Antarctica. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2021, 194, 104960. [Google Scholar] [CrossRef]
- Hunt, B.P.V.; Pakhomov, E.A.; Trotsenko, B.G. The macrozooplankton of the Cosmonaut Sea, east Antarctica (30°E–60°E), 1987–1990. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 1042–1069. [Google Scholar] [CrossRef]
- Liao, Y.; Miao, X.; Wang, R.; Zhang, R.; Li, H.; Lin, L. First pelagic fish biodiversity assessment of Cosmonaut Sea based on environmental DNA. Mar. Environ. Res. 2023, 106225. [Google Scholar] [CrossRef]
- Van de Putte, A.P.; Jackson, G.D.; Pakhomov, E.; Flores, H.; Volckaert, F.A.M. Distribution of squid and fish in the pelagic zone of the Cosmonaut Sea and Prydz Bay region during the BROKE-West campaign. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 956–967. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, R.; Miao, X.; Li, H.; Song, P.; Li, Y.; Lin, L. Demersal fish community in the near-shelf zone of the Cosmonaut Sea, Southern Ocean. Diversity 2024, 16, 156. [Google Scholar] [CrossRef]
- Eastman, J.T.; Eakin, R.R. Checklist of the species of notothenioid fishes. Antarct. Sci. 2021, 3, 273–280. [Google Scholar] [CrossRef]
- Fitzcharles, E.; Hollyman, P.R.; Goodall-Copestake, W.P.; Maclaine, J.S.; Collins, M.A. The taxonomic identity and distribution of the eel cod Muraenolepis (Gadiformes: Muraenolepididae) around South Georgia and the South Sandwich Islands. Polar Biol. 2021, 44, 637–651. [Google Scholar] [CrossRef]
- Steinke, D.; Zemlak, T.S.; Boutillier, J.A.; Hebert, P.D.N. DNA barcoding of Pacific Canada’s fishes. Mar. Biol. 2009, 156, 2641–2647. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, D. Sequencher 3.1.1. Biotech Softw. Internet Rep. 2000, 1, 24–30. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Ye, J.; McGinnis, S.; Madden, T.L. BLAST: Improvements for better sequence analysis. Nucleic Acids Res. 2006, 34, W6–W9. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. The BPP program for species tree estimation and species delimitation. Curr. Zool. 2015, 61, 854–865. [Google Scholar] [CrossRef]
- Yang, Z.; Rannala, B. Bayesian species identification under the multispecies coalescent provides significant improvements to DNA barcoding analyses. Mol. Ecol. 2017, 26, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and high-performance computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Stoeckle, M.Y.; Zemlak, T.S.; Francis, C.M.; Charles, G. Identification of birds through DNA barcodes. PLoS Biol. 2004, 2, e312. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Longueville, J.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey16. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.E.; Bond, C.E. Taxonomic methods: Morphology. In Methods for Fish Biology; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 109–140. [Google Scholar]
- Zhang, J.; Hanner, R. Molecular approach to the identification of fish in the South China Sea. PLoS ONE 2012, 7, e30621. [Google Scholar] [CrossRef] [PubMed]
- Batta-Lona, P.G.; Galindo-Sánchez, C.E.; Arteaga, M.C.; Robles-Flores, J.; Jiménez-Rosenberg, S.P.A. DNA barcoding and morphological taxonomy: Identification of lanternfish (Myctophidae) larvae in the Gulf of Mexico. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2019, 30, 375–383. [Google Scholar] [CrossRef]
