A Divergent Platelet Transcriptome in Patients with Lipedema and Lymphedema
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Diagnosis of Patients
2.3. Platelet Isolation and Transcriptomic Profiling
2.4. Bioinformatics and Statistical Analysis
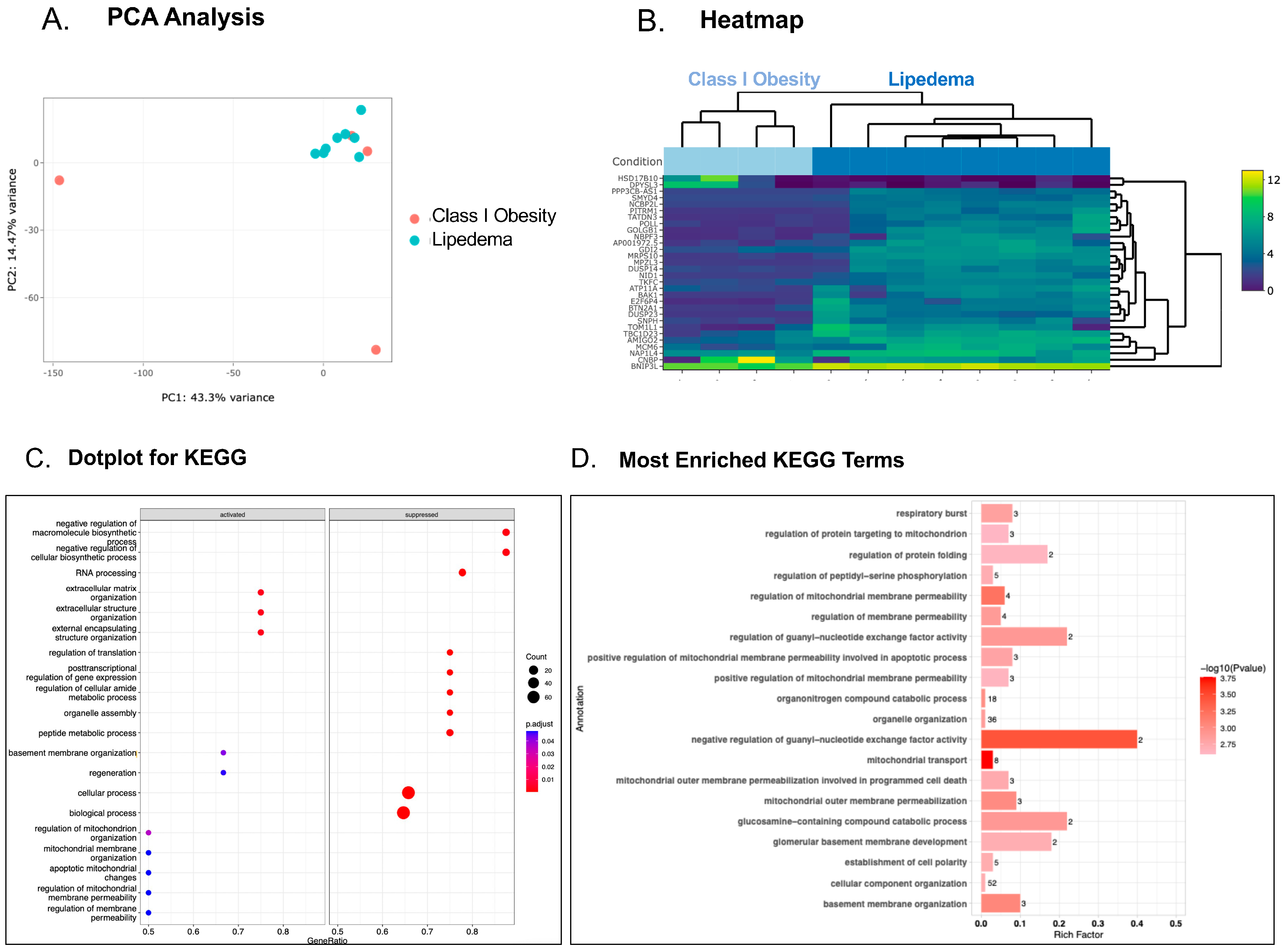
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gasparis, A.P.; Kim, P.S.; Dean, S.M.; Khilnani, N.M.; Labropoulos, N. Diagnostic approach to lower limb edema. Phlebology 2020, 35, 650–655. [Google Scholar] [CrossRef]
- Aday, A.W.; Donahue, P.M.; Garza, M.; Crain, V.N.; Patel, N.J.; Beasley, J.A.; Herbst, K.L.; Beckman, J.A.; Taylor, S.L.; Pridmore, M.; et al. National survey of patient symptoms and therapies among 707 women with a lipedema phenotype in the United States. Vasc. Med. 2024, 29, 36–41. [Google Scholar] [CrossRef]
- Rockson, S.G.; Tian, W.; Jiang, X.; Kuznetsova, T.; Haddad, F.; Zampell, J.; Mehrara, B.; Sampson, J.P.; Roche, L.; Kim, J.; et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 2018, 3, e123775. [Google Scholar] [CrossRef]
- Ezzaty Mirhashemi, M.; Shah, R.V.; Kitchen, R.R.; Rong, J.; Spahillari, A.; Pico, A.R.; Vitseva, O.; Levy, D.; Demarco, D.; Shah, S.; et al. The Dynamic Platelet Transcriptome in Obesity and Weight Loss. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 854–864. [Google Scholar] [CrossRef]
- Latshaw, S.P.; Bazaes, S.; Randolph, A.; Poorman, R.A.; Heinrikson, R.L.; Kemp, R.G. Identification of highly reactive cysteinyl and methionyl residues of rabbit muscle phosphofructokinase. J. Biol. Chem. 1987, 262, 10672–10677. [Google Scholar] [CrossRef]
- Barrachina, M.N.; Sueiro, A.M.; Izquierdo, I.; Hermida-Nogueira, L.; Guitian, E.; Casanueva, F.F.; Farndale, R.W.; Moroi, M.; Jung, S.M.; Pardo, M.; et al. GPVI surface expression and signalling pathway activation are increased in platelets from obese patients: Elucidating potential anti-atherothrombotic targets in obesity. Atherosclerosis 2019, 281, 62–70. [Google Scholar] [CrossRef]
- Khalid, M.U.; Prasada, S.; Jennings, C.; Bartholomew, J.R.; McCarthy, M.; Hornacek, D.A.; Joseph, D.; Chen, W.; Schwarz, G.; Bhandari, R.; et al. Venous thromboembolic outcomes in patients with lymphedema and lipedema: An analysis from the National Inpatient Sample. Vasc. Med. 2024, 29, 42–47. [Google Scholar] [CrossRef]
- Cushman, M. Epidemiology and risk factors for venous thrombosis. Semin. Hematol. 2007, 44, 62–69. [Google Scholar] [CrossRef]
- Barrachina, M.N.; Hermida-Nogueira, L.; Moran, L.A.; Casas, V.; Hicks, S.M.; Sueiro, A.M.; Di, Y.; Andrews, R.K.; Watson, S.P.; Gardiner, E.E.; et al. Phosphoproteomic Analysis of Platelets in Severe Obesity Uncovers Platelet Reactivity and Signaling Pathways Alterations. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 478–490. [Google Scholar] [CrossRef]
