Genome-Wide Identification of OsZIPs in Rice and Gene Expression Analysis under Manganese and Selenium Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. OsZIP Genes Structure Analysis
2.2. Construction of the ZIP Phylogenetic Tree
2.3. Chromosome Localization of OsZIPs
2.4. Analysis of OsZIPs Conserved Motif
2.5. Cis Acting Factor Analysis
2.6. Rice Culture Conditions
2.7. Analysis of OsZIP Genes Expression Levels in Rice
3. Results
3.1. Analysis of OsZIPs Basic Information
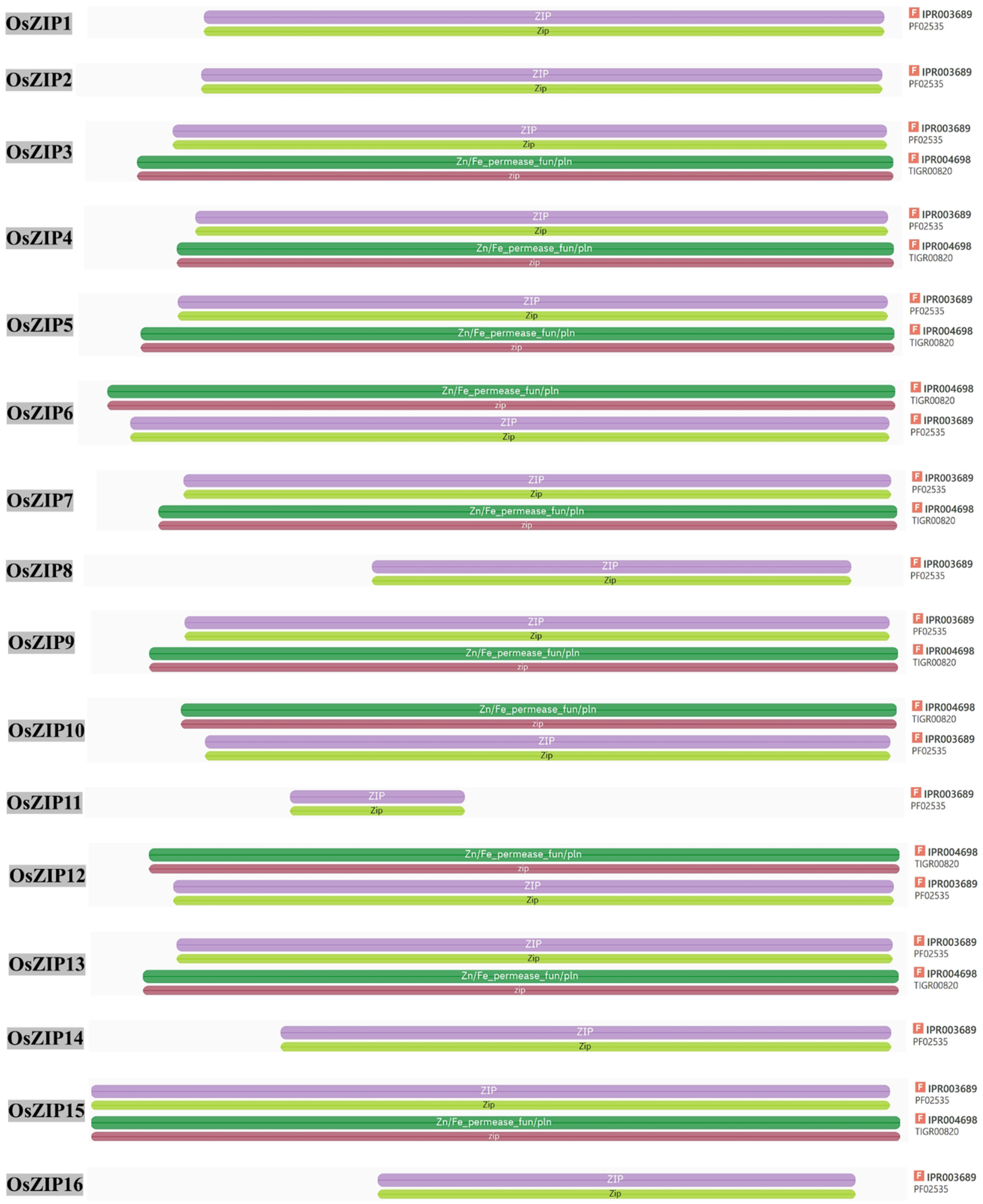
3.2. OsZIPs Structure Analysis
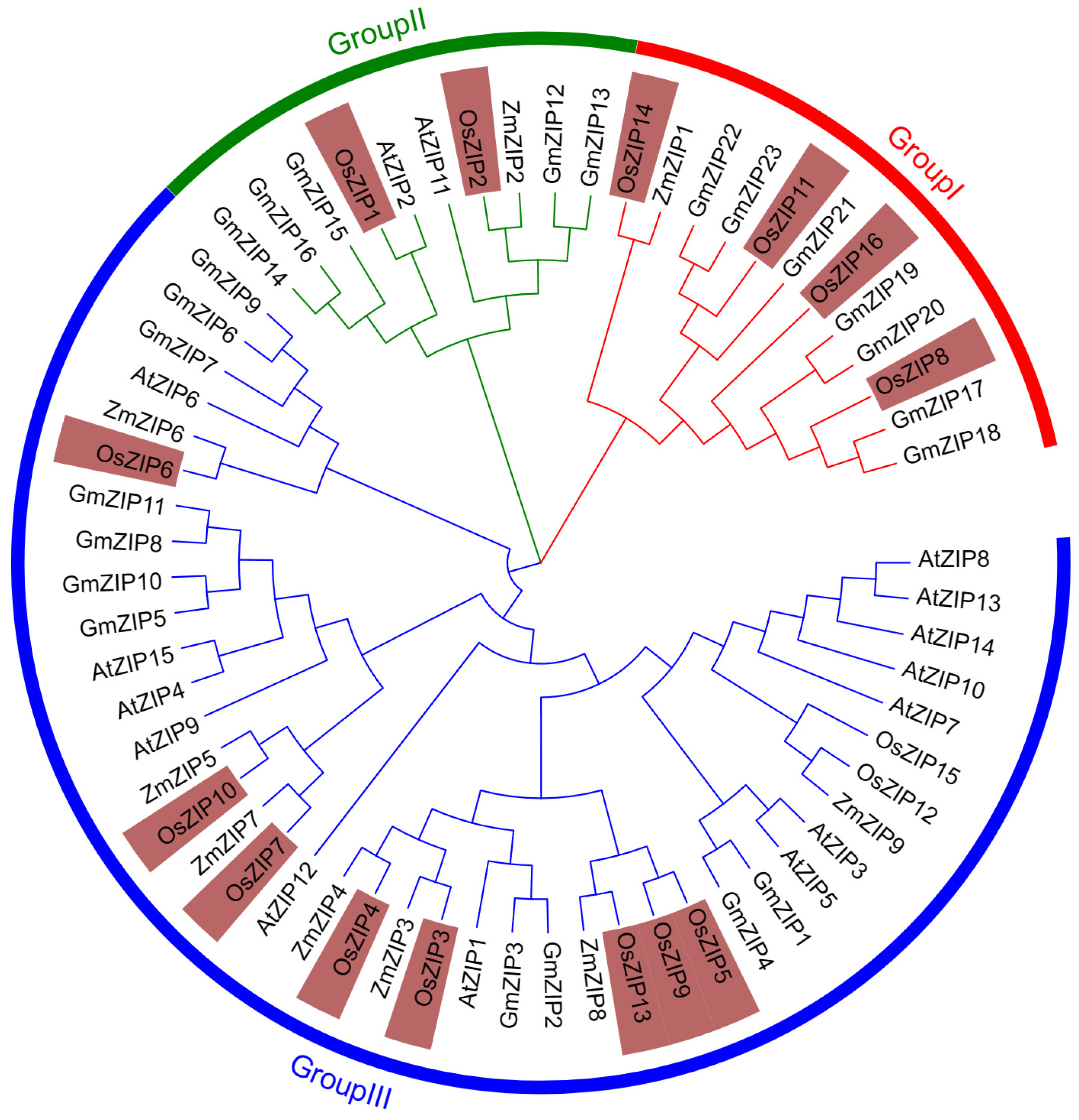
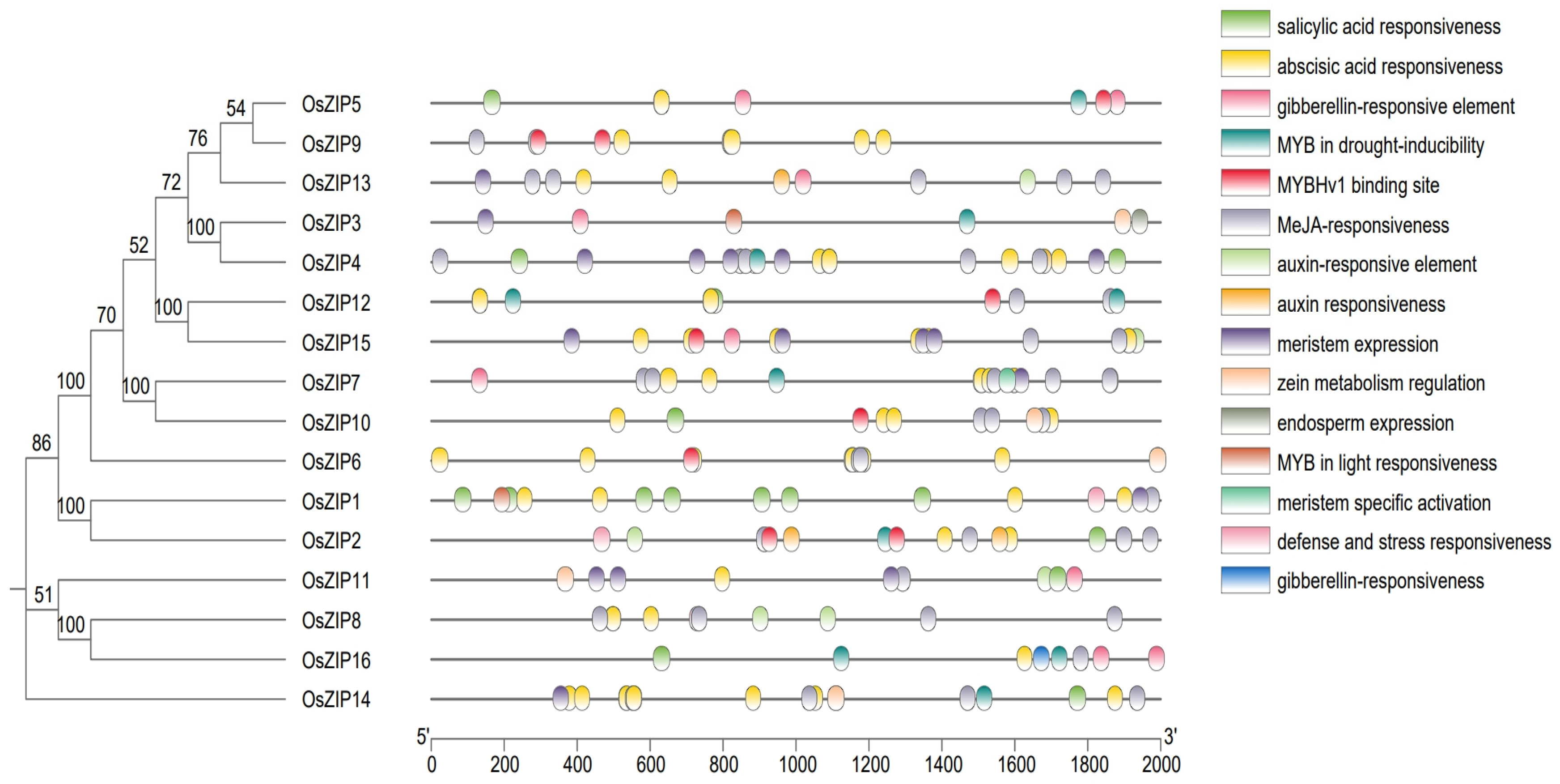
3.3. Phylogenetic Tree of ZIPs
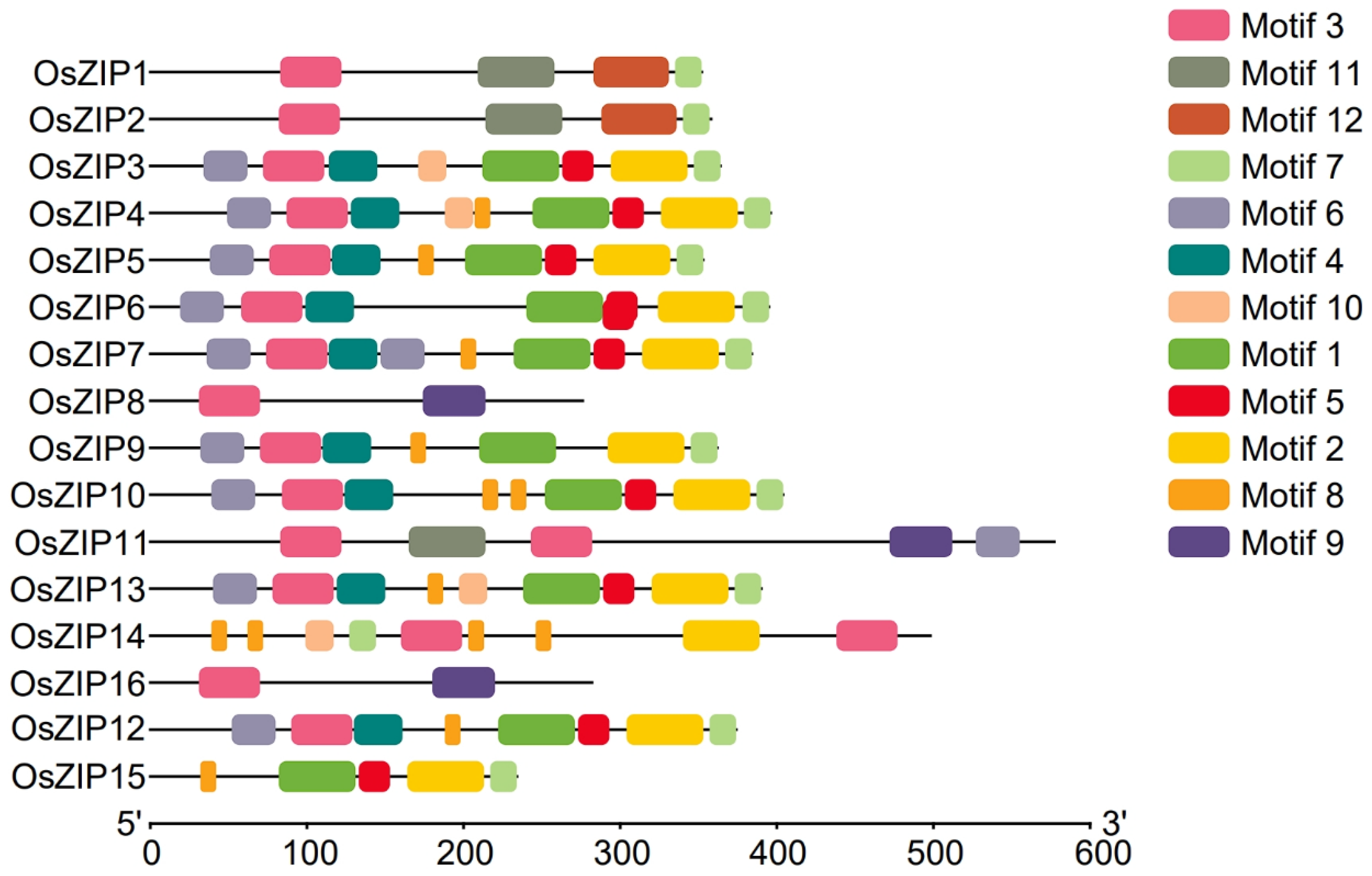
3.4. Analysis of the Conserved Motif of Rice ZIPs
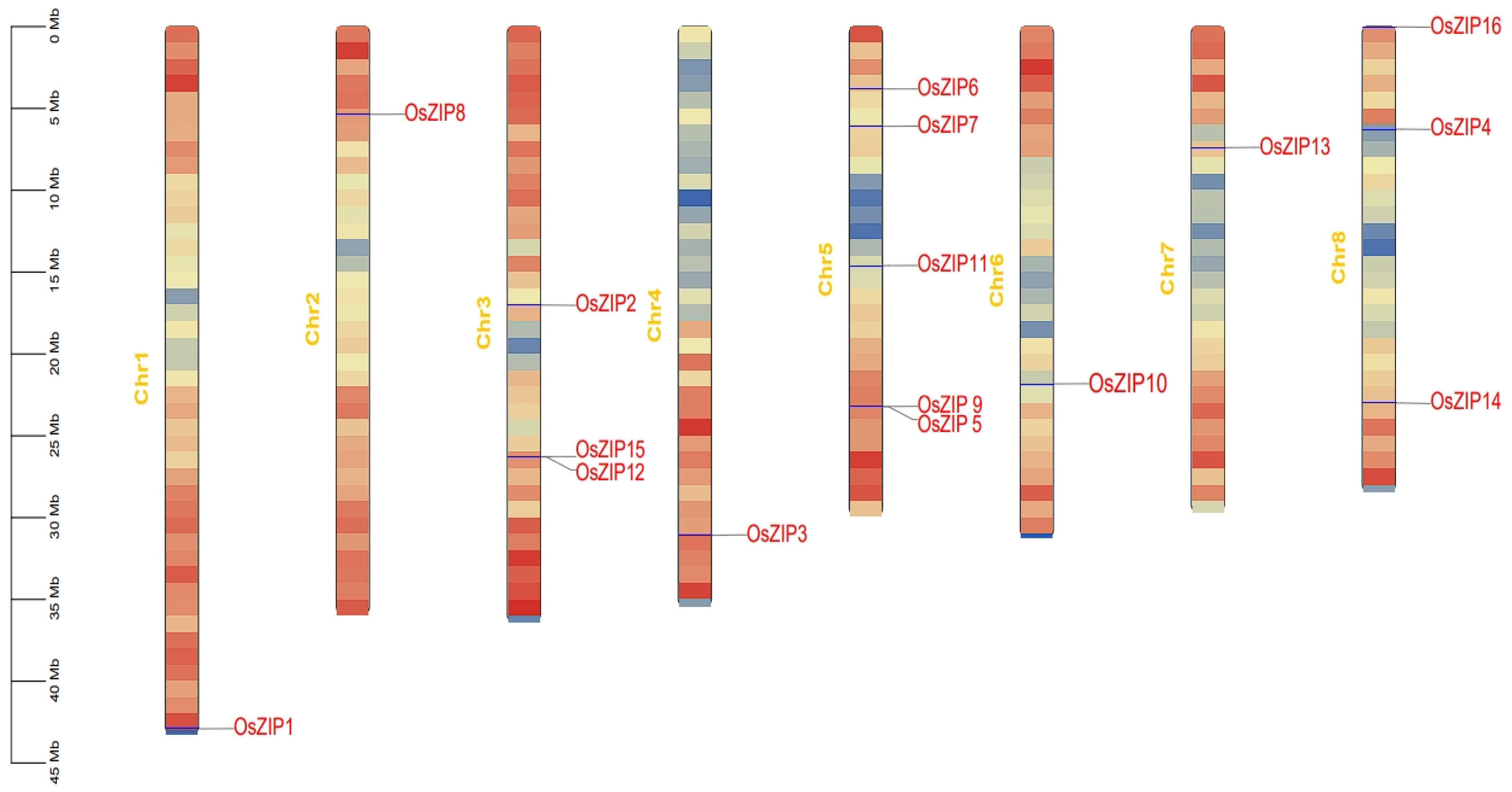
3.5. Chromosome Localization of Rice ZIPs
3.6. Verifying of OsZIP Cis Acting Elements
3.7. Analysis of OsZIPs Expression under Mn Poisoning
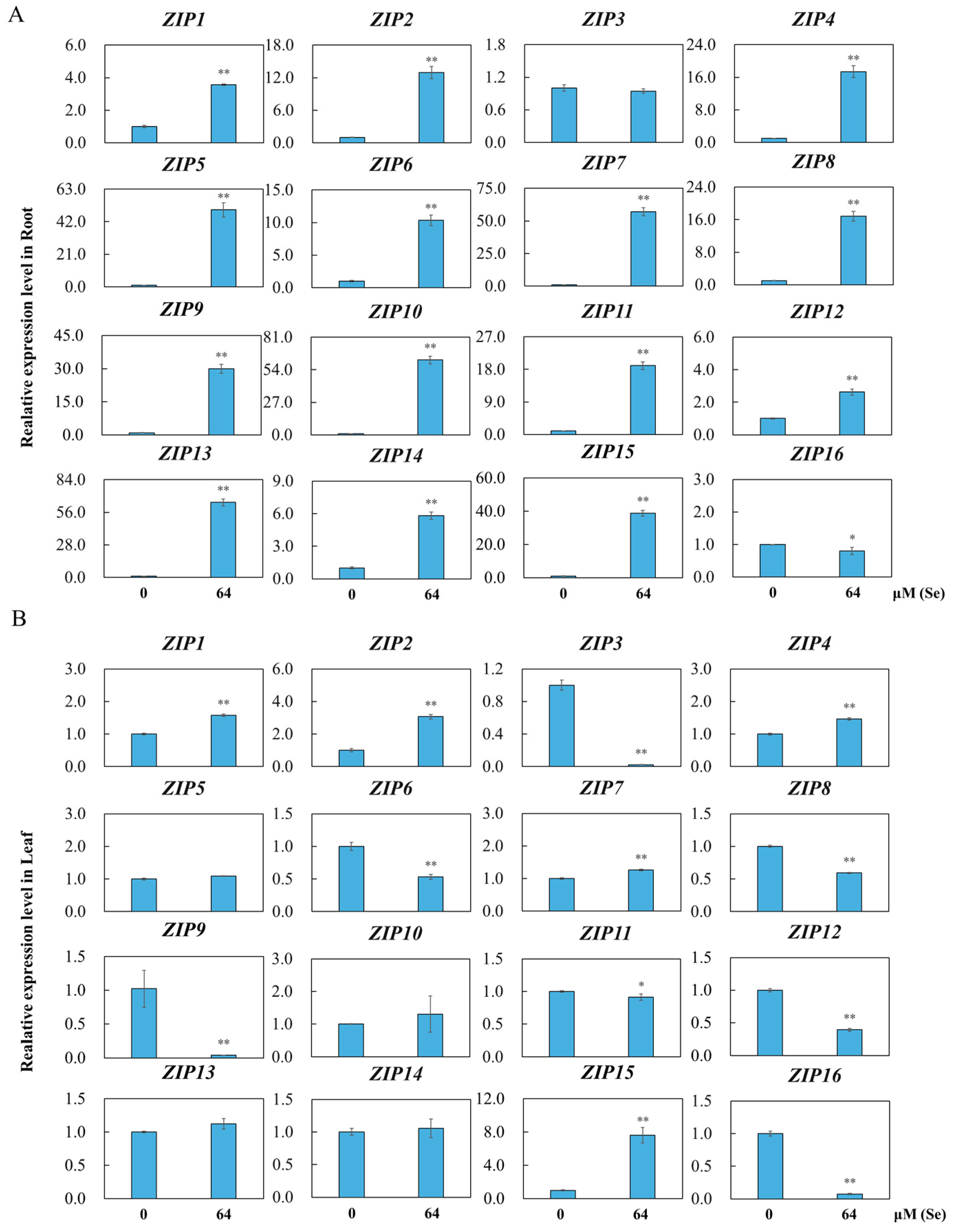
3.8. Analysis of OsZIPs Expression under Se Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Skórka, M.; Sieprawska, A.; Telk, A. The implication of manganese surplus on plant cell homeostasis: A review. J. Plant Growth Regul. 2023, 42, 1327–1341. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Pan, Y.; Chen, J.; Liu, Y. Metabolic responses to manganese toxicity in soybean roots and leaves. Plants 2023, 12, 3615. [Google Scholar] [CrossRef] [PubMed]
- Le¡ková, A.; Giehl, R.F.H.; Hartmann, A.; Farga¡ová, A.; Wirén, N. Heavy metals induce iron deficiency responses at different hierarchic and regulatory levels. Plant Physiol. 2017, 174, 1648–1668. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Jia, Y.D.; Dong, R.S.; Huang, R.; Liu, P.D.; Li, X.Y.; Wang, Z.Y.; Liu, G.D.; Chen, Z.J. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 517877. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Yamaguchi–Iwai, Y.; Sasaki, R.; Nagao, M. Overview of mammalian zinc transporters. Cell Mol. Life Sci. 2004, 61, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure–function analysis of HKE4, a member of the new LIV–1 subfamily of zinc transporters. Biochem. J. 2004, 377, 131–139. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Chao, D.Y. Localization and circulation: Vesicle trafficking in regulating plant nutrient homeostasis. Plant J. 2022, 112, 1350–1363. [Google Scholar] [CrossRef]
