Fine Mapping of QTLs for Alkaline Tolerance in Crucian Carp (Carassius auratus) Using Genome-Wide SNP Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mapping Family and Phenotypic Measurement
2.2. DNA Extraction
2.3. Library Construction and Sequencing
2.4. SNP Extraction
2.5. Linkage Map Construction
2.6. QTL Mapping
2.7. Candidate Genes Analysis
2.8. Statistical Analysis
3. Results
3.1. Summary of Sequencing Data
3.2. Phenotypic Data
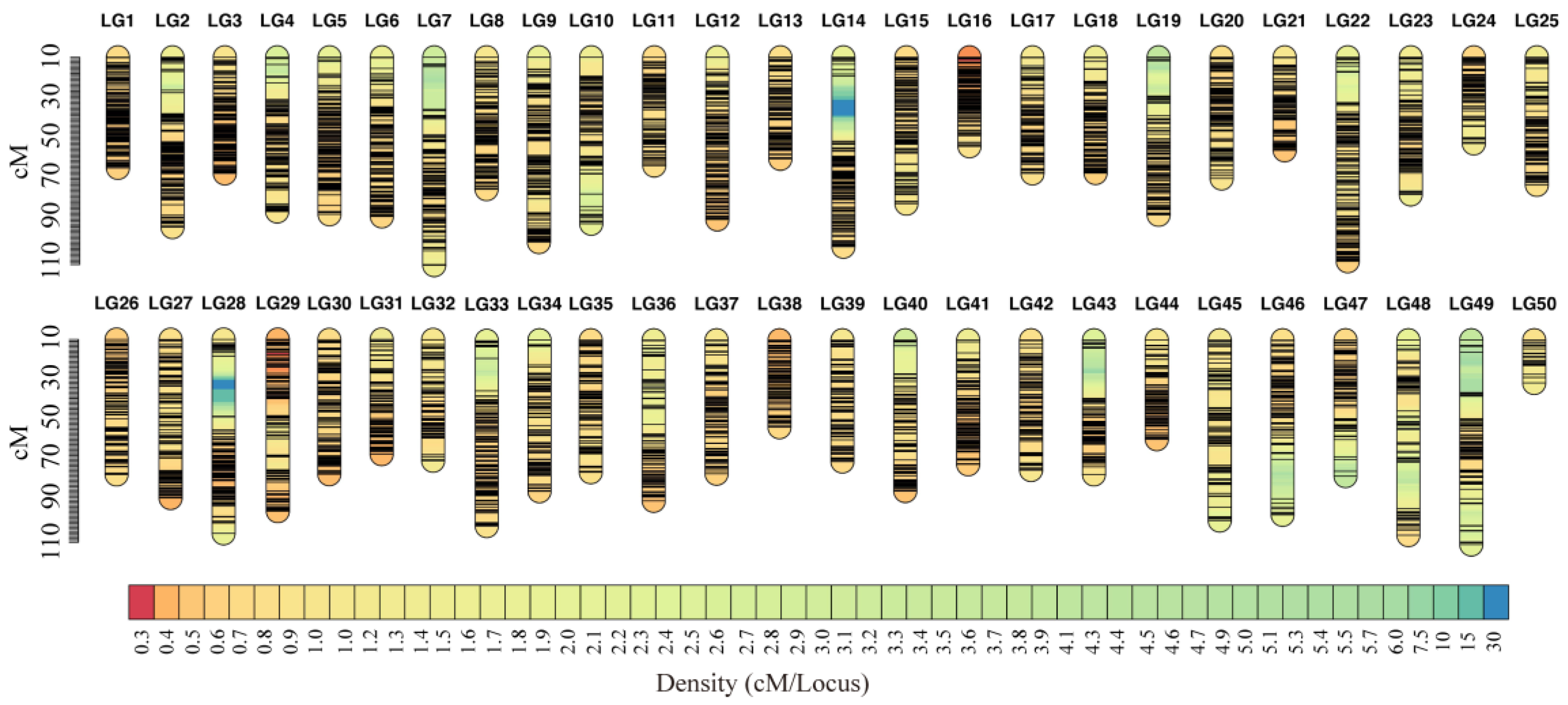
3.3. Linkage Map Construction
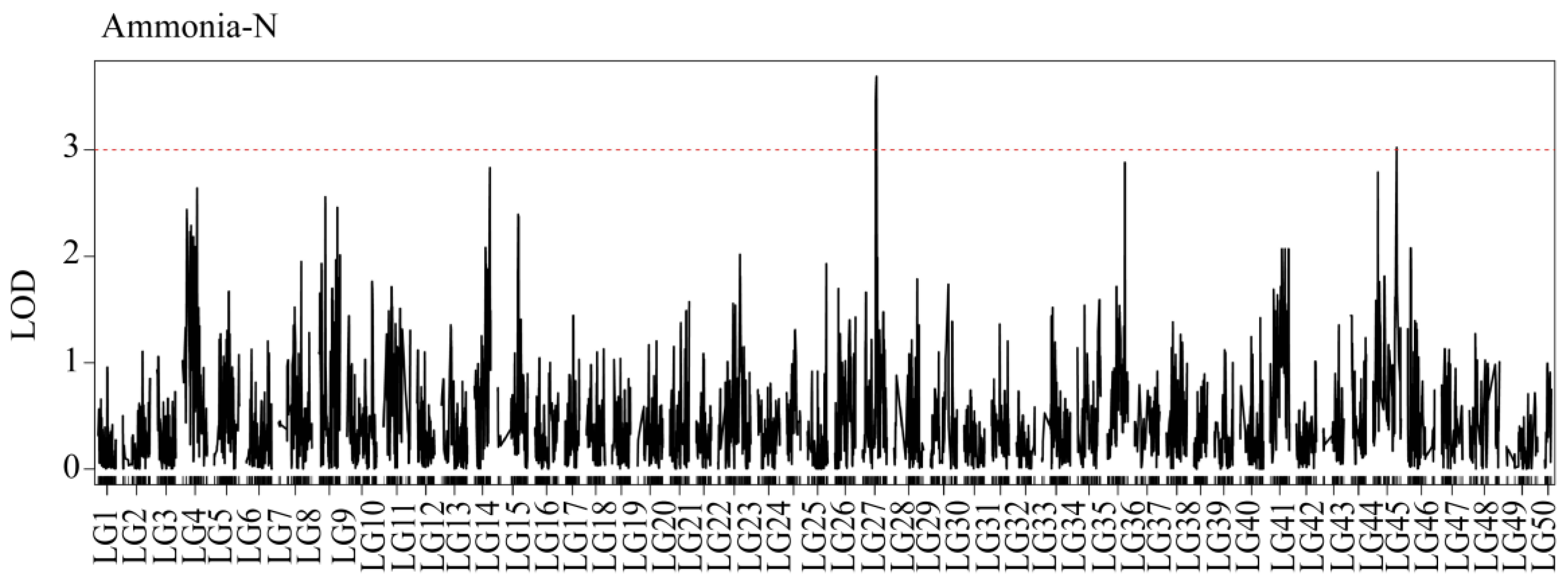
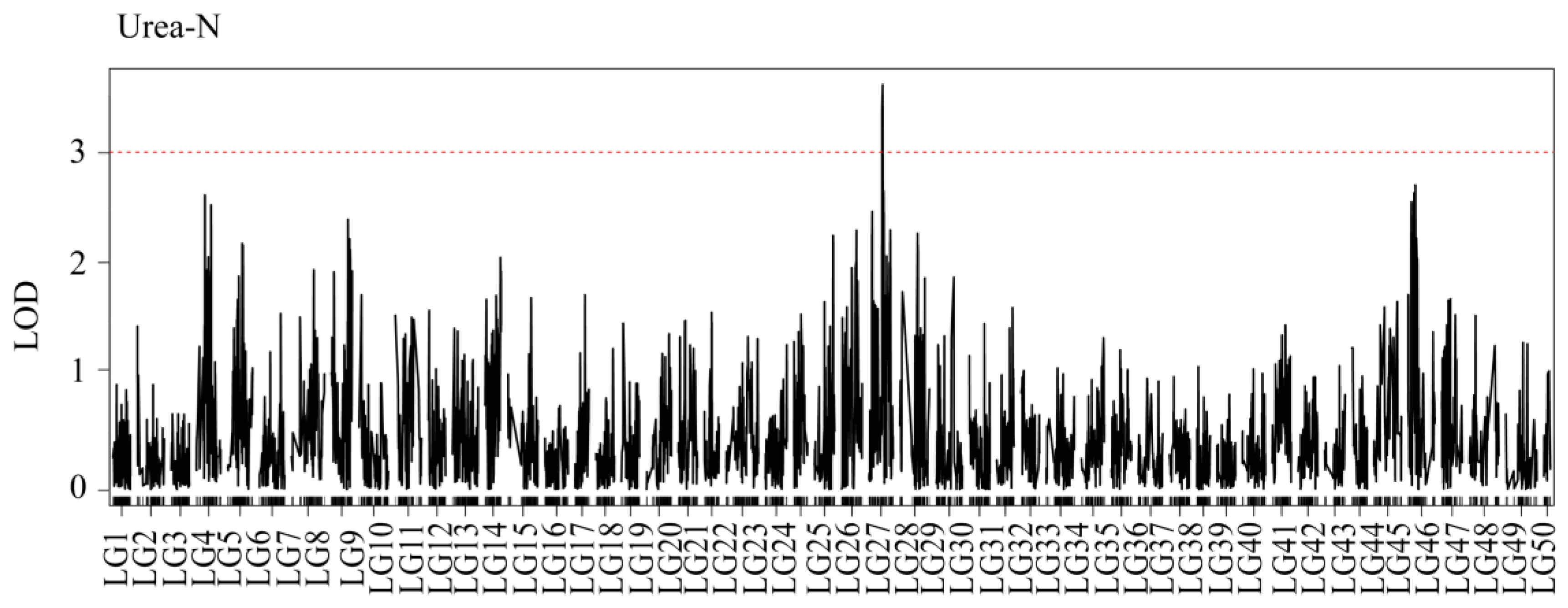
3.4. QTL Mapping
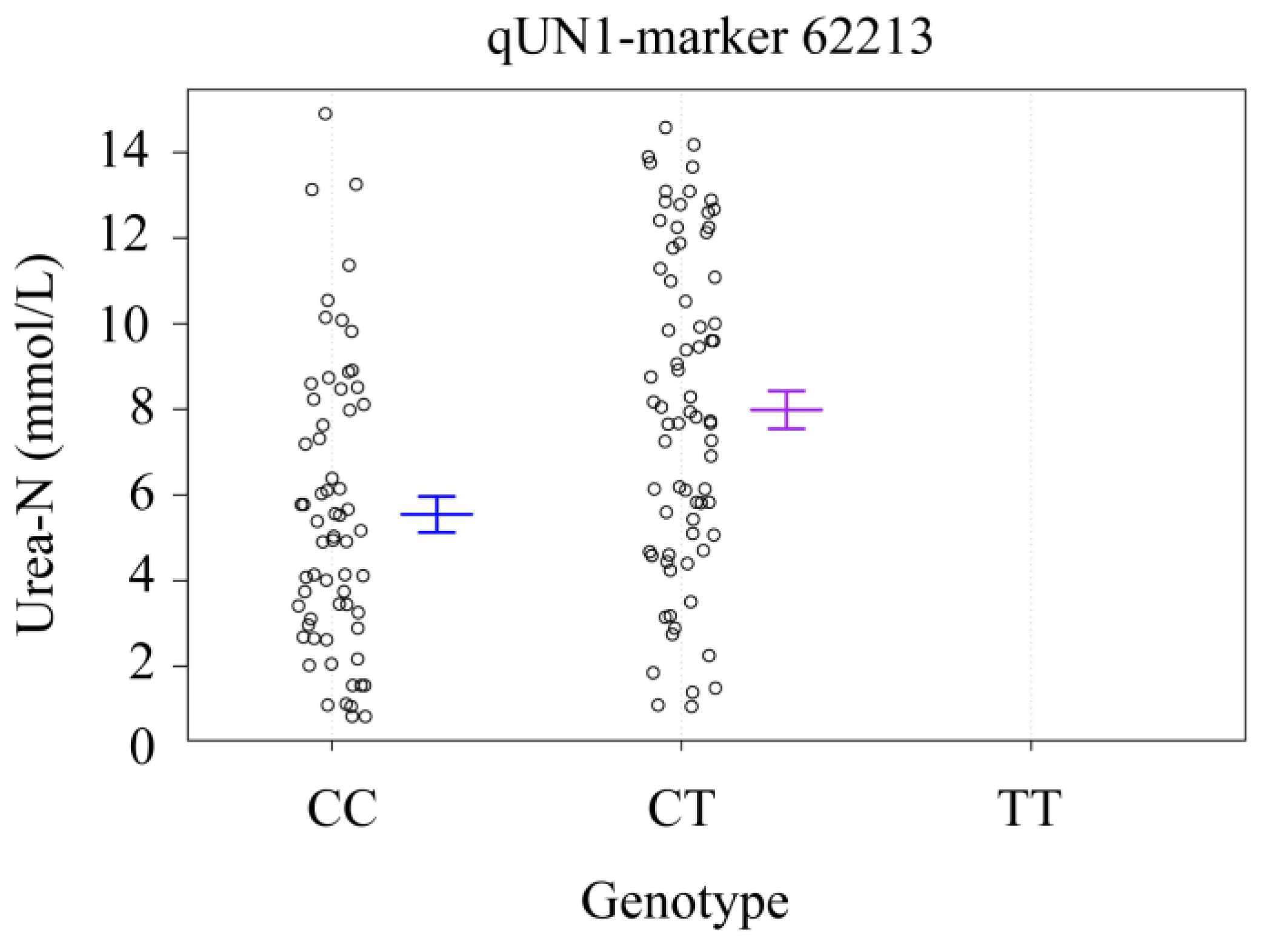
3.5. The Genotype Effect of QTL Peak
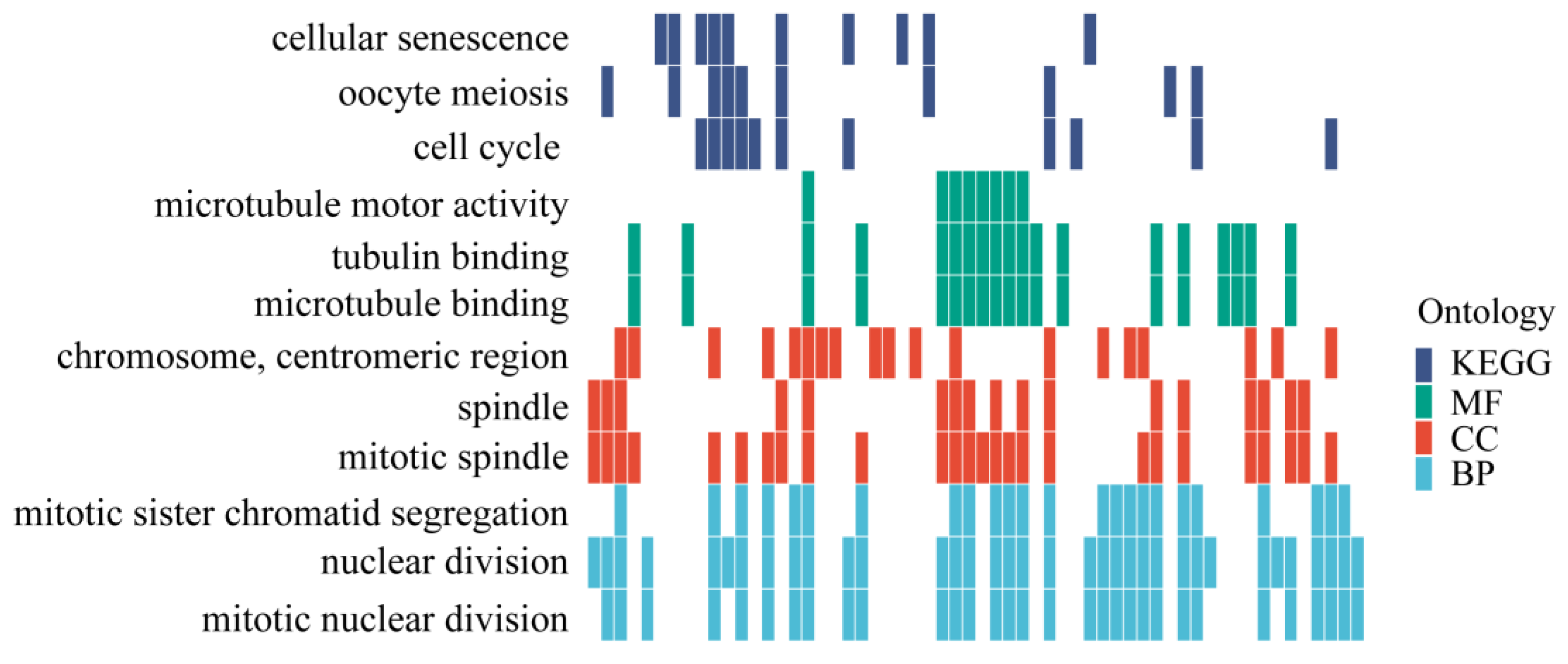
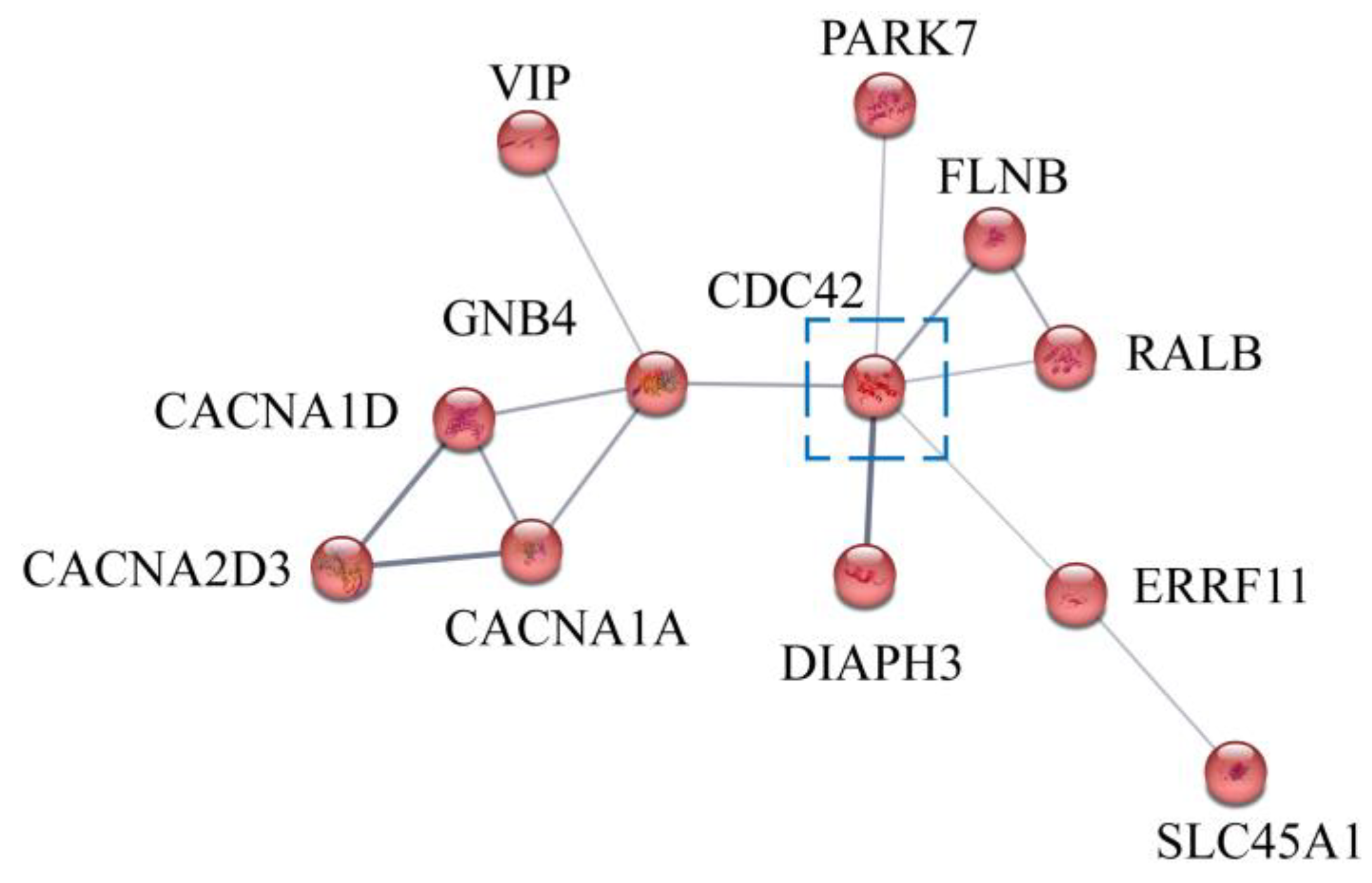
3.6. Candidate Genes Associated with Alkali-Tolerant Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef]
- Zhang, X.; Luan, P.; Cao, D.; Hu, G. A High-Density Genetic Linkage Map and Fine Mapping of QTL For Feed Conversion Efficiency in Common Carp (Cyprinus carpio). Front. Genet. 2021, 12, 778487. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, J.; Lv, H.; Zhou, T.; Zhao, J.; Bai, H.; Pu, F.; Xu, P. The adaptive evolution of Leuciscus waleckii in Lake Dali Nur and convergent evolution of Cypriniformes fishes inhabiting extremely alkaline environments. Genome Biol. Evol. 2023, 15, evad082. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.T.; Jiang, Y.; Peng, W.; Yao, Z.; Chen, B.; Jiang, L.; Feng, J.; Ji, P.; Liu, G.; et al. Genomic Basis of Adaptive Evolution: The Survival of Amur Ide (Leuciscus waleckii) in an Extremely Alkaline Environment. Mol. Biol. Evol. 2017, 34, 145–159. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F. Ammonia production, excretion, toxicity, and defense in fish: A review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, C.H.C.; Zhang, J.; Cao, L.; Chen, L.; Zhou, L.; Jin, Y.; Ye, H.; Deng, C.; Dai, Z.; et al. Transcriptomic and genomic evolution under constant cold in Antarctic notothenioid fish. Proc. Natl. Acad. Sci. USA 2008, 105, 12944–12949. [Google Scholar] [CrossRef]
- Wilkie, M.P. Ammonia excretion and urea handling by fish gills: Present understanding and future research challenges. J. Exp. Zool. 2002, 293, 284–301. [Google Scholar] [CrossRef]
- Nakada, T.; Hoshijima, K.; Esaki, M.; Nagayoshi, S.; Kawakami, K.; Hirose, S. Localization of ammonia transporter Rhcg1 in mitochondrion-rich cells of yolk sac, gill, and kidney of zebrafish and its ionic strength-dependent expression. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, R1743–R1753. [Google Scholar] [CrossRef]
- Randall, D.J.; Wood, C.M.; Perry, S.F.; Bergman, H.L.; Maloiy, G.M.O.; Mommsen, T.P.; Wright, P.A. Urea excretion as a strategy for survival in a fish living in a very alkaline environment. Nature 1989, 337, 165–166. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, P.J. High ammonia tolerance in fishes of the family Batrachoididae (Toadfish and Midshipmen). Aquat. Toxicol. 2000, 50, 205–219. [Google Scholar] [CrossRef]
- Sashaw, J.; Nawata, M.; Thompson, S.; Wood, C.M.; Wright, P.A. Rhesus glycoprotein and urea transporter genes in rainbow trout embryos are upregulated in response to alkaline water (pH 9.7) but not elevated water ammonia. Aquat. Toxicol. 2010, 96, 308–313. [Google Scholar] [CrossRef]
- Tong, C.; Fei, T.; Zhang, C.; Zhao, K. Comprehensive transcriptomic analysis of Tibetan Schizothoracinae fish Gymnocypris przewalskii reveals how it adapts to a high altitude aquatic life. BMC Evol. Biol. 2017, 17, 74. [Google Scholar] [CrossRef]
