Transcriptomic Analysis of Newborn Hanwoo Calves: Effects of Maternal Overnutrition during Mid- to Late Pregnancy on Subcutaneous Adipose Tissue and Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals, Dietary Regimens, and Management Practices
2.3. Collection and Preservation of SAT and Liver Samples from Newborn Calves
2.4. RNA Isolation, Quality Assurance, and Sequencing Protocol
2.5. Transcriptomic Data Preprocessing and Analysis
2.6. Analysis of Differential Gene Expression, Gene Ontology (GO), and Pathway Enrichment
2.7. Protein–Protein Interaction (PPI) Network Analysis
2.8. Data Analysis
3. Results
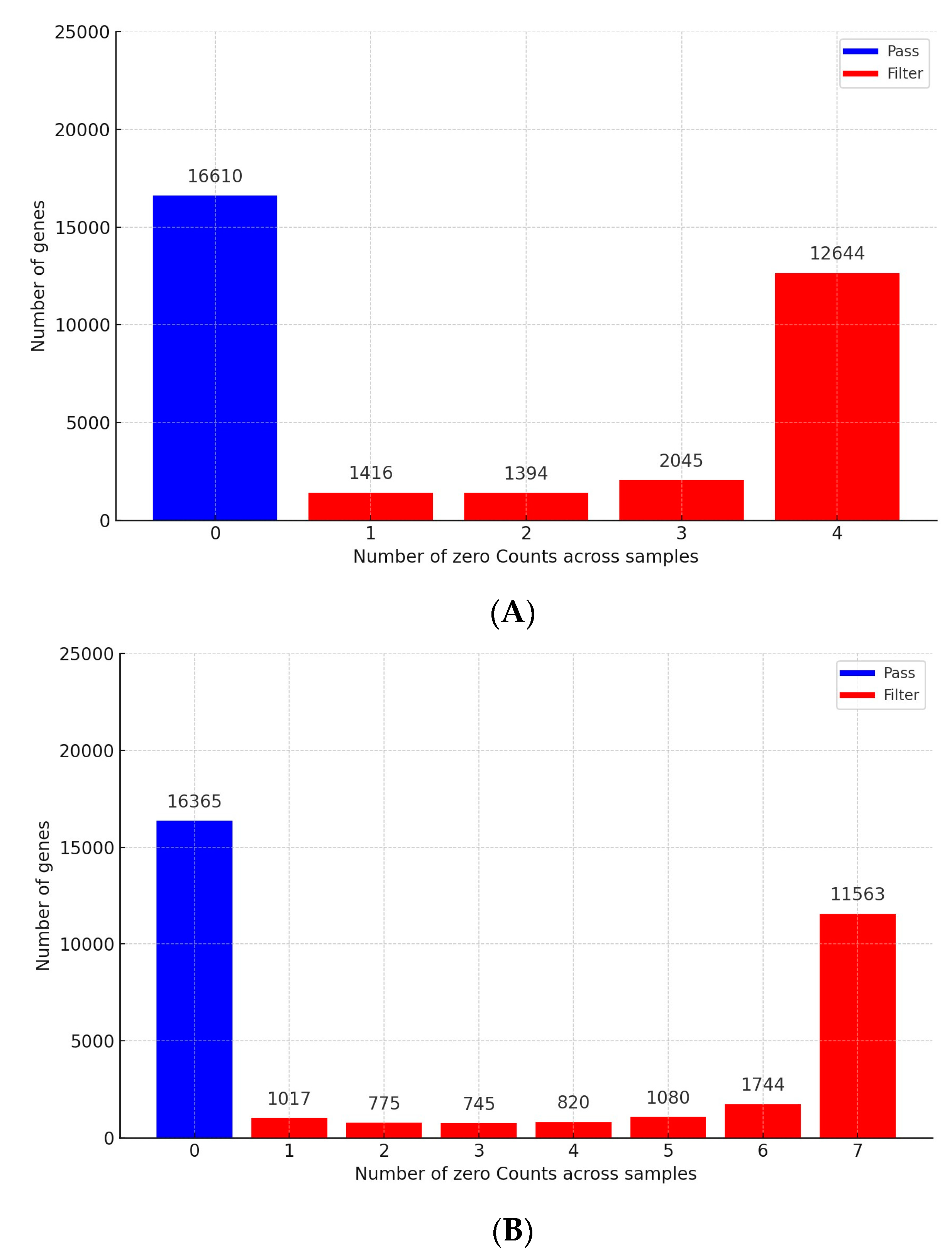
3.1. Analysis of RNA Sequencing Data
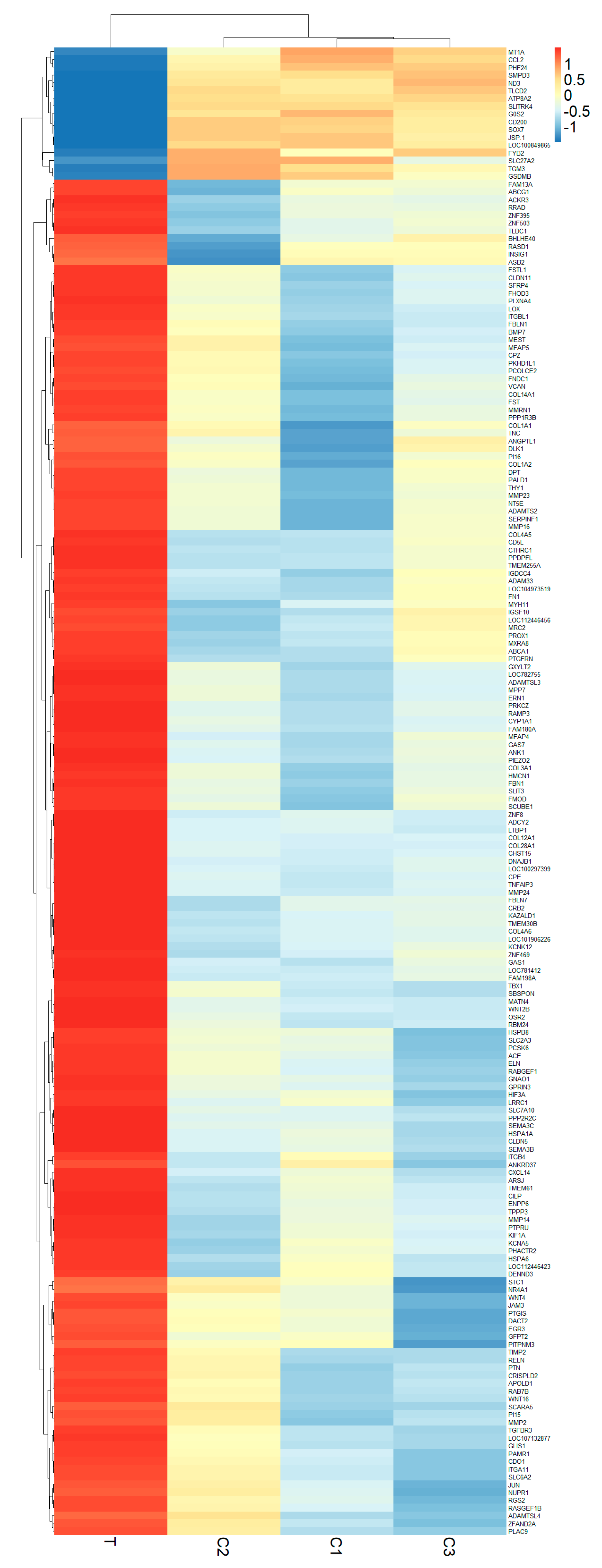
3.2. Analyzing Differential Gene Expression
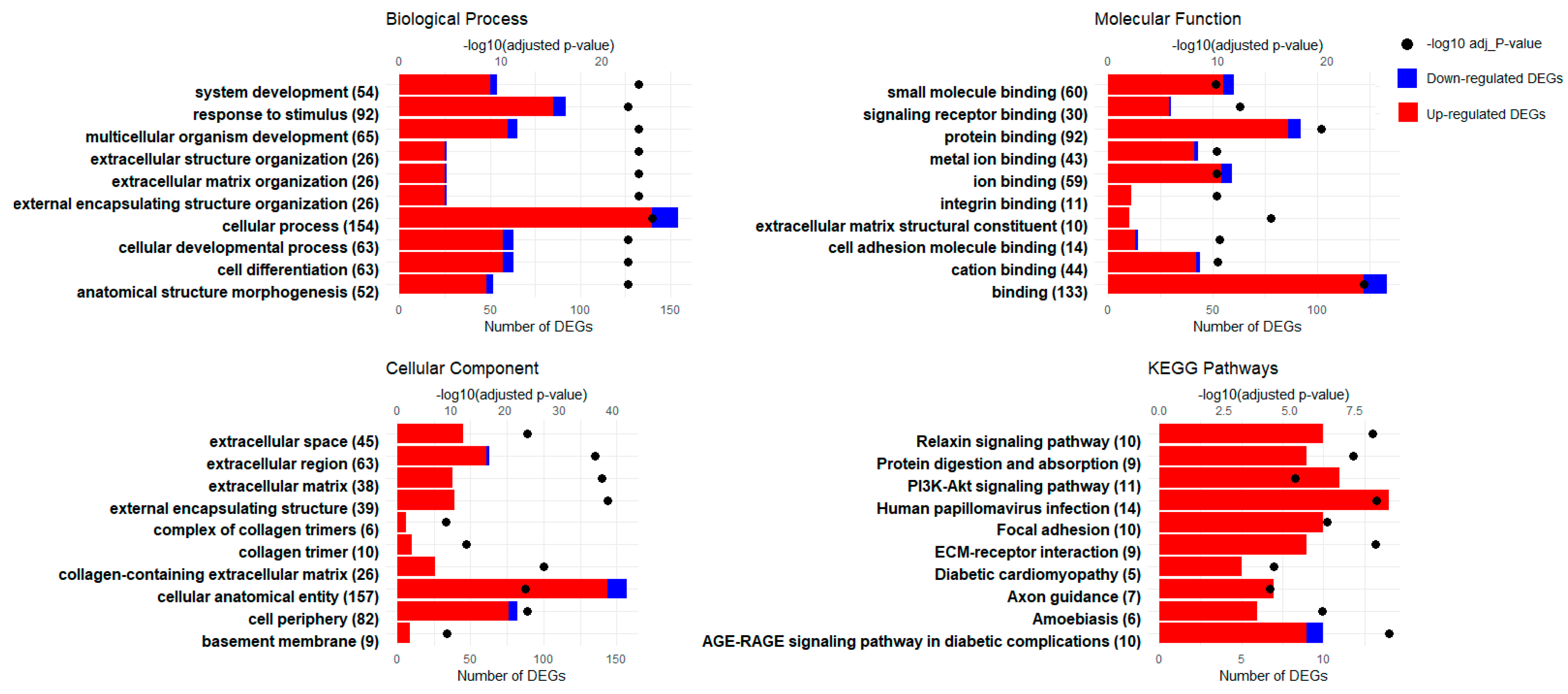
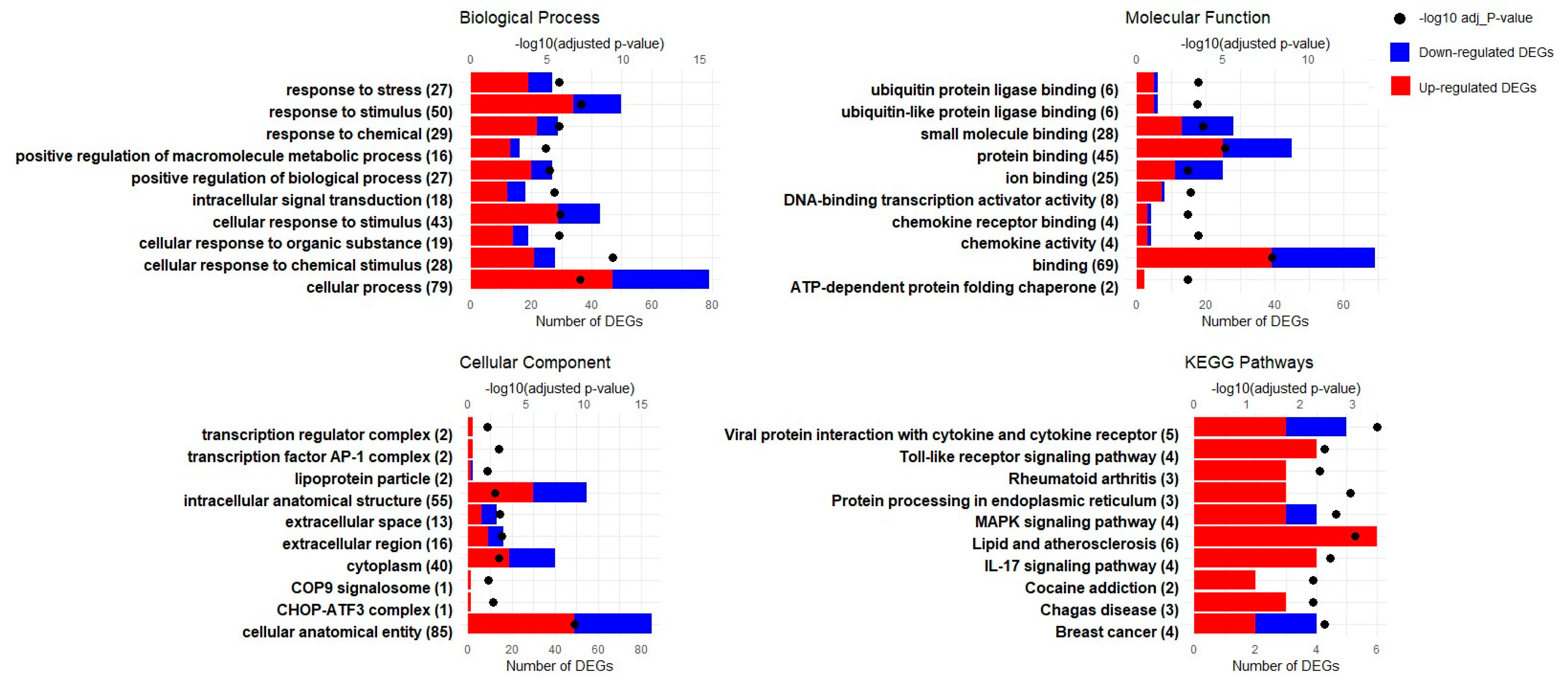
3.3. GO Enrichment Analysis
3.4. KEGG Pathway Enrichment Analysis
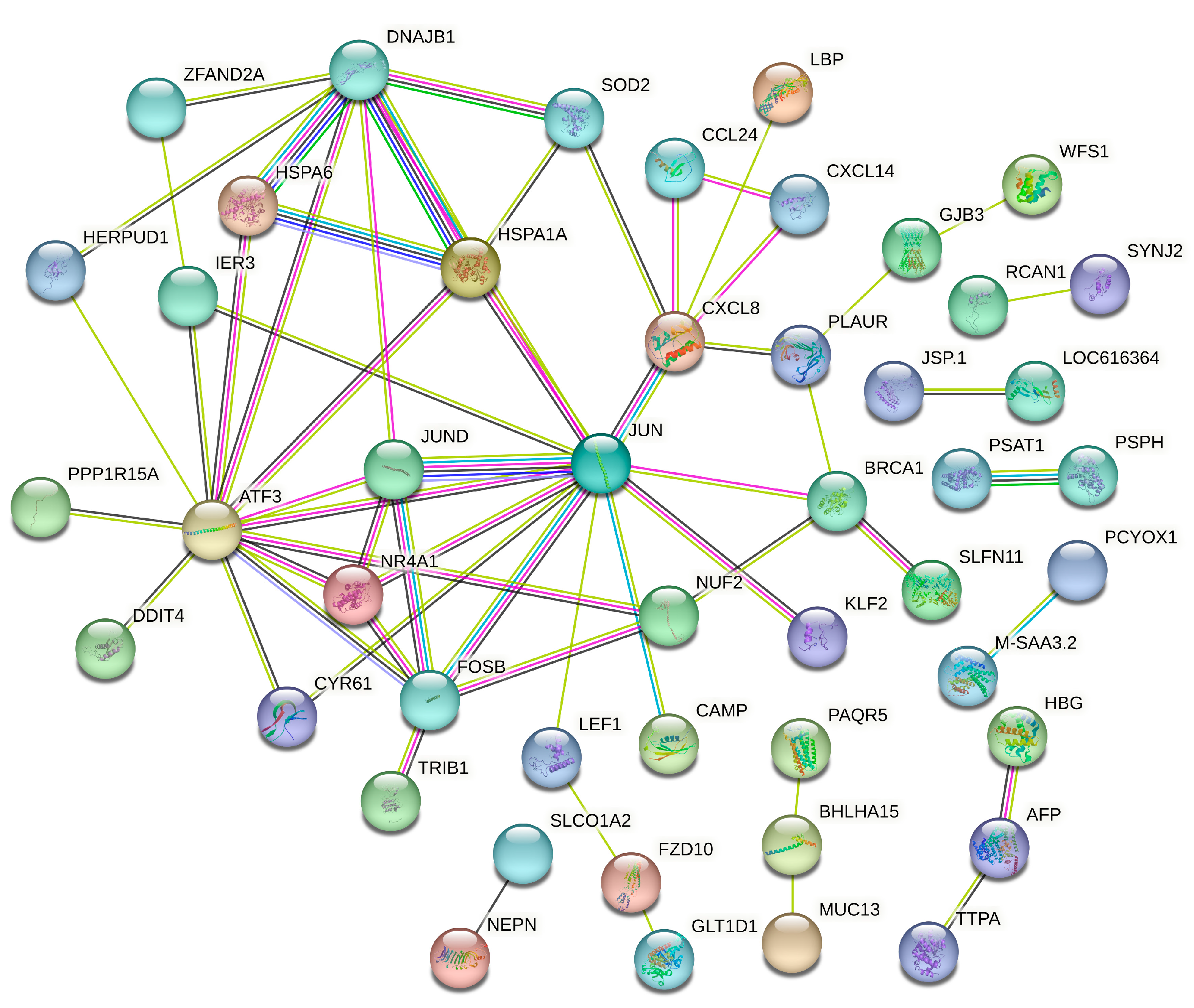
3.5. Protein–Protein Interaction Analysis of SAT and Liver Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Gianotti, T.F.; Burgueño, A.L.; Pirola, C.J. Fetal metabolic programming and epigenetic modifications: A systems biology approach. Pediatr. Res. 2013, 73, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Diniz, W.J.; Crouse, M.S.; Cushman, R.A.; McLean, K.J.; Caton, J.S.; Dahlen, C.R.; Reynolds, L.P.; Ward, A.K. Cerebrum, liver, and muscle regulatory networks uncover maternal nutrition effects in developmental programming of beef cattle during early pregnancy. Sci. Rep. 2021, 11, 2771. [Google Scholar] [CrossRef] [PubMed]
