Utilization of qPCR to Determine Duration and Environmental Drivers Contributing to the Persistence of Human DNA in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Donors, Sampling Location, and Sampling Collection
2.2. Weather and Environmental Data Collection
2.3. DNA Extraction and Quantitation
2.4. Statistical Analysis
3. Results
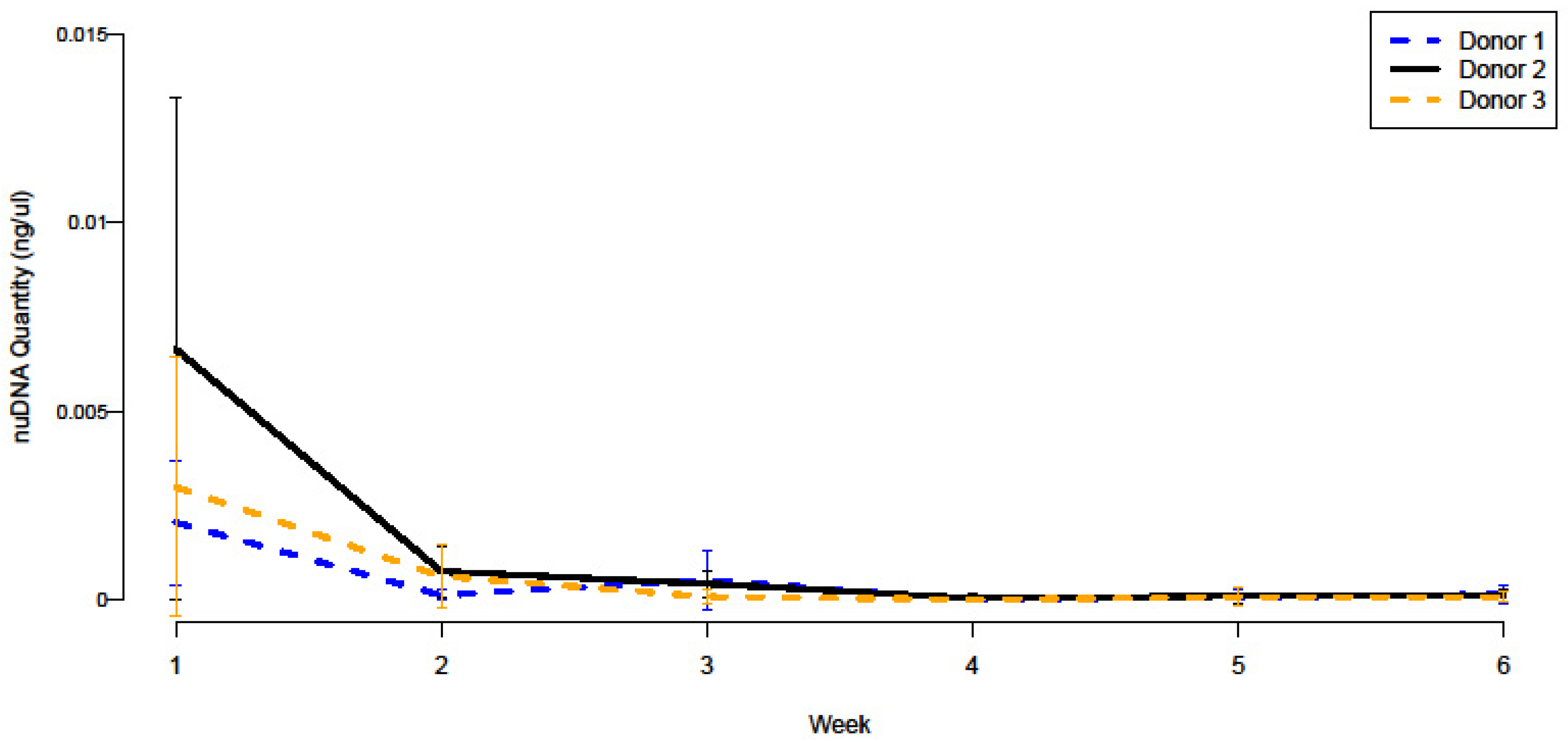
3.1. NuDNA Quantitation and Persistence
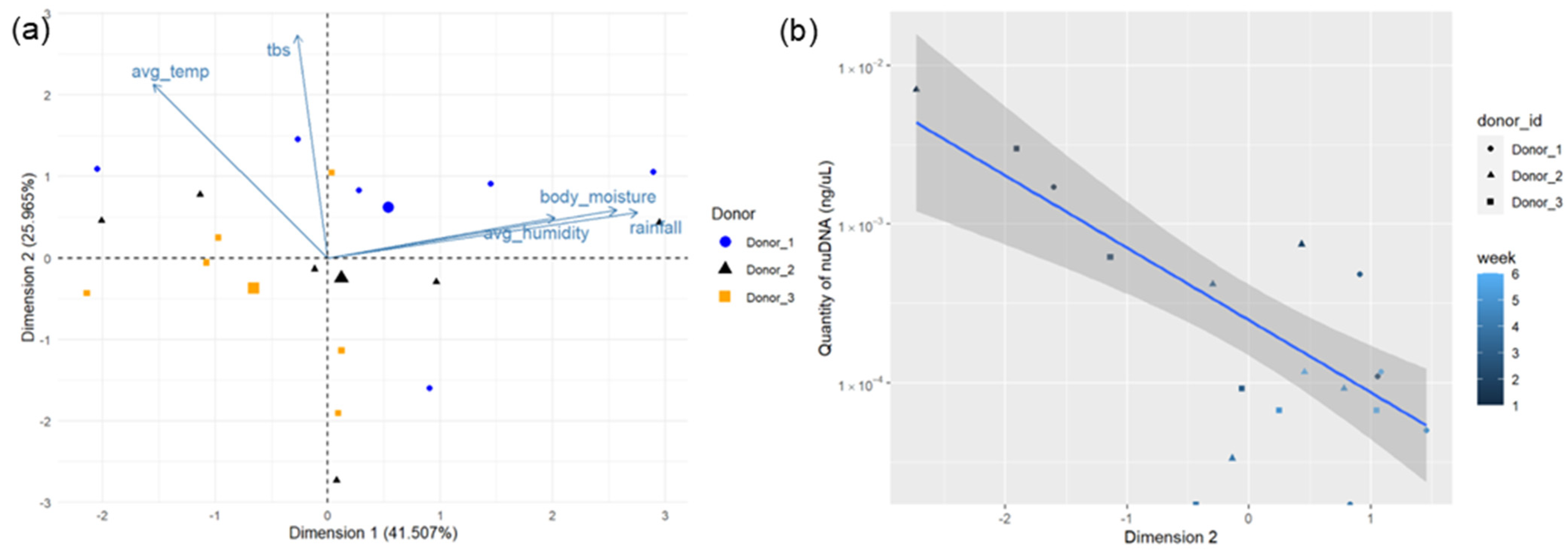
3.2. NuDNA and Environmental Drivers
3.3. MtDNA Quantitation and Persistence
3.4. MtDNA and Environmental Drivers
4. Discussion
4.1. Recovery of DNA from Soil
4.2. Effects of Environmental Variables on DNA Persistence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forbes, S. Decomposition chemistry in a burial environment. In Soil Analysis in Forensic Taphonomy: Chemical and Biological Effects of Buried Human Remains, 1st ed.; Tibbett, M., Carter, D., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 203–217. [Google Scholar]
- Levy-Booth, D.J.; Campbell, R.G.; Gulden, R.H.; Hart, M.M.; Powell, J.R.; Klironomos, J.N.; Pauls, K.P.; Swanton, C.J.; Trevors, J.T.; Dunfield, K.E. Cycling of extracellular DNA in the soil environment. Soil Biol. Biochem. 2007, 39, 2977–2991. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Johnsen, P.J.; Bensasson, D.; Daffonchio, D. Release and persistence of extracellular DNA in the environment. Environ. Biosafety Res. 2007, 6, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef]
- Morley, M.W.; Goldberg, P.; Uliyanov, V.A.; Kozlikin, M.B.; Shunkov, M.V.; Derevianko, A.P.; Jacobs, Z.; Roberts, R.G. Hominin and animal activities in the microstratigraphic record from Denisova Cave (Altai Mountains, Russia). Sci. Rep. 2019, 9, 13785. [Google Scholar] [CrossRef]
