Rare Pathogenic Variants in Pooled Whole-Exome Sequencing Data Suggest Hyperammonemia as a Possible Cause of Dementia Not Classified as Alzheimer’s Disease or Frontotemporal Dementia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Langa, K.M. Cognitive aging, dementia, and the future of an aging population. In Future Directions for the Demography of Aging: Proceedings of a Workshop; National Academies Press (US): Washington, DC, USA, 2018; pp. 249–268. [Google Scholar]
- Kamboh, M.I. Genomics and Functional Genomics of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 152–172. [Google Scholar] [CrossRef]
- Gratuze, M.; Leyns, C.E.G.; Holtzman, D.M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 66. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; Yang, Y.S.; Youn, Y.C.; An, S.S.A.; Kim, S.Y. Genetic analyses of early-onset Alzheimer’s disease using next generation sequencing. Sci. Rep. 2019, 9, 8368. [Google Scholar] [CrossRef]
- Cacace, R.; Sleegers, K.; Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimer’s Dement. 2016, 12, 733–748. [Google Scholar] [CrossRef]
- Chen, H.-H.; Petty, L.E.; Sha, J.; Zhao, Y.; Kuzma, A.; Valladares, O.; Bush, W.; Naj, A.C.; Gamazon, E.R.; Below, J.E.; et al. Genetically regulated expression in late-onset Alzheimer’s disease implicates risk genes within known and novel loci. Transl. Psychiatry 2021, 11, 618. [Google Scholar] [CrossRef]
- König, T.; Stögmann, E. Genetics of Alzheimer’s disease. Wien. Med. Wochenschr. (1946) 2021, 171, 249–256. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Finger, E.C. Frontotemporal Dementias. Continum 2016, 22, 464–489. [Google Scholar] [CrossRef]
- Rademakers, R.; Neumann, M.; Mackenzie, I.R. Advances in understanding the molecular basis of frontotemporal dementia. Nat. Rev. Neurol. 2012, 8, 423–434. [Google Scholar] [CrossRef]
- Greaves, C.V.; Rohrer, J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef]
- Olszewska, D.A.; Lonergan, R.; Fallon, E.M.; Lynch, T. Genetics of Frontotemporal Dementia. Curr. Neurol. Neurosci. Rep. 2016, 16, 107. [Google Scholar] [CrossRef]
- Salimi, S.; Irish, M.; Foxe, D.; Hodges, J.R.; Piguet, O.; Burrell, J.R. Visuospatial dysfunction in Alzheimer’s disease and behavioural variant frontotemporal dementia. J. Neurol. Sci. 2019, 402, 74–80. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Neumann, M. Molecular neuropathology of frontotemporal dementia: Insights into disease mechanisms from postmortem studies. J. Neurochem. 2016, 138 (Suppl. S1), 54–70. [Google Scholar] [CrossRef]
- Ferrari, R.; Wang, Y.; Vandrovcova, J.; Guelfi, S.; Witeolar, A.; Karch, C.M.; Schork, A.J.; Fan, C.C.; Brewer, J.B.; International FTD-Genomics Consortium; et al. Genetic architecture of sporadic frontotemporal dementia and overlap with Alzheimer’s and Parkinson’s diseases. J. Neurol. Neurosurg. Psychiatry 2017, 88, 152–164. [Google Scholar] [CrossRef]
- Goldman, J.S.; Van Deerlin, V.M. Alzheimer’s Disease and Frontotemporal Dementia: The Current State of Genetics and Genetic Testing Since the Advent of Next-Generation Sequencing. Mol. Diagn. Ther. 2018, 22, 505–513. [Google Scholar] [CrossRef]