- Gu, J.J.; Xu, J.H.; Guo, Y.P.; Zong, Y.; Chen, W.R.; Guo, W.D. Cloning and functional analysis of ZIP transporters in blueberry. Sci. Hortic. 2021, 278, 109871. [Google Scholar] [CrossRef]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. BBA–Mol. Cell Res. 2006, 1763, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, P.G.; Kuruvilla, S.; Mathew, M.K. Functional characterization of a transition metal ion transporter, OsZIP6 from rice (Oryza sativa L.). Plant Physiol. Bioch. 2015, 97, 165–174. [Google Scholar]
- Hu, J. Toward unzipping the ZIP metal transporters: Structure, evolution, and implications on drug discovery against cancer. FEBS J. 2021, 288, 5805–5825. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal–polluted land. Front. Plant Sci. 2020, 11, 513099. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z.; Zhou, X.J.; Huang, Y.Q.; Zhu, L.Y.; Zhang, S.J.; Zhao, Y.F.; Guo, J.J.; Chen, J.T.; Chen, R.M. Identification and characterization of the zinc–regulated transporters, iron–regulated transporter–like protein (ZIP) gene family in maize. BMC Plant Biol. 2013, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guerinot, M.L. The ZIP family of metal transporters. BBA–Biomembranes 2000, 1465, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Maser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.M.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Shah, Z.; Munir, I.; Iqbal, H.; Ahmad, M.Z.; Sultan, W.; Khan, A. Genome-wide screening and evolutionary analysis of ZIP (ZRT–IRT like proteins) family in cowpea (Vigna unguiculata L.). Genet. Resour. Crop. Evol. 2024, 71, 1145–1157. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.H.; Guo, L.L.; Li, H.J.; Nie, X.J.; Chai, S.C.; Zheng, W.J. Genome-wide identification of wheat ZIP gene family and functional characterization of the TaZIP13–B in plants. Front. Plant Sci. 2021, 12, 748146. [Google Scholar] [CrossRef]