- Massilani, D.; Morley, M.W.; Mentzer, S.M.; Aldeias, V.; Vernot, B.; Miller, C.; Stahlschmidt, M.; Kozlikin, M.B.; Shunkov, M.V.; Derevianko, A.P.; et al. Microstratigraphic preservation of ancient faunal and hominin DNA in Pleistocene cave sediments. Proc. Natl. Acad. Sci. USA 2022, 119, e2113666118. [Google Scholar] [CrossRef]
- Slon, V.; Hopfe, C.; Weiß, C.L.; Mafessoni, F.; de la Rasilla, M.; Lalueza-Fox, C.; Rosas, A.; Soressi, M.; Knul, M.V.; Miller, R.; et al. Neandertal and Denisovan DNA from Pleistocene sediments. Science 2017, 356, 605–608. [Google Scholar] [CrossRef]
- Vernot, B.; Zavala, E.I.; Gómez-Olivencia, A.; Jacobs, Z.; Slon, V.; Mafessoni, F.; Romagné, F.; Pearson, A.; Petr, M.; Sala, N.; et al. Unearthing Neanderthal population history using nuclear and mitochondrial DNA from cave sediments. Science 2021, 372, eabf1667. [Google Scholar] [CrossRef]
- Emmons, A.L.; DeBruyn, J.M.; Mundorff, A.Z.; Cobaugh, K.L.; Cabana, G.S. The persistence of human DNA in soil following surface decomposition. Sci. Justice 2017, 57, 341–348. [Google Scholar] [CrossRef]
- Crecchio, C.; Stotzky, G. Binding of DNA on humic acids: Effect on transformation of Bacillus Subtilis and resistance to DNase. Soil Biol. Biochem. 1998, 30, 1061–1067. [Google Scholar] [CrossRef]
- Ladd, J.N.; Foster, R.C.; Nannipieri, P.; Oades, J.M. Soil structure and biological activity. In Soil Biochemistry; Stotzky, G., Bollag, J.M., Eds.; Marcel Dekker: New York City, NY, USA, 1996; Volume 9, pp. 23–79. [Google Scholar]
- Pathan, S.I.; Arfaioli, P.; Ceccherini, M.T.; Ascher-Jenull, J.; Nannipieri, P.; Pietramellara, G.; D’Acqui, L.P. Physical protection of extracellular and intracellular DNA in soil aggregates against simulated natural oxidative processes. Appl. Soil Ecol. 2021, 165, 104002. [Google Scholar] [CrossRef]