- Cameron, S.J.; Ture, S.K.; Mickelsen, D.; Chakrabarti, E.; Modjeski, K.L.; McNitt, S.; Seaberry, M.; Field, D.J.; Le, N.T.; Abe, J.; et al. Platelet Extracellular Regulated Protein Kinase 5 Is a Redox Switch and Triggers Maladaptive Platelet Responses and Myocardial Infarct Expansion. Circulation 2015, 132, 47–58. [Google Scholar] [CrossRef]
- Soo Kim, B.; Auerbach, D.S.; Sadhra, H.; Godwin, M.; Bhandari, R.; Ling, F.S.; Mohan, A.; Yule, D.I.; Wagner, L., 2nd; Rich, D.Q.; et al. Sex-Specific Platelet Activation Through Protease-Activated Receptors Reverses in Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 390–400. [Google Scholar] [CrossRef]
- Morrell, C.N.; Mix, D.; Aggarwal, A.; Bhandari, R.; Godwin, M.; Owens, P., 3rd; Lyden, S.P.; Doyle, A.; Krauel, K.; Rondina, M.T.; et al. Platelet olfactory receptor activation limits platelet reactivity and growth of aortic aneurysms. J. Clin. Investig. 2022, 132, e152373. [Google Scholar] [CrossRef]
- Hilt, Z.T.; Pariser, D.N.; Ture, S.K.; Mohan, A.; Quijada, P.; Asante, A.A.; Cameron, S.J.; Sterling, J.A.; Merkel, A.R.; Johanson, A.L.; et al. Platelet-derived beta2M regulates monocyte inflammatory responses. JCI Insight 2019, 4, e122943. [Google Scholar] [CrossRef]
- Buck, D.W., 2nd; Herbst, K.L. Lipedema: A Relatively Common Disease with Extremely Common Misconceptions. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1043. [Google Scholar] [CrossRef]
- Herbst, K.L.; Kahn, L.A.; Iker, E.; Ehrlich, C.; Wright, T.; McHutchison, L.; Schwartz, J.; Sleigh, M.; Donahue, P.M.; Lisson, K.H.; et al. Standard of care for lipedema in the United States. Phlebology 2021, 36, 779–796. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Lurie, F.; Malgor, R.D.; Carman, T.; Dean, S.M.; Iafrati, M.D.; Khilnani, N.M.; Labropoulos, N.; Maldonado, T.S.; Mortimer, P.; O’Donnell, T.F., Jr.; et al. The American Venous Forum, American Vein and Lymphatic Society and the Society for Vascular Medicine expert opinion consensus on lymphedema diagnosis and treatment. Phlebology 2022, 37, 252–266. [Google Scholar] [CrossRef]
- Cameron, S.J.; Mix, D.S.; Ture, S.K.; Schmidt, R.A.; Mohan, A.; Pariser, D.; Stoner, M.C.; Shah, P.; Chen, L.; Zhang, H.; et al. Hypoxia and Ischemia Promote a Maladaptive Platelet Phenotype. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1594–1606. [Google Scholar] [CrossRef]
- Davi, G.; Guagnano, M.T.; Ciabattoni, G.; Basili, S.; Falco, A.; Marinopiccoli, M.; Nutini, M.; Sensi, S.; Patrono, C. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA 2002, 288, 2008–2014. [Google Scholar] [CrossRef]
- Heffron, S.P.; Marier, C.; Parikh, M.; Fisher, E.A.; Berger, J.S. Severe obesity and bariatric surgery alter the platelet mRNA profile. Platelets 2019, 30, 967–974. [Google Scholar] [CrossRef]
- Jones, C.I. Platelet function and ageing. Mamm. Genome 2016, 27, 358–366. [Google Scholar] [CrossRef]