- Yu, R.M.; Chang, Y.N.; Song, Y.J.; Tian, T.; Wang, H.J.; Gao, G. Genome-wide identification and expression analysis of the zinc transporter protein zip family in potato (Solanum tuberosum). Int. J. Agric. Biol. 2020, 26, 1533–1542. [Google Scholar]
- Montanari, S.; Salinitro, M.; Simoni, A.; Ciavatta, C.; Tassoni, A. Foraging for selenium: A comparison between hyperaccumulator and non–accumulator plant species. Sci. Rep. 2023, 13, 10661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef]
- Pilon–Smits, E.A.H. Selenium in plants. In Progress in Botany; Springer International Publishing: Cham, Switzerland, 2014; Volume 76, pp. 93–107. [Google Scholar]
- Pilon–Smits, E.A.H. Mechanisms of Plant Selenium Hyperaccumulation. In Plant Ecophysiology, Selenium in Plants; Pilon–Smits, E., Winkel, L., Lin, Z.Q., Eds.; Springer: Cham, Switzerland, 2017; Volume 11, pp. 53–66. [Google Scholar]
- Handa, N.; Bhardwaj, R.; Kaur, H.; Poonam; Kapoor, D.; Rattan, A.; Kaur, S.; Thukral, K.A.; Kaur, S.; Arora, S. Selenium: An antioxidative protectant in plants under stress. Plant Met. Interact. 2016, 7, 179–207. [Google Scholar]
- Oancea, F.; Szabolcs, L.; Oancea, A.O.; Lăcătuşu, R.; Abraham, B.; Stanciu–Burileanu, M.M.; Meszaros, A.; Lungu, M. Selenium biofortification biotechnologies of wheat grain in south–eastern part of Romania for a better human health. Studia. Univ. VG SSV (Life Sci. Ser.) 2014, 24, 47–56. [Google Scholar]
- Zhang, Y.; Pan, G.; Chen, J.; Hu, Q. Uptake and transport of selenite and selenate by soybean seedlings of two genotypes. Plant Soil. 2003, 253, 437–443. [Google Scholar] [CrossRef]
- Longchamp, M.; Angeli, N.; Castrec-Rouelle, M. Effects on the accumulation of calcium, magnesium, iron, manganese, copper and zinc of adding the two inorganic forms of selenium to solution cultures of Zea mays. Plant Physiol. Bioch. 2016, 98, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A.U. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol. Bioch. 