- Wang, Y.S.; Gonzalez, R.J.; Patrick, M.L.; Grosell, M.; Zhang, C.; Feng, Q.; Du, J.; Walsh, P.J.; Wood, C.M. Unusual physiology of scale-less carp, Gymnocypris przewalskii, in Lake Qinghai: A high altitude alkaline saline lake. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 134, 409–421. [Google Scholar] [CrossRef]
- Solier, S.; Muller, S.; Rodriguez, R. Whole-genome mapping of small-molecule targets for cancer medicine. Curr. Opin. Chem. Biol. 2020, 56, 42–50. [Google Scholar] [CrossRef]
- Ashton, D.T.; Ritchie, P.A.; Wellenreuther, M. Fifteen years of quantitative trait loci studies in fish: Challenges and future directions. Mol. Ecol. 2017, 26, 1465–1476. [Google Scholar] [CrossRef]
- Su, S.; Raouf, B.; He, X.; Cai, N.; Li, X.; Yu, J.; Li, J.; Yu, F.; Wang, M.; Tang, Y. Genome Wide Analysis for Growth at Two Growth Stages in A New Fast-Growing Common Carp Strain (Cyprinus carpio L.). Sci. Rep. 2020, 10, 7259. [Google Scholar] [CrossRef]
- Zheng, X.; Kuang, Y.; Lv, W.; Cao, D.; Sun, Z.; Sun, X. Genome-Wide Association Study for Muscle Fat Content and Abdominal Fat Traits in Common Carp (Cyprinus carpio). PLoS ONE 2016, 11, e169127. [Google Scholar] [CrossRef]
- Peng, W.; Xu, J.; Zhang, Y.; Feng, J.; Dong, C.; Jiang, L.; Feng, J.; Chen, B.; Gong, Y.; Chen, L.; et al. An ultra-high density linkage map and QTL mapping for sex and growth-related traits of common carp (Cyprinus carpio). Sci. Rep. 2016, 6, 26693. [Google Scholar] [CrossRef]
- Baranski, M.; Moen, T.; Vage, D.I. Mapping of quantitative trait loci for flesh colour and growth traits in Atlantic salmon (Salmo salar). Genet. Sel. Evol. 2010, 42, 17. [Google Scholar] [CrossRef]
- Evans, D.H. A brief history of the study of fish osmoregulation: The central role of the Mt. Desert Island Biological Laboratory. Front. Physiol. 2010, 1, 13. [Google Scholar] [CrossRef]