- Xiao, X.; Yuan, Z.; Guo, L.; Liao, X.; Zhou, Y.; Zhang, W.; Zhou, L.; Wang, X.; Liu, X.; Liu, H.; et al. The role of frontotemporal dementia associated genes in patients with Alzheimer’s disease. Neurobiol. Aging 2021, 107, 153–158. [Google Scholar] [CrossRef]
- Pasanen, P.; Myllykangas, L.; Pöyhönen, M.; Kiviharju, A.; Siitonen, M.; Hardy, J.; Bras, J.; Paetau, A.; Tienari, P.J.; Guerreiro, R.; et al. Genetics of dementia in a Finnish cohort. Eur. J. Hum. Genet. 2018, 26, 827–837. [Google Scholar] [CrossRef]
- Pagnon de la Vega, M.; Näslund, C.; Brundin, R.; Lannfelt, L.; Löwenmark, M.; Kilander, L.; Ingelsson, M.; Giedraitis, V. Mutation analysis of disease causing genes in patients with early onset or familial forms of Alzheimer’s disease and frontotemporal dementia. BMC Genom. 2022, 23, 99. [Google Scholar] [CrossRef]
- Adlimoghaddam, A.; Sabbir, M.G.; Albensi, B.C. Ammonia as a Potential Neurotoxic Factor in Alzheimer’s Disease. Front. Mol. Neurosci. 2016, 9, 57. [Google Scholar] [CrossRef]
- Jin, Y.Y.; Singh, P.; Chung, H.J.; Hong, S.T. Blood Ammonia as a Possible Etiological Agent for Alzheimer’s Disease. Nutrients 2018, 10, 564. [Google Scholar] [CrossRef]
- Bobermin, L.D.; Roppa, R.H.A.; Gonçalves, C.-A.; Quincozes-Santos, A. Ammonia-Induced Glial-Inflammaging. Mol. Neurobiol. 2020, 57, 3552–3567. [Google Scholar] [CrossRef]
- Jessy, J.; DeJoseph, M.R.; Hawkins, R.A. Hyperammonaemia depresses glucose consumption throughout the brain. Biochem. J. 1991, 277 Pt 3, 693–696. [Google Scholar] [CrossRef]
- Häberle, J.; Boddaert, N.; Burlina, A.; Chakrapani, A.; Dixon, M.; Huemer, M.; Karall, D.; Martinelli, D.; Crespo, P.S.; Santer, R.; et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J. Rare Dis. 2012, 7, 32. [Google Scholar] [CrossRef]
- Matsumoto, S.; Häberle, J.; Kido, J.; Mitsubuchi, H.; Endo, F.; Nakamura, K. Urea cycle disorders—Update. J. Hum. Genet. 2019, 64, 833–847. [Google Scholar] [CrossRef]
- Rüfenacht, V.; Häberle, J. Mini-Review: Challenges in Newborn Screening for Urea Cycle Disorders. Int. J. Neonatal Screen. 2015, 1, 27–35. [Google Scholar] [CrossRef]
- Rimoin, D.L.; Pyeritz, R.E.; Korf, B.R. Emery and Rimoin’s Principles and Practice of Medical Genetics; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Walker, V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes. Metab. 2009, 11, 823–835. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- Rascovsky, K.; Grossman, M. Clinical diagnostic criteria and classification controversies in frontotemporal lobar degeneration. Int. Rev. Psychiatry 2013, 25, 145–158. [Google Scholar] [CrossRef]
- Chang, X.; Wang, K. wANNOVAR: Annotating genetic variants for personal genomes via the web. J. Med. Genet. 2012, 49, 433–436. [Google Scholar] [CrossRef]
- Kircher, M.; Witten, D.M.; Jain, P.; O’Roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2005, 22, 195–201. [Google Scholar] [CrossRef]