2015, 94, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Saidi, I.; Nawel, N.; Djebali, W. Role of selenium in preventing manganese toxicity in sunflower (Helianthus annuus) seedling. S. Afr. J. Bot. 2014, 94, 88–94. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Li, J.; Chen, J.; Yang, S.; Zhao, M.; Xue, Y. Transcriptome sequencing analysis of root in soybean responding to Mn poisoning. Int. J. Mol. Sci. 2023, 24, 12727. [Google Scholar] [CrossRef]
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Hashmi, M.L.R.; Khan, M.B.; He, Z.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci. Total Environ. 2020, 712, 136497. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Perinelli, D.R.; Camilletti, D.; Carotenuto, F.; Belli, L.; Polzonetti, V. Nutritional properties of rice varieties commonly consumed in Italy and applicability in Gluten free diet. Foods 2021, 10, 1375. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, X. Understanding global rice trade flows: Network evolution and implications. Foods 2023, 12, 3298. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, J.; Soomar, A.M.; Khalid, F.; Abbasi, Y. Assessing rice (Oryza sativa L.) quality: A comprehensive review of current techniques and future directions. J. Agric. Food Res. 2023, 14, 100843. [Google Scholar] [CrossRef]
- Yuan, S.; Linquist, B.A.; Wilson, L.T.; Cassman, K.G.; Stuart, A.M.; Pede, V.; Miro, B.; Saito, K.; Agustiani, N.; Aristya, V.E.; et al. Sustainable intensification for a larger global rice bowl. Nat. Commun. 2021, 12, 7163. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Shi, H.; Qiu, J.; Ding, X.; Kou, Y. Recent progress in rice broad–spectrum disease resistance. Int. J. Mol. Sci. 2021, 22, 11658. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.B. Resilience in agriculture through crop diversification: Adaptive management for environmental change. BioScience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- Shao, J.F.; Yamaji, N.; Shen, R.F.; Ma, J.F. The key to Mn homeostasis in plants: Regulation of Mn transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Ishiyama, K.; Suganami, M.; Ushijima, T.; Fujii, T.; Tazoe, Y.; Kawasaki, M.; Noguchi, K.; Makino, A. Manganese toxicity disrupts indole acetic acid homeostasis and suppresses the CO2 assimilation reaction in rice leaves. Sci. Rep. 2021, 11, 20922. [Google Scholar] [CrossRef]