- Whittamore, J.M. Osmoregulation and epithelial water transport: Lessons from the intestine of marine teleost fish. J. Comp. Physiol. B 2012, 182, 1–39. [Google Scholar]
- Zhao, X.F.; Huang, J.; Li, W.; Wang, S.Y.; Liang, L.Q.; Zhang, L.M.; Liew, H.J.; Chang, Y.M. Rh proteins and H+ transporters involved in ammonia excretion in Amur Ide (Leuciscus waleckii) under high alkali exposure. Ecotoxicol. Environ. Saf. 2024, 273, 116160. [Google Scholar] [CrossRef]
- Li, T.; Chen, L.; Wang, M.; Huang, X.; Chen, L.; Duan, G.; Chen, Y.; Gong, L.; Yu, K. First Detection of Atractylodes Mild Mottle Virus in Atractylodes lancea (Thunb.) DC. in Hubei Province of China. Plant Dis. 2024. [Google Scholar] [CrossRef]
- Gaston, J.M.; Alm, E.J.; Zhang, A.N. Fast and accurate variant identification tool for sequencing-based studies. BMC Biol. 2024, 22, 90. [Google Scholar] [CrossRef]
- Rastas, P. Lep-MAP3: Robust linkage mapping even for low-coverage whole genome sequencing data. Bioinformatics 2017, 33, 3726–3732. [Google Scholar] [CrossRef]
- Broman, K.W.; Gatti, D.M.; Simecek, P.; Furlotte, N.A.; Prins, P.; Sen, Ś.; Yandell, B.S.; Churchill, G.A. R/qtl2: Software for Mapping Quantitative Trait Loci with High-Dimensional Data and Multiparent Populations. Genetics 2019, 211, 495–502. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, T.; Liang, Z.; Chen, X.; Xu, Y.N.; Zhang, T.; Zhou, D. Prednisone combined with Dihydroartemisinin attenuates systemic lupus erythematosus by regulating M1/M2 balance through the MAPK signaling pathway. Mol. Immunol. 2024, 170, 144–155. [Google Scholar] [CrossRef]
- Mosier, S.; Apfelbaum, S.; Byck, P.; Calderon, F.; Teague, R.; Thompson, R.; Cotrufo, M.F. Adaptive multi-paddock grazing enhances soil carbon and nitrogen stocks and stabilization through mineral association in southeastern U.S. grazing lands. J. Environ. Manag. 2021, 288, 112409. [Google Scholar] [CrossRef]