- Maloney, C.A.; Rees, W.D. Gene-nutrient interactions during fetal development. Reproduction 2005, 130, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Ward, A.K.; Caton, J.S. Epigenetics and developmental programming in ruminants: Long-term impacts on growth and development. In Biology of Domestic Animals; CRC Press: Boca Raton, FL, USA, 2017; pp. 85–121. [Google Scholar]
- Caton, J.S.; Crouse, M.S.; McLean, K.J.; Dahlen, C.R.; Ward, A.K.; Cushman, R.A.; Grazul-Bilska, A.T.; Neville, B.W.; Borowicz, P.P.; Reynolds, L.P. Maternal periconceptual nutrition, early pregnancy, and developmental outcomes in beef cattle. J. Anim. Sci. 2020, 98, skaa358. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.P.; Nilsson, E.; Skinner, M.K. Environmental epigenetics and epigenetic inheritance in domestic farm animals. Anim. Reprod. Sci. 2020, 220, 106316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, X.; Gomez, N.A.; Gao, Y.; Son, J.S.; Chae, S.A.; Zhu, M.-J.; Du, M. Stage-specific nutritional management and developmental programming to optimize meat production. J. Anim. Sci. Biotechnol. 2023, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Crouse, M.S.; Dahlen, C.R.; Ward, A.K. Developmental programming of fetal growth and development. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Copping, K.; Hernandez-Medrano, J.; Hoare, A.; Hummitzsch, K.; McMillen, I.; Morrison, J.; Rodgers, R.; Perry, V. Maternal periconceptional and first trimester protein restriction in beef heifers: Effects on placental parameters and fetal and neonatal calf development. Reprod. Fertil. Dev. 2020, 32, 495–507. [Google Scholar] [CrossRef]
- Palmer, E.A.; Peñagaricano, F.; Vedovatto, M.; Oliveira, R.A.; Field, S.L.; Laporta, J.; Moriel, P. Effects of maternal gestational diet, with or without methionine, on muscle transcriptome of Bos indicus-influenced beef calves following a vaccine-induced immunological challenge. PLoS ONE 2021, 16, e0253810. [Google Scholar] [CrossRef]
- Shao, T.; McCann, J.C.; Shike, D.W. Effects of late gestation supplements differing in fatty acid amount and profile to beef cows on cow performance, steer progeny growth performance through weaning, and relative mrna expression of genes associated with muscle and adipose tissue development. Animals 2023, 13, 437. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.B.; Costa, T.C.; Sanglard, L.P.; Nascimento, K.B.; Meneses, J.A.; Galvão, M.C.; Serão, N.V.; Duarte, M.S.; Gionbelli, M.P. Transcriptome profile in the skeletal muscle of cattle progeny as a function of maternal protein supplementation during mid-gestation. Livest. Sci. 2022, 263, 104995. [Google Scholar] [CrossRef]