- Qian, F.; Le Breton, G.C.; Chen, J.; Deng, J.; Christman, J.W.; Wu, D.; Ye, R.D. Role for the guanine nucleotide exchange factor phosphatidylinositol-3,4,5-trisphosphate-dependent rac exchanger 1 in platelet secretion and aggregation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gil, H.J.; Escobedo, N.; Benito-Martin, A.; Ximenez-Embun, P.; Munoz, J.; Peinado, H.; Rockson, S.G.; Oliver, G. Platelet factor 4 is a biomarker for lymphatic-promoted disorders. JCI Insight 2020, 5, e135109. [Google Scholar] [CrossRef]
- Corbould, A.M.; Bawden, M.J.; Lavranos, T.C.; Rodgers, R.J.; Judd, S.J. The effect of obesity on the ratio of type 3 17beta-hydroxysteroid dehydrogenase mRNA to cytochrome P450 aromatase mRNA in subcutaneous abdominal and intra-abdominal adipose tissue of women. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 165–175. [Google Scholar] [CrossRef]
- Marques, A.T.; Antunes, A.; Fernandes, P.A.; Ramos, M.J. Comparative evolutionary genomics of the HADH2 gene encoding Abeta-binding alcohol dehydrogenase/17beta-hydroxysteroid dehydrogenase type 10 (ABAD/HSD10). BMC Genom. 2006, 7, 202. [Google Scholar] [CrossRef]
- Keith, L.; Seo, C.A.; Rowsemitt, C.; Pfeffer, M.; Wahi, M.; Staggs, M.; Dudek, J.; Gower, B.; Carmody, M. Ketogenic diet as a potential intervention for lipedema. Med. Hypotheses 2021, 146, 110435. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Lu, Y.; Zheng, Y.; Pan, L.; Wu, X.; Yuan, Y.; Shen, Z.; Ma, S.; Zhang, X.; et al. BNIP3L/NIX-mediated mitophagy: Molecular mechanisms and implications for human disease. Cell Death Dis. 2021, 13, 14. [Google Scholar] [CrossRef]
- Dosunmu-Ogunbi, A.; Yuan, S.; Reynolds, M.; Giordano, L.; Sanker, S.; Sullivan, M.; Stolz, D.B.; Kaufman, B.A.; Wood, K.C.; Zhang, Y.; et al. SOD2 V16A amplifies vascular dysfunction in sickle cell patients by curtailing mitochondria complex IV activity. Blood 2022, 139, 1760–1765. [Google Scholar] [CrossRef]
- Jiang, H.; Nechipurenko, D.Y.; Panteleev, M.A.; Xu, K.; Qiao, J. Redox regulation of platelet function and thrombosis. J. Thromb. Haemost. 2024, in press. [CrossRef]
- Richman, T.R.; Ermer, J.A.; Baker, J.; Siira, S.J.; Kile, B.T.; Linden, M.D.; Rackham, O.; Filipovska, A. Mitochondrial gene expression is required for platelet function and blood clotting. Cell Rep. 2023, 42, 113312. [Google Scholar] [CrossRef]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair. Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ai, Y.; Ni, L.; Wu, L.; Huang, X.; Chen, S. Platelet-derived TGF-beta1 is related to portal vein thrombosis in cirrhosis by promoting hypercoagulability and endothelial dysfunction. Front. Cardiovasc. Med. 2022, 9, 938397. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.P.; Ekhlak, M.; Dash, D. Energy metabolism in platelets fuels thrombus formation: Halting the thrombosis engine with small-molecule modulators of platelet metabolism. Metabolism 2023, 145, 155596. [Google Scholar] [CrossRef] [PubMed]