- Lin, T.; Lai, Q.; Yao, Z.; Lu, J.; Zhou, K.; Wang, H. Combined effects of carbonate alkalinity and pH on survival, growth and haemocyte parameters of the Venus clam Cyclina sinensis. Fish Shellfish. Immunol. 2013, 35, 525–531. [Google Scholar] [CrossRef]
- Fanzo, J.; de Steenhuijsen Piters, B.; Soto-Caro, A.; Saint Ville, A.; Mainuddin, M.; Battersby, J. Global and local perspectives on food security and food systems. Commun. Earth Environ. 2024, 5, 227. [Google Scholar] [CrossRef]
- Schneider, K.R.; Fanzo, J.; Haddad, L.; Herrero, M.; Moncayo, J.R.; Herforth, A.; Remans, R.; Guarin, A.; Resnick, D.; Covic, N.; et al. The state of food systems worldwide in the countdown to 2030. Nat. Food 2023, 4, 1090–1110. [Google Scholar] [CrossRef]
- Hill, E.F.; Henny, C.J.; Grove, R.A. Mercury and drought along the lower Carson River, Nevada: II. Snowy egret and black-crowned night-heron reproduction on Lahontan Reservoir, 1997–2006. Ecotoxicology 2008, 17, 117–131. [Google Scholar] [CrossRef]
- Hung, C.C.; Nawata, C.M.; Wood, C.M.; Wright, P.A. Rhesus glycoprotein and urea transporter genes are expressed in early stages of development of rainbow trout (Oncorhynchus mykiss). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2008, 309, 262–268. [Google Scholar] [CrossRef]
- Harris, R.J.; Jenner, R.A. Evolutionary Ecology of Fish Venom: Adaptations and Consequences of Evolving a Venom System. Toxins 2019, 11, 60. [Google Scholar] [CrossRef]
- Cowan, M.; Azpeleta, C.; Lopez-Olmeda, J.F. Rhythms in the endocrine system of fish: A review. J. Comp. Physiol. B 2017, 187, 1057–1089. [Google Scholar] [CrossRef]