- Bonnet, M.; Cassar-Malek, I.; Chilliard, Y.; Picard, B. Ontogenesis of muscle and adipose tissues and their interactions in ruminants and other species. Animal 2010, 4, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Funston, R.N.; Larson, D.M.; Vonnahme, K. Effects of maternal nutrition on conceptus growth and offspring performance: Implications for beef cattle production. J. Anim. Sci. 2010, 88, E205–E215. [Google Scholar] [CrossRef]
- Underwood, K.; Tong, J.; Price, P.; Roberts, A.; Grings, E.; Hess, B.; Means, W.; Du, M. Nutrition during mid to late gestation affects growth, adipose tissue deposition, and tenderness in cross-bred beef steers. Meat Sci. 2010, 86, 588–593. [Google Scholar] [CrossRef]
- Zhang, Y.; Otomaru, K.; Oshima, K.; Goto, Y.; Oshima, I.; Muroya, S.; Sano, M.; Saneshima, R.; Nagao, Y.; Kinoshita, A. Effects of low and high levels of maternal nutrition consumed for the entirety of gestation on the development of muscle, adipose tissue, bone, and the organs of Wagyu cattle fetuses. Anim. Sci. J. 2021, 92, e13600. [Google Scholar] [CrossRef]
- Zhang, Y.; Otomaru, K.; Muroya, S.; Gotoh, T. Maternal nutrition during gestation alters histochemical properties, and mRNA and microRNA expression in adipose tissue of wagyu fetuses. Front. Endocrinol. 2022, 12, 797680. [Google Scholar] [CrossRef] [PubMed]
- Jennings, T.; Underwood, K.; Wertz-Lutz, A.; Weaver, A. Effect of Maternal Nutrition on Fetal Adipocyte Development. 2010. Available online: https://openprairie.sdstate.edu/sd_beefreport_2010/12/ (accessed on 24 March 2024).