- Alshomer, F.; Lee, S.J.; Kim, Y.; Hong, D.W.; Pak, C.J.; Suh, H.P.; Hong, J.P. Lipedema associated with Skin Hypoperfusion and Ulceration: Soft Tissue Debulking Improving Skin Perfusion. Arch. Plast. Surg. 2024, 51, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Berthollier, C.; Ricard-Blum, S. The glycosaminoglycan interactome 2.0. Am. J. Physiol. Cell Physiol. 2022, 322, C1271–C1278. [Google Scholar] [CrossRef] [PubMed]
- Esmer, M.; Schingale, F.J. Intracellular and Extracellular Water Balance in Patients with Lipedema. Lymphat. Res. Biol. 2023, 21, 501–503. [Google Scholar] [CrossRef]
- Capitanio, A.M.; Niewiarowski, S.; Rucinski, B.; Tuszynski, G.P.; Cierniewski, C.S.; Hershock, D.; Kornecki, E. Interaction of platelet factor 4 with human platelets. Biochim. Biophys. Acta 1985, 839, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Cheng, B.; Farrugia, B.L.; McCarthy, S.; Whitelock, J.M. Platelet Factor 4 Binds to Vascular Proteoglycans and Controls Both Growth Factor Activities and Platelet Activation. J. Biol. Chem. 2017, 292, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.A.; Morrell, C.N.; Ling, F.S.; Simlote, P.; Fernandez, G.; Rich, D.Q.; Adler, D.; Gervase, J.; Cameron, S.J. The platelet phenotype in patients with ST-segment elevation myocardial infarction is different from non-ST-segment elevation myocardial infarction. Transl. Res. 2018, 195, 1–12. [Google Scholar] [CrossRef]
- Morrell, C.N.; Matsushita, K.; Chiles, K.; Scharpf, R.B.; Yamakuchi, M.; Mason, R.J.; Bergmeier, W.; Mankowski, J.L.; Baldwin, W.M., 3rd; Faraday, N.; et al. Regulation of platelet granule exocytosis by S-nitrosylation. Proc. Natl. Acad. Sci. USA 2005, 102, 3782–3787. [Google Scholar] [CrossRef]
- Matsushita, K.; Morrell, C.N.; Cambien, B.; Yang, S.X.; Yamakuchi, M.; Bao, C.; Hara, M.R.; Quick, R.A.; Cao, W.; O’Rourke, B.; et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 2003, 115, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Joshi, S.; Chanzu, H.; Alfar, H.R.; Shravani Prakhya, K.; Whiteheart, S.W. Alpha-Synuclein is the major platelet isoform but is dispensable for activation, secretion, and thrombosis. Platelets 2023, 34, 2267147. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Savage, J.S.; Harper, M.T.; Moore, S.F.; Hers, I.; Poole, A.W. Identification of roles for the SNARE-associated protein, SNAP29, in mouse platelets. Platelets 2016, 27, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Samocha-Bonet, D.; Justo, D.; Rogowski, O.; Saar, N.; Abu-Abeid, S.; Shenkerman, G.; Shapira, I.; Berliner, S.; Tomer, A. Platelet counts and platelet activation markers in obese subjects. Mediat. Inflamm. 2008, 2008, 834153. [Google Scholar] [CrossRef] [PubMed]