- Heileson, J.L.; Machek, S.B.; Harris, D.R.; Tomek, S.; de Souza, L.C.; Kieffer, A.J.; Barringer, N.D.; Gallucci, A.; Forsse, J.S.; Funderburk, L.K. The effect of fish oil supplementation on resistance training-induced adaptations. J. Int. Soc. Sports Nutr. 2023, 20, 2174704. [Google Scholar] [CrossRef]
- Kwong, R.W.; Kumai, Y.; Perry, S.F. The physiology of fish at low pH: The zebrafish as a model system. J. Exp. Biol. 2014, 217 Pt 5, 651–662. [Google Scholar] [CrossRef]
- Chang, Y.M.; Gao, G.Q.; Xu, L.H.; Liu, J.L.; Liu, C.L.; Liang, L.Q. Correlation and location of EST markers with cold tolerance trait of common carp (Cyprinus carpio L.). Dongwuxue Yanjiu 2010, 31, 581–586. [Google Scholar]
- Zhang, Y.; Liang, L.Q.; Chang, Y.M.; Hou, N.; Lu, C.Y.; Sun, X.W. Mapping and genetic effect analysis of quantitative trait loci related to body size in common carp (Cyprinus carpio L.). Yi Chuan 2007, 29, 1243–1248. [Google Scholar] [CrossRef]
- Laghari, M.Y.; Lashari, P.; Zhang, X.; Xu, P.; Xin, B.; Zhang, Y.; Narejo, N.T.; Sun, X. Mapping quantitative trait loci (QTL) for body weight, length and condition factor traits in backcross (BC1) family of Common carp (Cyprinus carpio L.). Mol. Biol. Rep. 2014, 41, 721–731. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Yu, X.; Zhang, X.; Lu, C.; Sun, X.; Tong, J. Microsatellite-centromere mapping in common carp through half-tetrad analysis in diploid meiogynogenetic families. Chromosoma 2015, 124, 67–79. [Google Scholar] [CrossRef]
- Laghari, M.Y.; Lashari, P.; Zhang, X.; Xu, P.; Narejo, N.T.; Xin, B.; Zhang, Y.; Sun, X. QTL mapping for economically important traits of common carp (Cyprinus carpio L.). J. Appl. Genet. 2015, 56, 65–75. [Google Scholar] [CrossRef]
- Deslauriers, S.D. High-resolution imaging as a tool for identifying quantitative trait loci that regulate photomorphogenesis in Arabidopsis thaliana. AoB Plants 2021, 13, b63. [Google Scholar] [CrossRef]