- Hou, G.; Wang, J.; Liu, L.; Chen, Y.; Pan, C.; Lin, J.; Zhang, H. Assemblage structure of the ichthyoplankton and its relationship with environmental factors in spring and autumn off the Pearl River Estuary. Front. Mar. Sci. 2021, 8, 732970. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Xing, B.; Lin, H.; Zhang, Z.; Wang, C.; Wang, Y.; Wang, J. DNA barcoding for identification of fish species in the Taiwan Strait. PLoS ONE 2018, 13, e198109. [Google Scholar]
- Dettai, A.; Lautredou, A.C.; Bonillo, C.; Goimbault, E.; Busson, F.; Causse, R.; Couloux, A.; Cruaud, C.; Duhamel, G.; Denys, G.; et al. The actinopterygian diversity of the CEAMARC cruises: Barcoding and molecular taxonomy as a multi-level tool for new findings. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 250–263. [Google Scholar] [CrossRef]
- Eastman, J.T. Bathymetric distributions of notothenioid fishes. Polar Biol. 2017, 40, 2077–2095. [Google Scholar] [CrossRef]
- Hanchet, S.; Dunn, A.; Parker, S.; Horn, P.; Stevens, D.; Mormede, S. Antarctic toothfish (Dissostichus mawsoni): Biology, ecology, and life history in the Ross Sea region. Hydrobiologia 2015, 761, 397–414. [Google Scholar] [CrossRef]
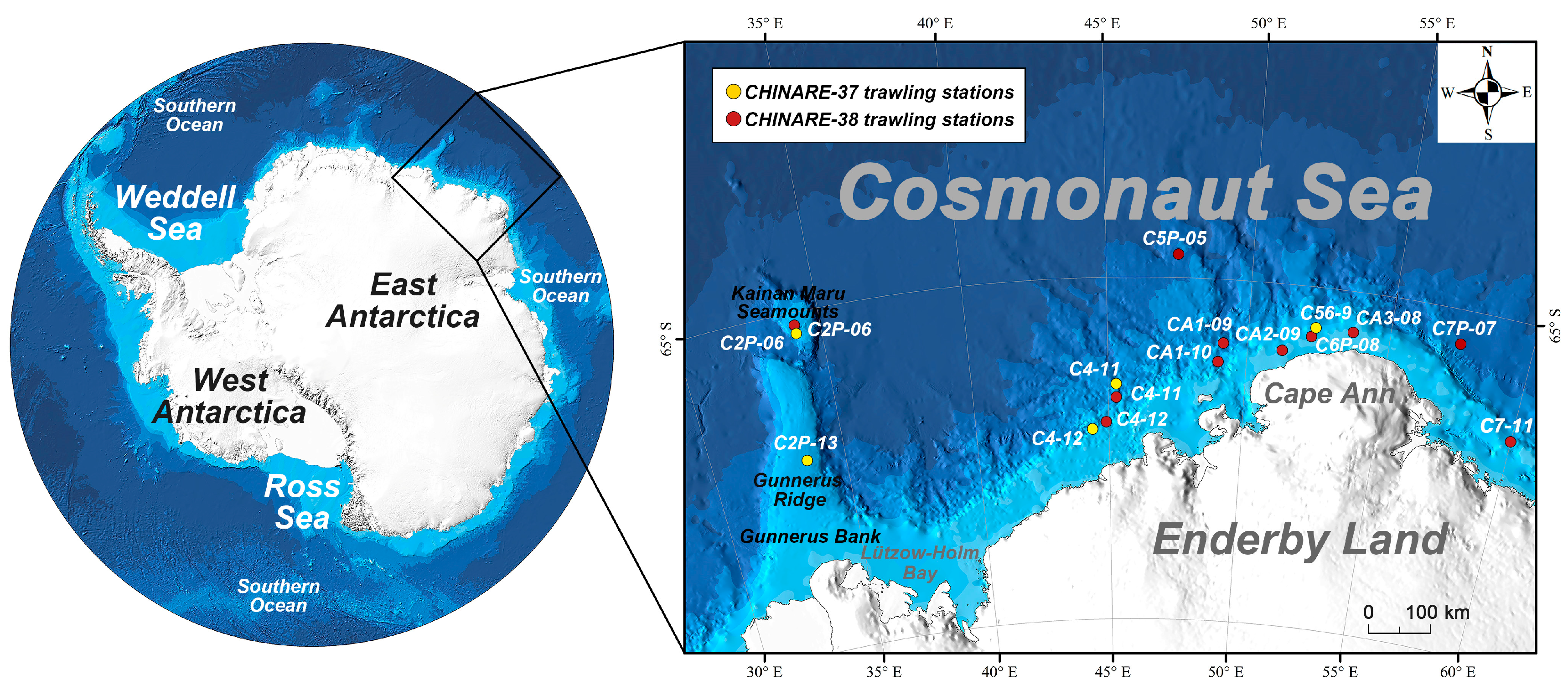
| Sample ID | Cruise | Trawling Station | Longitude (°/E) | Latitude (°/S) | Trawling Depth (m) | Trawling Duration (min) | Morphological Taxonomy | Molecular Taxonomy |
|---|---|---|---|---|---|---|---|---|
| 1 | CHINARE-37 | C2P-13 | 33.7361 | 67.3241 | 1270 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 2 | CHINARE-37 | C2P-13 | 33.7361 | 67.3241 | 1270 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 3 | CHINARE-37 | C2P-13 | 33.7361 | 67.3241 | 1270 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 4 | CHINARE-37 | C2P-13 | 33.7361 | 67.3241 | 1270 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 5 | CHINARE-37 | C2P-13 | 33.7361 | 67.3241 | 1270 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 6 | CHINARE-37 | C2P-13 | 33.7361 | 67.3241 | 1270 | 15 | Electrona antarctica (Günther, 1878) | Electrona antarctica (Günther, 1878) |
| 7 | CHINARE-37 | C2P-13 | 33.7361 | 67.3241 | 1270 | 15 | Electrona antarctica (Günther, 1878) | Electrona antarctica (Günther, 1878) |
| 8 | CHINARE-37 | C2P-13 | 33.7361 | 67.3241 | 1270 | 15 | Bathylagus antarcticus Günther, 1878 | Bathylagus antarcticus Günther, 1878 |
| 9 | CHINARE-37 | C4-12 | 44.3405 | 67.1106 | 986 | 15 | Bathylagus antarcticus Günther, 1878 | Bathylagus antarcticus Günther, 1878 |
| 10 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Electrona antarctica (Günther, 1878) | Electrona antarctica (Günther, 1878) |
| 11 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Electrona antarctica (Günther, 1878) | Electrona antarctica (Günther, 1878) |
| 12 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Gymnoscopelus opisthopterus Fraser-Brunner, 1949 | Gymnoscopelus opisthopterus Fraser-Brunner, 1949 |
| 13 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Coryphaenoides lecointei (Dollo, 1900) | Coryphaenoides lecointei (Dollo, 1900) |
| 14 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 15 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Bathydraco antarcticus Günther, 1878 | Bathydraco antarcticus Günther, 1878 |
| 16 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 17 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Careproctus longipectoralis Duhamel, 1992 | Careproctus longipectoralis Duhamel, 1992 |