- Molnár, A.; Kolbert, Z.; Kéri, K.; Feigl, G.; Ördög, A.; Szőllősi, R.; Erdei, L. Selenite–induced nitro–oxidative stress processes in Arabidopsis thaliana and Brassica juncea. Ecotox. Environ. Safe. 2018, 148, 664–674. [Google Scholar] [CrossRef]
- He, L.X.; Zheng, A.X.; Du, B.; Luo, H.W.; Lu, R.H.; DU, P.; Chen, Y.L.; Zhang, T.T.; Lai, R.F.; Tang, X.R. Low-concentration sodium selenite applications improve oxidation resistance of filling-stage rice. Appl. Ecol. Environ. Res. 2019, 17, 989–998. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Alho, L.; Carvalho, M. Toxic levels of manganese in an acidic Cambisol alters antioxidant enzymes activity, element uptake and subcellular distribution in Triticum aestivum. Ecotox. Environ. Safe. 2020, 193, 110355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, Y.B.; Xie, B.X.; Zhu, S.N.; Lu, X.; Liang, C.Y.; Tian, J. Complex gene regulation between young and old soybean leaves in responses to manganese toxicity. Plant Physiol. Bioch. 2020, 155, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.W.; Zhao, P.P.; Zhu, Y.M.; Yang, J.G.; Wai, X.Q.; Yang, L.; Liu, H.; Rensing, C.; Ding, Y.Z. Application of inorganic selenium to reduce accumulation and toxicity of heavy metals (metalloids) in plants: The main mechanisms, concerns, and risks. Sci. Total Environ. 2021, 771, 144776. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium toxicity in plants and environment: Biogeochemistry and remediation possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef]
- Huang, S.; Sasaki, A.; Yamaji, N.; Okada, H.; Mitani-Ueno, N.; Ma, J.F. The ZIP transporter family member OsZIP9 contributes to root zinc uptake in rice under zinc-limited conditions. Plant Physiol. 2020, 183, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W. The MEME suite. Nucleic Acids Res. 2015, 43, 39–49. [Google Scholar] [CrossRef]