- Baud, A.; Flint, J. Identifying genes for neurobehavioural traits in rodents: Progress and pitfalls. Dis. Model. Mech. 2017, 10, 373–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Wang, L.; Yang, M.; Hu, K.; Li, W.; Sun, X.; Xue, H.; Dong, Q.; Zhang, M.; et al. Identifying Quantitative Trait Loci and Candidate Genes Conferring Resistance to Soybean Mosaic Virus SC7 by Quantitative Trait Loci-Sequencing in Soybean. Front. Plant. Sci. 2022, 13, 843633. [Google Scholar] [CrossRef]
- Hu, H.; Meng, Y.; Wang, H.; Liu, H.; Chen, S. Identifying quantitative trait loci and determining closely related stalk traits for rind penetrometer resistance in a high-oil maize population. Theor. Appl. Genet. 2012, 124, 1439–1447. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Huang, J.; Xiao, Q.; Hayward, A.; Li, F.; Gong, Y.; Liu, Q.; Ma, M.; Fu, D. Mapping and Identifying Candidate Genes Enabling Cadmium Accumulation in Brassica napus Revealed by Combined BSA-Seq and RNA-Seq Analysis. Int. J. Mol. Sci. 2023, 24, 10163. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Qin, J.; Liu, Y.; Wang, S.; Wu, S.; Zhang, M.; Zhong, J.; Wang, J. GbyE: An integrated tool for genome widely association study and genome selection based on genetic by environmental interaction. BMC Genom. 2024, 25, 386. [Google Scholar] [CrossRef]
- Fogel, E.J.; Samouha, A.; Goel, S.; Maitra, R. Transcriptome Signature of Immune Cells Post Reovirus Treatment in KRAS Mutated Colorectal Cancer. Cancer Manag. Res. 2021, 13, 6743–6754. [Google Scholar] [CrossRef]
- Chen, K.; Kwon, S.H.; Henn, D.; Kuehlmann, B.A.; Tevlin, R.; Bonham, C.A.; Griffin, M.; Trotsyuk, A.A.; Borrelli, M.R.; Noishiki, C.; et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat. Commun. 2021, 12, 5256. [Google Scholar] [CrossRef]