| 18 | CHINARE-37 | C2P-06 | 34.1758 | 65.3239 | 1763 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 19 | CHINARE-37 | C2P-06 | 34.1758 | 65.3239 | 1763 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 20 | CHINARE-37 | C2P-06 | 34.1758 | 65.3239 | 1763 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 21 | CHINARE-37 | C2P-06 | 34.1758 | 65.3239 | 1763 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 22 | CHINARE-37 | C2P-06 | 34.1758 | 65.3239 | 1763 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 23 | CHINARE-37 | C2P-06 | 34.1758 | 65.3239 | 1763 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 24 | CHINARE-37 | C2P-06 | 34.1758 | 65.3239 | 1763 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 25 | CHINARE-37 | C2P-06 | 34.1758 | 65.3239 | 1763 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 26 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 27 | CHINARE-37 | C4-11 | 45.0542 | 66.4743 | 2085 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 28 | CHINARE-37 | C56-09 | 52.5742 | 65.5688 | 1940 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 29 | CHINARE-37 | C56-09 | 52.5742 | 65.5688 | 1940 | 15 | Gymnoscopelus opisthopterus Fraser-Brunner, 1949 | Gymnoscopelus opisthopterus Fraser-Brunner, 1949 |
| 30 | CHINARE-38 | C2P-06 | 34.1713 | 65.1852 | 1492 | 15 | Muraenolepis orangiensis Vaillant, 1888 | Muraenolepis orangiensis Vaillant, 1888 |
| 31 | CHINARE-38 | C2P-06 | 34.1713 | 65.1852 | 1492 | 15 | Bathylagus Günther, 1878 sp. | Bathylagus antarcticus Günther, 1878 * |
| 32 | CHINARE-38 | C2P-06 | 34.1713 | 65.1852 | 1492 | 15 | Coryphaenoides Gunnerus, 1765 sp.1 | Macrourus whitsoni (Regan, 1913) * |
| 33 | CHINARE-38 | C6P-08 | 52.4918 | 65.6517 | 270 | 15 | Lycodichthys antarcticus Pappenheim, 1911 | Lycodichthys antarcticus Pappenheim, 1911 |
| 34 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Muraenolepis microps Lönnberg, 1905 | Notomuraenobathys microcephalus (Norman, 1937) * |
| 35 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Coryphaenoides Gunnerus, 1765 sp.2 | Macrourus whitsoni (Regan, 1913) * |
| 36 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Bathydraco joannae DeWitt, 1985 | Bathydraco antarcticus Günther, 1878 * |
| 37 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Bathydraco joannae DeWitt, 1985 | Bathydraco antarcticus Günther, 1878 * |
| 38 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Bathydraco joannae DeWitt, 1985 | Bathydraco antarcticus Günther, 1878 * |
| 39 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Bathydraco antarcticus Günther, 1878 | Bathydraco antarcticus Günther, 1878 |
| 40 | CHINARE-38 | C5P-05 | 47.4897 | 64.6615 | 250 | 30 | Chionobathyscus dewitti Andriashev & Neelov, 1978 | Chionobathyscus dewitti Andriashev & Neelov, 1978 |
| 41 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 42 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 43 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 44 | CHINARE-38 | C4-11 | 45.0173 | 66.6722 | 2159 | 15 | Lycenchelys antarctica Regan, 1913 | Lycenchelys aratrirostris Andriashev & Permitin, 1968 * |
| 45 | CHINARE-38 | C4-12 | 44.9958 | 67.0017 | 1679 | 15 | Notolepis Dollo, 1908 sp.1 | Notolepis coatsorum Dollo, 1908 * |
| 46 | CHINARE-38 | C4-12 | 44.9958 | 67.0017 | 1679 | 15 | Notolepis Dollo, 1908 sp.2 | Notolepis coatsorum Dollo, 1908 * |