- Shen, G.; Jia, Y.; Wang, W.L. Evolutionary divergence of motifs in B–class MADS–box proteins of seed plants. J. Biol. Res.–Thessalon. 2021, 28, 12. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Chen, X.J.; Wang, P.J.; Sun, Y.; Yue, C.; Ye, N.X. Genome-wide and expression pattern analysis of JAZ family involved in stress responses and postharvest processing treatments in Camellia sinensis. Sci. Rep. 2020, 10, 2792. [Google Scholar] [CrossRef] [PubMed]
- Cock, J.; Yoshida, S.; Forno, D.A. Laboratory manual for physiological studies of rice. IRRI 1976, 61, 156–188. [Google Scholar]
- Hu, A.Y.; Zheng, M.M.; Sun, L.M.; Zhao, X.Q.; Shen, R.F. Ammonium alleviates manganese toxicity and accumulation in rice by down–regulating the transporter gene OsNramp5 through rhizosphere acidification. Front. Plant Sci. 2019, 10, 464209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, M.; Chen, J.Y.; Yang, S.X.; Chen, J.P.; Xue, Y.B. Comparative transcriptome analysis reveals complex physiological response and gene regulation in peanut roots and leaves under manganese toxicity stress. Int. J. Mol. Sci. 2023, 24, 1161. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real–time PCR. Biochem. Bioph. Res. Co. 2006, 345, 646–651. [Google Scholar] [CrossRef]
- Krishna, T.P.A.; Maharajan, T.; Roch, G.V.; Ignacimuthu, S.; Ceasar, A.S. Structure, function, regulation and phylogenetic relationship of ZIP family transporters of plants. Front. Plant Sci. 2020, 11, 533228. [Google Scholar]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.H.; Yuan, Y.; Han, X.W.; Han, S.; Li, Y.T.; Ma, D.F.; Fang, Z.W.; Gong, S.J.; Yin, J.L. Genome-wide identification, characterization, and expression profiling of TaDUF668 gene family in Triticum aestivum. Agronomy 2023, 13, 2178. [Google Scholar] [CrossRef]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef]
- Colangelo, E.P.; Guerinot, M.L. Put the metal to the petal: Metal uptake and transport throughout plants. Curr. Opin. Plant Biol. 2006, 9, 322–330. [Google Scholar] [CrossRef]
- Tang, M.J.; Zhang, X.L.; Xu, L.; Wang, Y.; Chen, S.; Dong, J.H.; Liu, L.W. Genome–and transcriptome–wide characterization of ZIP gene family reveals their potential role in radish (Raphanus sativus) response to heavy metal stresses. Sci. Hortic.–Amst. 2024, 324, 112564. [Google Scholar] [CrossRef]