- Long, N.; Prado-Cooper, M.; Krehbiel, C.; DeSilva, U.; Wettemann, R. Effects of nutrient restriction of bovine dams during early gestation on postnatal growth, carcass and organ characteristics, and gene expression in adipose tissue and muscle. J. Anim. Sci. 2010, 88, 3251–3261. [Google Scholar] [CrossRef] [PubMed]
- Muhlhausler, B.S.; Duffield, J.; McMillen, I. Increased maternal nutrition increases leptin expression in perirenal and subcutaneous adipose tissue in the postnatal lamb. Endocrinology 2007, 148, 6157–6163. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Budge, H.; Symonds, M.E. Early developmental influences on hepatic organogenesis. Organogenesis 2008, 4, 170–175. [Google Scholar] [CrossRef]
- Smith, B.I.; Liefeld, A.; Vásquez-Hidalgo, M.A.; Vonnahme, K.A.; Grazul-Bilska, A.T.; Swanson, K.C.; Mishra, N.; Reed, S.A.; Zinn, S.A.; Govoni, K.E. Mid-to late-gestational maternal nutrient restriction followed by realimentation alters development and lipid composition of liver and skeletal muscles in ovine fetuses. J. Anim. Sci. 2021, 99, skab299. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, M.; Gardner, D.; Sebert, S.; Wilson, V.; Davidson, N.; Nigmatullina, Y.; Chan, L.; Budge, H.; Symonds, M. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction 2011, 141, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, M.; Gopalakrishnan, G.; Bispham, J.; Gentili, S.; McMillen, I.; Rhind, S.; Rae, M.; Kyle, C.; Brooks, A.; Jones, C. Maternal nutrient restriction in early pregnancy programs hepatic mRNA expression of growth-related genes and liver size in adult male sheep. J. Endocrinol. 2007, 192, 87–98. [Google Scholar] [CrossRef]
- Prezotto, L.; Camacho, L.; Lemley, C.; Keomanivong, F.; Caton, J.; Vonnahme, K.; Swanson, K. Nutrient restriction and realimentation in beef cows during early and mid-gestation and maternal and fetal hepatic and small intestinal in vitro oxygen consumption. Animal 2016, 10, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Diniz, W.J.; Ward, A.K.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Reynolds, L.P.; Borowicz, P.P.; Sedivec, K.K.; Kirsch, J.D.; Dorsam, S.T. Periconceptual maternal nutrition affects fetal liver programming of energy- and lipid-related genes. Animals 2023, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Otomaru, K.; Oshima, K.; Oshima, I.; Ojima, K.; Gotoh, T. DNA Methylation of Genes Participating in Hepatic Metabolisms and Function in Fetal Calf Liver Is Altered by Maternal Undernutrition during Gestation. Int. J. Mol. Sci. 2023, 24, 10682. [Google Scholar] [CrossRef] [PubMed]
- NIAS. Korean Feeding Standard for Hanwoo; National Institute of Animal Science, Rural Development Administration: Wanju-gun, Republic of Korea, 2017. Available online: https://www.nias.go.kr/ (accessed on 12 December 2020).