| 47 | CHINARE-38 | C4-12 | 44.9958 | 67.0017 | 1679 | 15 | Gymnoscopelus braueri (Lönnberg, 1905) | Gymnoscopelus braueri (Lönnberg, 1905) |
| 48 | CHINARE-38 | C4-12 | 44.9958 | 67.0017 | 1679 | 15 | Electrona antarctica (Günther, 1878) | Electrona antarctica (Günther, 1878) |
| 49 | CHINARE-38 | CA1-10 | 48.7242 | 66.3123 | 1004 | 15 | Bathydraco antarcticus Günther, 1878 | Bathydraco antarcticus Günther, 1878 |
| 50 | CHINARE-38 | CA1-10 | 48.7242 | 66.3123 | 1004 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 51 | CHINARE-38 | CA1-10 | 48.7242 | 66.3123 | 1004 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 52 | CHINARE-38 | CA1-10 | 48.7242 | 66.3123 | 1004 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 53 | CHINARE-38 | CA1-10 | 48.7242 | 66.3123 | 1004 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 54 | CHINARE-38 | CA1-10 | 48.7242 | 66.3123 | 1004 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 55 | CHINARE-38 | CA1-10 | 48.7242 | 66.3123 | 1004 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 56 | CHINARE-38 | CA2-09 | 51.2150 | 65.9750 | 229 | 15 | Chionodraco hamatus (Lönnberg, 1905) | Chionodraco hamatus (Lönnberg, 1905) |
| 57 | CHINARE-38 | CA2-09 | 51.2150 | 65.9750 | 229 | 15 | Trematomus pennellii Regan, 1914 | Trematomus pennellii Regan, 1914 |
| 58 | CHINARE-38 | CA2-09 | 51.2150 | 65.9750 | 229 | 15 | Artedidraco shackletoni Waite, 1911 | Artedidraco shackletoni Waite, 1911 |
| 59 | CHINARE-38 | CA2-09 | 51.2150 | 65.9750 | 229 | 15 | Cygnodraco mawsoni Waite, 1916 | Cygnodraco mawsoni Waite, 1916 |
| 60 | CHINARE-38 | CA2-09 | 51.2150 | 65.9750 | 229 | 15 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914) |
| 61 | CHINARE-38 | CA2-09 | 51.2150 | 65.9750 | 229 | 15 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914) |
| 62 | CHINARE-38 | CA2-09 | 51.2150 | 65.9750 | 229 | 15 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914) |
| 63 | CHINARE-38 | CA2-09 | 51.2150 | 65.9750 | 229 | 15 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914 |
| 64 | CHINARE-38 | CA1-09 | 49.0147 | 65.9546 | 1872 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1914) |
| 65 | CHINARE-38 | CA1-09 | 49.0147 | 65.9546 | 1872 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 66 | CHINARE-38 | CA1-09 | 49.0147 | 65.9546 | 1872 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 67 | CHINARE-38 | CA1-09 | 49.0147 | 65.9546 | 1872 | 15 | Macrourus whitsoni (Regan, 1913) | Macrourus whitsoni (Regan, 1913) |
| 68 | CHINARE-38 | CA1-09 | 49.0147 | 65.9546 | 1872 | 15 | Electrona antarctica (Günther, 1878) | Electrona antarctica (Günther, 1878) |
| 69 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 15 | Racovitzia glacialis Dollo, 1900 | Racovitzia glacialis Dollo, 1900 |
| 70 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Racovitzia glacialis Dollo, 1900 | Racovitzia glacialis Dollo, 1900 |
| 71 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Pogonophryne Regan, 1914 sp.1 | Pogonophryne scotti Regan, 1914 * |
| 72 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Pogonophryne Regan, 1914 sp.2 | Pogonophryne scotti Regan, 1914 * |
| 73 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Trematomus scotti (Boulenger, 1907) |
| 74 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Trematomus scotti (Boulenger, 1907) |