- Nishida, S.; Tsuzuki, C.; Kato, A.; Aisu, A.; Yoshida, J.; Mizuno, T. AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 1433–1442. [Google Scholar] [CrossRef]
- Fan, S.K.; Fang, X.Z.; Guan, M.Y.; Ye, Y.Q.; Lin, X.Y.; Du, S.T.; Jin, C.W. Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1–mediated cadmium uptake. Front. Plant Sci. 2014, 5, 721. [Google Scholar] [CrossRef] [PubMed]
- Assunção, A.G.L.; Herrero, E.; Lin, Y.F.; Huettel, B.; Talukdar, S.; Smaczniak, C.; Immink, R.G.H.; Eldik, M.; Fiers, M.; Schat, H.; et al. Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. USA 2010, 107, 10296–10301. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, Z.B.; Chen, J.H.; Huang, Y.F.; Liu, Z.L.; Zou, J.W.; Chen, Y.H.; Su, N.N.; Cui, J. Transcriptome analysis revealed pivotal transporters involved in the reduction of cadmium accumulation in pak choi (Brassica chinensis L.) by exogenous hydrogen–rich water. Chemosphere 2019, 216, 684–697. [Google Scholar] [CrossRef]
- Mora, M.L.; Rosas, A.; Ribera, A.; Rengel, Z. Differential tolerance to Mn toxicity in perennial ryegrass genotypes: Involvement of antioxidative enzymes and root exudation of carboxylates. Plant Soil 2009, 320, 79–89. [Google Scholar] [CrossRef]
- Chen, Z.J.; Sun, L.L.; Liu, P.D.; Liu, G.D.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese–enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2015, 167, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.G.D.B.; dos Reis, A.R. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an anti–oxidant and pro–oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]


| Gene Number | Gene ID | Chr | CDS (bp) | Portion Length | MW (kda) | PI | Subcellular Prediction |
|---|---|---|---|---|---|---|---|
| OsZIP1 | LOC_Os01g74110.1 | 01 | 1059 | 352 | 37.45 | 8.89 | Cell membrane [48] |
| OsZIP2 | LOC_Os03g29850.1 | 03 | 1077 | 358 | 36.65 | 6.07 | Cell membrane [48] |
| OsZIP3 | LOC_Os04g52310.1 | 04 | 1095 | 364 | 38.14 | 8.75 | Cell membrane [48] |