- Park, M.S.; Shokrollahi, B.; Kim, U.H.; Won, J.I.; Cho, S.-H.; Jin, S.; Kang, S.S.; Moon, S.J.; Um, K.-H.; Jang, K.S. Effects of mid-to-late prepartum feed supplementation in Hanwoo beef cows on their performance, blood metabolites, and the carcass characteristics and metabolites of their neonatal calves. Front. Vet. Sci. 2023, 10, 1287119. [Google Scholar] [CrossRef] [PubMed]
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics: Cambridge, UK, 2010. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Von Mering, C.; Jensen, L.J.; Snel, B.; Hooper, S.D.; Krupp, M.; Foglierini, M.; Jouffre, N.; Huynen, M.A.; Bork, P. STRING: Known and predicted protein–protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005, 33, D433–D437. [Google Scholar] [CrossRef] [PubMed]
- Du, M. Prenatal development of muscle and adipose and connective tissues and its impact on meat quality. Meat Muscle Biol. 2023, 7, 16230. [Google Scholar] [CrossRef]
- Herring, C.M.; Bazer, F.W.; Johnson, G.A.; Wu, G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp. Biol. Med. 2018, 243, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Amaya-Farfan, J. Dietary nutrients and bioactive substances modulate heat shock protein (HSP) expression: A review. Nutrients 2018, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Kuppuswami, J.; Senthilkumar, G.P. Nutri-stress, mitochondrial dysfunction, and insulin resistance—Role of heat shock proteins. Cell Stress Chaperones 2023, 28, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Hyder, I.; Pasumarti, M.; Reddy, P.R.; Prasad, C.S.; Kumar, K.A.; Sejian, V. Thermotolerance in domestic ruminants: A HSP70 perspective. In Heat Shock Proteins in Veterinary Medicine and Sciences; Springer: Cham, Switzerland, 2017; pp. 3–35. [Google Scholar]
- Banerjee, D.; Upadhyay, R.C.; Chaudhary, U.B.; Kumar, R.; Singh, S.; Ashutosh; Polley, S.; Mukherjee, A.; Das, T.K.; De, S. Seasonal variation in expression pattern of genes under HSP70: Seasonal variation in expression pattern of genes under HSP70 family in heat-and cold-adapted goats (Capra hircus). Cell Stress Chaperones 2014, 19, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mohanarao, G.J.; Mukherjee, A.; Banerjee, D.; Gohain, M.; Dass, G.; Brahma, B.; Datta, T.K.; Upadhyay, R.C.; De, S. HSP70 family genes and HSP27 expression in response to heat and cold stress in vitro in peripheral blood mononuclear cells of goat (Capra hircus). Small Rumin. Res. 2014, 116, 94–99. [Google Scholar] [CrossRef]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.d.F.B.; Baena, M.M.; de Cássia Estopa, A.; Gervásio, I.C.; Ibelli, A.M.G.; Gionbelli, T.R.S.; Gionbelli, M.P.; de Freitas, R.T.F.; Meirelles, S.L.C. Differential expression of HSF1 and HSPA6 genes and physiological responses in Angus and Simmental cattle breeds. J. Therm. Biol. 2019, 84, 92–98. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Li, L.; He, M.L.; Wang, K.; Zhang, Y.S. Buffering agent via insulin-mediated activation of PI3K/AKT signaling pathway to regulate lipid metabolism in lactating goats. Physiol. Res. 2018, 67, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Schulien, I.; Hockenjos, B.; Schmitt-Graeff, A.; Perdekamp, M.G.; Follo, M.; Thimme, R.; Hasselblatt, P. The transcription factor c-Jun/AP-1 promotes liver fibrosis during non-alcoholic steatohepatitis by regulating Osteopontin expression. Cell Death Differ. 2019, 26, 1688–1699. [Google Scholar] [CrossRef]