- Li, Z.; Hannigan, M.; Mo, Z.; Liu, B.; Lu, W.; Wu, Y.; Smrcka, A.V.; Wu, G.; Li, L.; Liu, M.; et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 2003, 114, 215–227. [Google Scholar] [CrossRef]



| Trait | Minimum | Maximum | Mean ± SD | Coefficient of Variation |
|---|---|---|---|---|
| Ammonia-N | 0.827 | 14.903 | 6.856 ± 0.093 | 0.418 |
| Urea-N | 0.143 | 1.308 | 0.681 ± 0.166 | 0.547 |
| Trait | Linkage Group | QTL Name | Linkage Group Size (cM) | LOD | Var | Nearest Marker |
|---|---|---|---|---|---|---|
| Ammonia-N | LG27 | qAN1 | 48.9 | 3.63 | 1.14 | snp62213 |
| Ammonia-N | LG45 | qAN2 | 83 | 3.02 | 6.75 | snp42497 |
| Urea-N | LG27 | qUN1 | 48.9 | 3.52 | 1.88 | snp62213 |
| Term ID | Term Name | Genes in Term |
|---|---|---|
| HAS-896461 | G β γ signaling through CDC42 | CDC42 GNB4 |
| WP382 | MAPK signaling pathway | CDC42 FLNB |
| WP437 | EGF/EGF2R signaling pathway | CDC42 RALB ERRF11 |
| WP4223 | Ras signaling pathway | CDC42 RALB GNB4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Su, B.; Huang, J.; Zhang, L.; Chang, Y.; Hu, G. Fine Mapping of QTLs for Alkaline Tolerance in Crucian Carp (Carassius auratus) Using Genome-Wide SNP Markers. Genes 2024, 15, 751. https://doi.org/10.3390/genes15060751
Zhang L, Su B, Huang J, Zhang L, Chang Y, Hu G. Fine Mapping of QTLs for Alkaline Tolerance in Crucian Carp (Carassius auratus) Using Genome-Wide SNP Markers. Genes. 2024; 15(6):751. https://doi.org/10.3390/genes15060751
Chicago/Turabian StyleZhang, Liang, Baofeng Su, Jing Huang, Limin Zhang, Yumei Chang, and Guo Hu. 2024. "Fine Mapping of QTLs for Alkaline Tolerance in Crucian Carp (Carassius auratus) Using Genome-Wide SNP Markers" Genes 15, no. 6: 751. https://doi.org/10.3390/genes15060751