| 75 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Trematomus scotti (Boulenger, 1907) |
| 76 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti Boulenger, 1907 | Artedidraco skottsbergi Lönnberg, 1905 * |
| 77 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti Boulenger, 1907 | Trematomus scotti (Boulenger, 1907) |
| 78 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914 |
| 79 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914 |
| 80 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914 |
| 81 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914 |
| 82 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914 |
| 83 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914 |
| 84 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Prionodraco evansii Regan, 1914 | Prionodraco evansii Regan, 1914 |
| 85 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 86 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 87 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 88 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii (Regan, 1914 * |
| 89 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 90 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 91 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 92 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 93 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 94 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 95 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 96 | CHINARE-38 | CA3-08 | 53.8283 | 65.5457 | 240 | 5 | Trematomus scotti (Boulenger, 1907) | Prionodraco evansii Regan, 1914 * |
| 97 | CHINARE-38 | C7-11 | 59.9998 | 66.6647 | 854 | 15 | Electrona antarctica (Günther, 1878) | Electrona antarctica (Günther, 1878) |
| 98 | CHINARE-38 | C7P-07 | 59.9968 | 65.3302 | 250 | 15 | Gymnoscopelus braueri (Lönnberg, 1905) | Gymnoscopelus nicholsi (Gilbert, 1911) * |
| Historical Records | CHINARE Results | ||
|---|---|---|---|
| Family | Species | Family | Species |
| Rajidae | Amblyraja georgiana (Norman, 1938) | ||
| Bathyraja eatonii (Günther, 1876) | |||
| Bathyraja maccaini Springer, 1971 | |||
| Paralepididae | Notolepis coatsorum | Paralepididae | Notolepis coatsorum |
| Macrouridae | Macrourus caml McMillan, Iwamoto, Stewart & Smith, 2012 | Macrouridae | Coryphaenoides lecointei Macrourus whitsoni |
| Macrourus whitsoni | |||
| Antimora rostrata (Günther, 1878) | |||
| Muraenolepididae | Muraenolepis marmorata Günther, 1880 | Muraenolepididae | Muraenolepis orangiensis |
| Notomuraenobathys microcephalus | |||
| Myctophidae | Electrona antarctica Gymnoscopelus braueri Gymnoscopelus nicholsi | Myctophidae | Electrona antarctica |
| Gymnoscopelus braueri | |||
| Gymnoscopelus nicholsi | |||
| Gymnoscopelus opisthopterus | |||
| Bathylagidae | Bathylagus sp. | Bathylagidae | Bathylagus antarcticus |
| Artedidraconidae | Artedidraco spp. | Artedidraconidae | Artedidraco shackletoni |
| Pogonophryne permitini Andriashev, 1967 | Pogonophryne scotti | ||
| Bathydraconidae | Cygnodraco mawsoni | Bathydraconidae | Cygnodraco mawsoni |
| Gerlachea australis Dollo, 1900 | Bathydraco antarcticus | ||
| Prionodraco evansii | Prionodraco evansii | ||
| Gymnodraco acuticeps Boulenger, 1902 | Racovitzia glacialis | ||
| Channichthyidae | Chaenodraco wilsoni Regan, 1914 | Channichthyidae | Chionodraco hamatus |
| Chionobathyscus dewitti | Chionobathyscus dewitti | ||
| Cryodraco spp. | |||