- Giles, S.B.; Harrison, K.; Errickson, D.; Márquez-Grant, N. The effect of seasonality on the application of accumulated degree-days to estimate the early post-mortem interval. Forensic Sci. Int. 2020, 315, 110419. [Google Scholar] [CrossRef]
- Carter, D.O.; Metcalf, J.L.; Bibat, A.; Knight, R. Seasonal variation of postmortem microbial communities. Forensic Sci. Med. Pathol. 2015, 11, 202–207. [Google Scholar] [CrossRef]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Moisture can be the dominant environmental parameter governing cadaver decomposition in soil. Forensic Sci. Int. 2010, 200, 60–66. [Google Scholar] [CrossRef]
- Erkol, Z.; Hösükler, E. Postmortem animal attacks on human corpses. In Post Mortem Examination and Autopsy—Current Issues from Death to Laboratory Analysis; Dogan, K.H., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Megyesi, M.S.; Nawrocki, S.P.; Haskell, N.H. Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. J. Forensic Sci. 2005, 50, 618–626. [Google Scholar] [CrossRef]
- Applied Biosystems. User Guide: Quantifiler HP and Trio DNA Quantification Kits; Publication No. 4485354 Rev. H; Applied Biosystems: Waltham, MA, USA, 2018. [Google Scholar]
- Kavlick, M.F. Development of a triplex mtDNA qPCR assay to assess quantification, degradation, inhibition, and amplification target copy numbers. Mitochondrion 2019, 46, 41–50. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 29 May 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R package version 1.1.3. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 29 May 2024).
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 29 May 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 29 May 2024).
- Zhu, H. kableExtra: Construct Complex Table with ‘kable’ and Pipe Syntax. R Package Version 1.4.0. 2024. Available online: https://CRAN.R-project.org/package=kableExtra (accessed on 29 May 2024).