- Pan, C.; Wang, S.; Yang, C.; Hu, C.; Sheng, H.; Xue, X.; Hu, H.; Lei, Z.; Yang, M.; Ma, Y. Genome-wide identification and expression profiling analysis of Wnt family genes affecting adipocyte differentiation in cattle. Sci. Rep. 2022, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Griner, J.D.; Rogers, C.J.; Zhu, M.-J.; Du, M. Lysyl oxidase propeptide promotes adipogenesis through inhibition of FGF-2 signaling. Adipocyte 2017, 6, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Olijnyk, D.; Ibrahim, A.; Ferrier, R.; Tsuda, T.; Chu, M.-L.; Gusterson, B.; Stein, T.; Morris, J. Fibulin-2 is involved in early extracellular matrix development of the outgrowing mouse mammary epithelium. Cell. Mol. Life Sci. 2014, 71, 3811–3828. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, G.C.; Almos, P.; McNeill, R.V.; Jansch, C.; Lesch, K.P. Cellular effects and clinical implications of SLC2A3 copy number variation. J. Cell. Physiol. 2020, 235, 9021–9036. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Silva, D.B.; Fonseca, L.F.S.; Pinheiro, D.G.; Muniz, M.M.M.; Magalhães, A.F.B.; Baldi, F.; Ferro, J.A.; Chardulo, L.A.L.; De Albuquerque, L.G. Prediction of hub genes associated with intramuscular fat content in Nelore cattle. BMC Genom. 2019, 20, 520. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.C.; Gross, J.J.; Bruckmaier, R.; Albrecht, C. Cholesterol metabolism, transport, and hepatic regulation in dairy cows during transition and early lactation. J. Dairy Sci. 2014, 97, 5481–5490. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef]
- Duarte, M.; Gionbelli, M.; Paulino, P.; Serão, N.; Nascimento, C.; Botelho, M.; Martins, T.; Filho, S.; Dodson, M.; Guimarães, S. Maternal overnutrition enhances mRNA expression of adipogenic markers and collagen deposition in skeletal muscle of beef cattle fetuses. J. Anim. Sci. 2014, 92, 3846–3854. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, T. Integrins modulate cellular fibrogenesis at multiple levels: Regulation of TGF-β signaling. Endocr. Metab. Immune Disord. Drug Targets 2010, 10, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhang, X.; Qi, Y.; Wang, Y.; Pang, Y.; Zhang, Z.; Liu, P. The relationships of collagen and ADAMTS2 expression levels with meat quality traits in cattle. Indian J. Anim. Res. 2018, 52, 167–172. [Google Scholar]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 2019, 10, 491100. [Google Scholar] [CrossRef] [PubMed]


| Item | Concentrate | Rice Straw |
|---|---|---|
| Dry Matter (%) | 88.43 | 97.87 |
| % of Dry Matter | ||
| Crude Protein | 16.43 | 6.22 |
| TDN 1 | 75.40 | 60.64 |
| NDF 2 | 35.64 | 71.47 |
| ADF 3 | 13.83 | 38.56 |
| Ash | 7.55 | 11.31 |
| Ether extract | 3.42 | 0.64 |
| Crude fiber | 8.14 | 29.99 |
| Lignin | 2.88 | 4.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokrollahi, B.; Lee, H.-J.; Baek, Y.C.; Jin, S.; Jang, G.-S.; Moon, S.J.; Um, K.-H.; Jang, S.S.; Park, M.S. Transcriptomic Analysis of Newborn Hanwoo Calves: Effects of Maternal Overnutrition during Mid- to Late Pregnancy on Subcutaneous Adipose Tissue and Liver. Genes 2024, 15, 704. https://doi.org/10.3390/genes15060704
Shokrollahi B, Lee H-J, Baek YC, Jin S, Jang G-S, Moon SJ, Um K-H, Jang SS, Park MS. Transcriptomic Analysis of Newborn Hanwoo Calves: Effects of Maternal Overnutrition during Mid- to Late Pregnancy on Subcutaneous Adipose Tissue and Liver. Genes. 2024; 15(6):704. https://doi.org/10.3390/genes15060704
Chicago/Turabian StyleShokrollahi, Borhan, Hyun-Jeong Lee, Youl Chang Baek, Shil Jin, Gi-Suk Jang, Sung Jin Moon, Kyung-Hwan Um, Sun Sik Jang, and Myung Sun Park. 2024. "Transcriptomic Analysis of Newborn Hanwoo Calves: Effects of Maternal Overnutrition during Mid- to Late Pregnancy on Subcutaneous Adipose Tissue and Liver" Genes 15, no. 6: 704. https://doi.org/10.3390/genes15060704
APA StyleShokrollahi, B., Lee, H.-J., Baek, Y. C., Jin, S., Jang, G.-S., Moon, S. J., Um, K.-H., Jang, S. S., & Park, M. S. (2024). Transcriptomic Analysis of Newborn Hanwoo Calves: Effects of Maternal Overnutrition during Mid- to Late Pregnancy on Subcutaneous Adipose Tissue and Liver. Genes, 15(6), 704. https://doi.org/10.3390/genes15060704