- Aleman, J.O.; Iyengar, N.M.; Walker, J.M.; Milne, G.L.; Da Rosa, J.C.; Liang, Y.; Giri, D.D.; Zhou, X.K.; Pollak, M.N.; Hudis, C.A.; et al. Effects of Rapid Weight Loss on Systemic and Adipose Tissue Inflammation and Metabolism in Obese Postmenopausal Women. J. Endocr. Soc. 2017, 1, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Goggs, R.; Williams, C.M.; Mellor, H.; Poole, A.W. Platelet Rho GTPases-a focus on novel players, roles and relationships. Biochem. J. 2015, 466, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Williamson, J.K.; Aronova, M.A.; Prince, A.A.; Pokrovskaya, I.D.; Leapman, R.D.; Storrie, B. Golgi proteins in circulating human platelets are distributed across non-stacked, scattered structures. Platelets 2017, 28, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Mignon-Ravix, C.; Riccardi, F.; Daquin, G.; Cacciagli, P.; Lamoureux-Toth, S.; Villard, L.; Villeneuve, N.; Molinari, F. NAPB and developmental and epileptic encephalopathy: Description of the electroclinical profile associated with a novel pathogenic variant. Epilepsia 2023, 64, e127–e134. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Tanriverdi, K.; Somasundaran, M.; Liu, P.; Soofi, S.; Bhandari, R.; Godwin, M.; Parsi, K.M.; et al. SARS-CoV-2 Initiates Programmed Cell Death in Platelets. Circ. Res. 2021, 129, 631–646. [Google Scholar] [CrossRef]
- Cookson, P.; Sutherland, J.; Turner, C.; Bashir, S.; Wiltshire, M.; Hancock, V.; Smith, K.; Cardigan, R. Platelet apoptosis and activation in platelet concentrates stored for up to 12 days in plasma or additive solution. Transfus. Med. 2010, 20, 392–402. [Google Scholar] [CrossRef]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef] [PubMed]
- Nassa, G.; Giurato, G.; Cimmino, G.; Rizzo, F.; Ravo, M.; Salvati, A.; Nyman, T.A.; Zhu, Y.; Vesterlund, M.; Lehtio, J.; et al. Splicing of platelet resident pre-mRNAs upon activation by physiological stimuli results in functionally relevant proteome modifications. Sci. Rep. 2018, 8, 498. [Google Scholar] [CrossRef] [PubMed]



| Lipedema (n = 8) | Lymphedema (n = 8) | Obesity I (n = 4) | Obesity II (n = 4) | |
|---|---|---|---|---|
| Age, y; mean (SD) | 57.1 (9.7) | 50 (21) | 53.3 (5.3) | 51.3 (14.9) |
| Female gender, n (%) | 8 (100) | 8 (100) a | 4 (100) b | 4 (100) c |
| BMI, kg/m2; mean (SD) | 37.9 (6.2) | 34.1 d (5) | 30.9 e (5.3) | 37.7 f (4.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalise, A.; Aggarwal, A.; Sangwan, N.; Hamer, A.; Guntupalli, S.; Park, H.E.; Aleman, J.O.; Cameron, S.J. A Divergent Platelet Transcriptome in Patients with Lipedema and Lymphedema. Genes 2024, 15, 737. https://doi.org/10.3390/genes15060737
Scalise A, Aggarwal A, Sangwan N, Hamer A, Guntupalli S, Park HE, Aleman JO, Cameron SJ. A Divergent Platelet Transcriptome in Patients with Lipedema and Lymphedema. Genes. 2024; 15(6):737. https://doi.org/10.3390/genes15060737
Chicago/Turabian StyleScalise, Alliefair, Anu Aggarwal, Naseer Sangwan, Annelise Hamer, Suman Guntupalli, Huijun Edelyn Park, Jose O. Aleman, and Scott J. Cameron. 2024. "A Divergent Platelet Transcriptome in Patients with Lipedema and Lymphedema" Genes 15, no. 6: 737. https://doi.org/10.3390/genes15060737