- Maussion, G.; Thomas, R.A.; Demirova, I.; Gu, G.; Cai, E.; Chen, C.X.-Q.; Abdian, N.; Strauss, T.J.P.; Kelaï, S.; Nauleau-Javaudin, A.; et al. Auto-qPCR; a Python-based web app for automated and reproducible analysis of qPCR data. Sci. Rep. 2021, 11, 21293. [Google Scholar] [CrossRef]
- Sutherland, A.; Myburgh, J.; Steyn, M.; Becker, P.J. The effect of body size on the rate of decomposition in a temperate region of South Africa. Forensic Sci. Int. 2013, 231, 257–262. [Google Scholar] [CrossRef]
- Mann, R.W.; Bass, W.M.; Meadows, L. Time since death and decomposition of the human body: Variables and observations in case and experimental field studies. J. Forensic Sci. 1990, 31, 103–111. [Google Scholar] [CrossRef]
- Carter, D.O.; Tibbett, M. Cadaver decomposition and soil: Processes. In Soil Analysis in Forensic Taphonomy: Chemical and Biological Effects of Buried Human Remains, 1st ed.; Tibbett, M., Carter, D., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 29–45. [Google Scholar]
- Tumer, A.R.; Karacaoglu, E.; Namli, A.; Keten, A.; Farasat, S.; Akcan, R.; Sert, O.; Odabaşi, A.B. Effects of different types of soil on decomposition: An experimental study. Leg. Med. 2013, 15, 149–156. [Google Scholar] [CrossRef]


| Donor | Week | Small Target (ng/µL) | Large Target (ng/µL) | Y Target (ng/µL) | Degradation Index |
|---|---|---|---|---|---|
| 1 | 1 | 1.71 × 10−3 | 3.27 × 10−4 | 0 | 5.22 |
| 2 | 1.09 × 10−4 | 9.09 × 10−6 | 0 | 12.00 | |
| 3 | 4.82 × 10−4 | 0 | 0 | * | |
| 4 | 0 | 3.33 × 10−5 | 0 | 0.00 | |
| 5 | 5.00 × 10−5 | 0 | 0 | * | |
| 6 | 1.17 × 10−4 | 5.00 × 10−5 | 0 | 2.33 | |
| 2 | 1 | 7.07 × 10−3 | 3.74 × 10−3 | 6.91 × 10−3 | 1.89 |
| 2 | 7.45 × 10−4 | 1.18 × 10−4 | 3.27 × 10−4 | 6.31 | |
| 3 | 4.17 × 10−4 | 0 | 0 | * | |
| 4 | 3.33 × 10−5 | 8.33 × 10−5 | 0 | 0.40 | |
| 5 | 9.17 × 10−5 | 0 | 0 | * | |
| 6 | 1.17 × 10−4 | 1.67 × 10−5 | 0 | 7.00 | |
| 3 | 1 | 2.98 × 10−3 | 9.00 × 10−4 | 1.18 × 10−3 | 3.31 |
| 2 | 6.18 × 10−4 | 2.36 × 10−4 | 1.67 × 10−5 | 2.62 | |
| 3 | 1.42 × 10−4 | 2.50 × 10−5 | 0 | 5.67 | |
| 4 | 0 | 0 | 0 | * | |
| 5 | 6.67 × 10−5 | 0 | 0 | * | |
| 6 | 6.67 × 10−5 | 8.33 × 10−6 | 0 | 8.00 |
| Relationship | Spearman’s ρ | T-Statistic | DF | p-Value |
|---|---|---|---|---|
| Week into Decomposition Period | −0.6594 | 3.5082 | 16 | 0.0029 * |
| TBS | −0.4977 | 2.2951 | 16 | 0.0356 * |
| Weekly Body Moisture Content | 0.2668 | 1.1074 | 16 | 0.2845 |
| Total Weekly Rainfall | 0.1729 | 0.7023 | 16 | 0.4926 |
| Weekly Average Humidity | −0.0167 | 0.0667 | 16 | 0.9477 |
| Weekly Average Temperature | −0.6184 | 3.1479 | 16 | 0.0062 * |
| Sample | Calibrator | |||||
|---|---|---|---|---|---|---|
| Donor | Week | Average Ct (105 bp) | Average Ct (316 bp) | Average Ct (105 bp) | Average Ct (316 bp) | ΔΔCt (Degradation) |
| 1 | 1 | 27.93 | 35.10 | 24.66 | 30.10 | 1.73 |
| 2 | 31.10 | 33.24 | 25.88 | 26.98 | 1.04 | |
| 3 | 27.37 | 30.99 | 24.15 | 30.27 | −2.50 | |