| OsZIP4 | LOC_Os08g10630.1 | 08 | 1191 | 396 | 39.97 | 8.29 | Cell membrane [48] |
| OsZIP5 | LOC_Os05g39560.1 | 05 | 1062 | 354 | 36.76 | 6.85 | Cell membrane [48] |
| OsZIP6 | LOC_Os05g07210.1 | 05 | 1188 | 395 | 41.33 | 6.82 | Cell membrane [48] |
| OsZIP7 | LOC_Os05g10940.1 | 05 | 1155 | 384 | 39.73 | 7.06 | Cell membrane [48] |
| OsZIP8 | LOC_Os02g10230.1 | 02 | 831 | 276 | 29.28 | 7.88 | Cell membrane [48] |
| OsZIP9 | LOC_Os05g39540.1 | 05 | 1089 | 362 | 37.90 | 6.41 | Cell membrane [48] |
| OsZIP10 | LOC_Os06g37010.1 | 06 | 1215 | 404 | 41.53 | 7.03 | Chloroplast [48] |
| OsZIP11 | LOC_Os05g25194.1 | 05 | 1734 | 577 | 60.36 | 8.01 | Cell membrane [NCBI] |
| OsZIP12 | LOC_Os03g46470.1 | 03 | 1125 | 374 | 39.06 | 8.69 | Cell membrane [NCBI] |
| OsZIP13 | LOC_Os07g12890.1 | 07 | 1173 | 390 | 40.26 | 6.79 | Cell membrane [NCBI] |
| OsZIP14 | LOC_Os08g36420.1 | 08 | 1497 | 498 | 53.58 | 6.70 | Cell membrane [NCBI] |
| OsZIP15 | LOC_Os03g46454.1 | 03 | 705 | 234 | 24.15 | 8.04 | Cell membrane [NCBI] |
| OsZIP16 | LOC_Os08g01030.1 | 08 | 849 | 282 | 30.14 | 6.51 | Cell membrane [NCBI] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Yang, S.; Li, F.; Yao, Y.; Wu, Z.; Xue, Y.; Liu, Y. Genome-Wide Identification of OsZIPs in Rice and Gene Expression Analysis under Manganese and Selenium Stress. Genes 2024, 15, 696. https://doi.org/10.3390/genes15060696
Zeng X, Yang S, Li F, Yao Y, Wu Z, Xue Y, Liu Y. Genome-Wide Identification of OsZIPs in Rice and Gene Expression Analysis under Manganese and Selenium Stress. Genes. 2024; 15(6):696. https://doi.org/10.3390/genes15060696
Chicago/Turabian StyleZeng, Xiang, Shaoxia Yang, Feng Li, Yushuang Yao, Zhengwei Wu, Yingbin Xue, and Ying Liu. 2024. "Genome-Wide Identification of OsZIPs in Rice and Gene Expression Analysis under Manganese and Selenium Stress" Genes 15, no. 6: 696. https://doi.org/10.3390/genes15060696
APA StyleZeng, X., Yang, S., Li, F., Yao, Y., Wu, Z., Xue, Y., & Liu, Y. (2024). Genome-Wide Identification of OsZIPs in Rice and Gene Expression Analysis under Manganese and Selenium Stress. Genes, 15(6), 696. https://doi.org/10.3390/genes15060696