| Nototheniidae | Lepidonotothen squamifrons (Günther, 1880) | Nototheniidae | |
| Dissostichus mawsoni Norman, 1937 | |||
| Pleuragramma antarctica | |||
| Trematomus brachysoma Pappenheim, 1912 | Trematomus pennellii | ||
| Trematomus spp. | Trematomus scotti | ||
| Zoarcidae | Lycodichthys Pappenheim, 1911 spp. | Zoarcidae | Lycodichthys antarcticus |
| Pachycara Zugmayer, 1911 spp. | Lycenchelys aratrirostris | ||
| Liparidae | Paraliparis leobergi Andriashev, 1982 | Liparidae | Careproctus longipectoralis |
| Maximum Divergence (%) | Minimum Divergence (%) | Mean Divergence (%) | SE Divergence (%) | |
|---|---|---|---|---|
| Within species | 3.29 | 0 | 0.63 | 0.20 |
| Within genus | 7.27 | 0 | 1.68 | 0.33 |
| Within family | 10.91 | 1.14 | 3.98 | 0.56 |
| Species Name | Minimum Interspecific Distance | Mean Interspecific Distance | Maximum Intraspecific Distance | Mean Intraspecific Distance |
|---|---|---|---|---|
| Artedidraco shackletoni | 0.0235 | 0.1779 | 0.0015 | 0.0010 |
| Artedidraco skottsbergi | 0.0235 | 0.1795 | 0.0046 | 0.0031 |
| Bathydraco antarcticus | 0.0358 | 0.1810 | 0.0093 | 0.0031 |
| Bathylagus antarcticus | 0.2350 | 0.2612 | 0.0310 | 0.0209 |
| Careproctus longipectoralis | 0.2386 | 0.2679 | 0.0171 | 0.0114 |
| Chionobathyscus dewitti | 0.0303 | 0.1905 | 0.0061 | 0.0041 |
| Chionodraco hamatus | 0.0303 | 0.1885 | 0.0031 | 0.0021 |
| Coryphaenoides lecointei | 0.2108 | 0.2531 | 0 | 0 |
| Cygnodraco mawsoni | 0.0694 | 0.1816 | 0.0015 | 0.0010 |
| Electrona antarctica | 0.1776 | 0.2297 | 0.0031 | 0.0012 |
| Gymnoscopelus braueri | 0.0262 | 0.2256 | 0.0015 | 0.0010 |
| Gymnoscopelus nicholsi | 0.0262 | 0.2344 | 0.0077 | 0.0062 |
| Gymnoscopelus opisthopterus | 0.0568 | 0.2273 | 0.0015 | 0.0010 |
| Lycenchelys aratrirostris | 0.0306 | 0.2299 | 0.0031 | 0.0021 |
| Lycodichthys antarcticus | 0.0306 | 0.2313 | 0.0218 | 0.0156 |
| Macrourus whitsoni | 0.2108 | 0.2622 | 0.0093 | 0.0013 |
| Notomuraenobathys microcephalus | 0.0825 | 0.2665 | NA | NA |
| Muraenolepis orangiensis | 0.0825 | 0.2596 | NA | NA |
| Notolepis coatsorum | 0.2128 | 0.2486 | 0.0398 | 0.0329 |
| Pogonophryne scotti | 0.0256 | 0.1824 | 0.0077 | 0.0062 |
| Prionodraco evansii | 0.0508 | 0.1796 | 0.0061 | 0.0022 |
| Racovitzia glacialis | 0.0358 | 0.1738 | 0.0031 | 0.0021 |
| Trematomus pennellii | 0.1001 | 0.2229 | 0.0015 | 0.0010 |
| Trematomus scotti | 0.1001 | 0.2201 | 0.0031 | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Miao, X.; Wang, R.; Liao, Y.; Wen, Y.; Zhang, R.; Lin, L. Biodiversity of Demersal Fish Communities in the Cosmonaut Sea Revealed by DNA Barcoding Analyses. Genes 2024, 15, 691. https://doi.org/10.3390/genes15060691
Li H, Miao X, Wang R, Liao Y, Wen Y, Zhang R, Lin L. Biodiversity of Demersal Fish Communities in the Cosmonaut Sea Revealed by DNA Barcoding Analyses. Genes. 2024; 15(6):691. https://doi.org/10.3390/genes15060691
Chicago/Turabian StyleLi, Hai, Xing Miao, Rui Wang, Yuzhuo Liao, Yilin Wen, Ran Zhang, and Longshan Lin. 2024. "Biodiversity of Demersal Fish Communities in the Cosmonaut Sea Revealed by DNA Barcoding Analyses" Genes 15, no. 6: 691. https://doi.org/10.3390/genes15060691
APA StyleLi, H., Miao, X., Wang, R., Liao, Y., Wen, Y., Zhang, R., & Lin, L. (2024). Biodiversity of Demersal Fish Communities in the Cosmonaut Sea Revealed by DNA Barcoding Analyses. Genes, 15(6), 691. https://doi.org/10.3390/genes15060691