| 4 | 31.75 | 33.51 | 25.32 | 26.52 | 0.56 | |
| 5 | 29.56 | 34.53 | 24.55 | 26.56 | 2.96 | |
| 6 | 30.62 | 33.25 | 25.73 | 26.98 | 1.38 | |
| 7 | 30.37 | 37.31 | 24.30 | 26.93 | 4.31 | |
| 8 | 29.81 | 39.02 | 21.03 | 26.80 | 3.44 | |
| 9 | 32.22 | 38.16 | 25.03 | 26.88 | 4.09 | |
| 10 | 33.94 | 38.72 | 26.31 | 28.12 | 2.97 | |
| 11 | 33.86 | Undetermined | 26.31 | 28.12 | * | |
| 2 | 1 | 24.82 | 30.81 | 24.66 | 30.10 | 0.55 |
| 2 | 27.12 | 28.66 | 25.88 | 26.98 | 0.44 | |
| 3 | 22.55 | 26.56 | 24.15 | 30.27 | −2.11 | |
| 4 | 29.85 | 31.70 | 25.32 | 26.52 | 0.65 | |
| 5 | 29.62 | 33.89 | 24.55 | 26.56 | 2.26 | |
| 6 | 29.19 | 35.07 | 25.73 | 26.98 | 4.63 | |
| 7 | 29.50 | 35.68 | 24.30 | 26.93 | 3.55 | |
| 8 | 29.29 | 37.36 | 21.03 | 26.80 | 2.30 | |
| 9 | 30.98 | 35.16 | 25.03 | 26.88 | 2.33 | |
| 10 | 31.02 | 34.35 | 26.31 | 28.12 | 1.52 | |
| 11 | 31.18 | 37.70 | 26.31 | 28.12 | 4.71 | |
| 3 | 1 | 25.70 | 32.93 | 24.66 | 30.10 | 1.79 |
| 2 | 31.15 | 34.13 | 25.88 | 26.98 | 1.88 | |
| 3 | 30.15 | 37.02 | 24.15 | 30.27 | 0.75 | |
| 4 | 33.22 | 36.24 | 25.32 | 26.52 | 1.82 | |
| 5 | 29.66 | 35.04 | 24.55 | 26.56 | 3.37 | |
| 6 | 29.75 | 35.15 | 25.73 | 26.98 | 4.15 | |
| 7 | 29.13 | 36.80 | 24.30 | 26.93 | 5.04 | |
| 8 | 27.93 | 36.82 | 21.03 | 26.80 | 3.12 | |
| 9 | 30.35 | 34.46 | 25.03 | 26.88 | 2.26 | |
| 10 | 31.78 | 35.16 | 26.31 | 28.12 | 1.57 | |
| 11 | 30.74 | 35.43 | 26.31 | 28.12 | 2.88 | |
| Relationship | Spearman’s ρ | T-Statistic | DF | p-Value |
|---|---|---|---|---|
| Week into Decomposition Period | −0.6219 | 4.422 | 31 | 0.0001 * |
| TBS | −0.4687 | 2.9539 | 31 | 0.0059 * |
| Weekly Body Moisture Content | 0.3274 | 1.9290 | 31 | 0.0629 |
| Total Weekly Rainfall | 0.1275 | 0.7160 | 31 | 0.4794 |
| Weekly Average Humidity | −0.3439 | 2.0391 | 31 | 0.0500 * |
| Weekly Average Temperature | −0.7200 | 5.7759 | 31 | 2.3245 × 10−6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noel, H.L.; George, R.L.; Bintz, B.; Hickman, M.P.; West, F. Utilization of qPCR to Determine Duration and Environmental Drivers Contributing to the Persistence of Human DNA in Soil. Genes 2024, 15, 741. https://doi.org/10.3390/genes15060741
Noel HL, George RL, Bintz B, Hickman MP, West F. Utilization of qPCR to Determine Duration and Environmental Drivers Contributing to the Persistence of Human DNA in Soil. Genes. 2024; 15(6):741. https://doi.org/10.3390/genes15060741
Chicago/Turabian StyleNoel, Hannah L., Rebecca L. George, Brittania Bintz, Maureen Peters Hickman, and Frankie West. 2024. "Utilization of qPCR to Determine Duration and Environmental Drivers Contributing to the Persistence of Human DNA in Soil" Genes 15, no. 6: 741. https://doi.org/10.3390/genes15060741
APA StyleNoel, H. L., George, R. L., Bintz, B., Hickman, M. P., & West, F. (2024). Utilization of qPCR to Determine Duration and Environmental Drivers Contributing to the Persistence of Human DNA in Soil. Genes, 15(6), 741. https://doi.org/10.3390/genes15060741