- Karlberg, T.; Collins, R.; van den Berg, S.; Flores, A.; Hammarström, M.; Högbom, M.; Holmberg Schiavone, L.; Uppenberg, J. Structure of human argininosuccinate synthetase. Acta Crystallogr. Sect. D Biol. Crystallogr. 2008, 64, 279–286. [Google Scholar] [CrossRef]
- Kimani, J.K.; Wei, T.; Chol, K.; Li, Y.; Yu, P.; Ye, S.; Huang, X.; Qi, M. Functional analysis of novel splicing and missense mutations identified in the ASS1 gene in classical citrullinemia patients. Clin. Chim. Acta 2015, 438, 323–329. [Google Scholar] [CrossRef]
- Sen, K.; Whitehead, M.; Castillo Pinto, C.; Caldovic, L.; Gropman, A. Fifteen years of urea cycle disorders brain research: Looking back, looking forward. Anal. Biochem. 2022, 636, 114343. [Google Scholar] [CrossRef]
- Berning, C.; Bieger, I.; Pauli, S.; Vermeulen, T.; Vogl, T.; Rummel, T.; Höhne, W.; Koch, H.G.; Rolinski, B.; Gempel, K.; et al. Investigation of citrullinemia type I variants by in vitro expression studies. Hum. Mutat. 2008, 29, 1222–1227. [Google Scholar] [CrossRef]
- Kose, E.; Unal, O.; Bulbul, S.; Gunduz, M.; Häberle, J.; Arslan, N. Identification of three novel mutations in fourteen patients with citrullinemia type 1. Clin. Biochem. 2017, 50, 686–689. [Google Scholar] [CrossRef]
- Lisjak, M.; Iaconcig, A.; Guarnaccia, C.; Vicidomini, A.; Moretti, L.; Collaud, F.; Ronzitti, G.; Zentilin, L.; Muro, A.F. Lethality rescue and long-term amelioration of a citrullinemia type I mouse model by neonatal gene-targeting combined to SaCRISPR-Cas9. Mol. Ther.-Methods Clin. Dev. 2023, 31, 101103. [Google Scholar] [CrossRef]
- Daou, M.; Souaid, M.; Yammine, T.; Khneisser, I.; Mansour, H.; Salem, N.; Nemr, A.; Awwad, J.; Moukarzel, A.; Farra, C. Analysis of ASS1 gene in ten unrelated middle eastern families with citrullinemia type 1 identifies rare and novel variants. Mol. Genet. Genom. Med. 2023, 11, e2058. [Google Scholar] [CrossRef]
- Diez-Fernandez, C.; Rüfenacht, V.; Häberle, J. Mutations in the Human Argininosuccinate Synthetase (ASS1) Gene, Impact on Patients, Common Changes, and Structural Considerations. Hum. Mutat. 2017, 38, 471–484. [Google Scholar] [CrossRef]
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Rangroo Thrane, V.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538. [Google Scholar] [CrossRef]
- Kalyta, K.; Stelmaszczyk, W.; Szczęśniak, D.; Kotuła, L.; Dobosz, P.; Mroczek, M. The Spectrum of the Heterozygous Effect in Biallelic Mendelian Diseases—The Symptomatic Heterozygote Issue. Genes 2023, 14, 1562. [Google Scholar] [CrossRef]
- Raabe, W. Effects of Hyperammonemia on Neuronal Function: NH4+, IPSP and Cl−-Extrusion. In Cirrhosis, Hyperammonemia, and Hepatic Encephalopathy; Grisolía, S., Felipo, V., Eds.; Springer US: Boston, MA, USA, 1993; pp. 71–82. [Google Scholar]
- Ghosh, S.; Durgvanshi, S.; Agarwal, S.; Raghunath, M.; Sinha, J.K. Current Status of Drug Targets and Emerging Therapeutic Strategies in the Management of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 883–903. [Google Scholar] [CrossRef]
- Lemberg, A.; Alejandra Fernández, M. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann. Hepatol. 2009, 8, 95–102. [Google Scholar] [CrossRef]
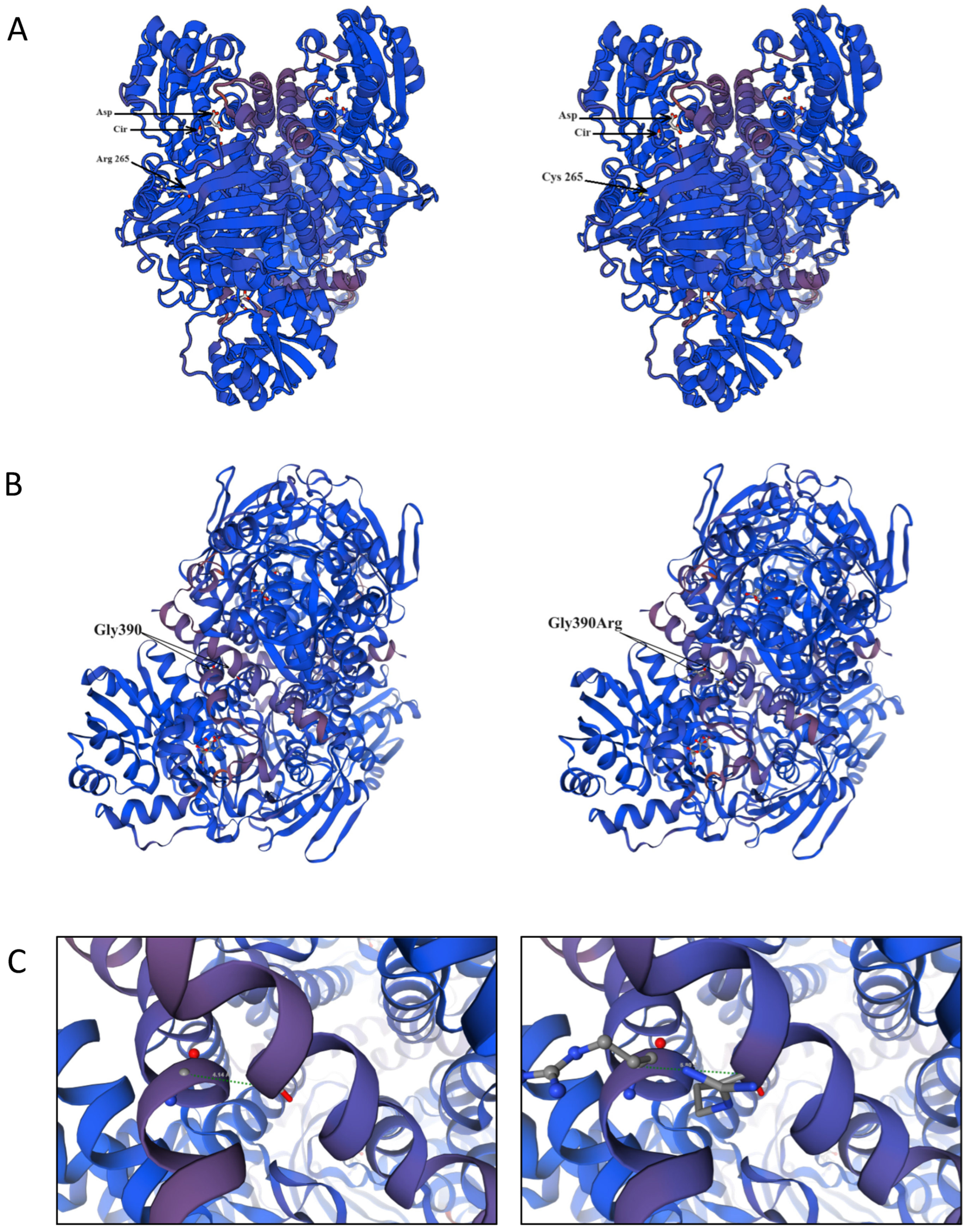
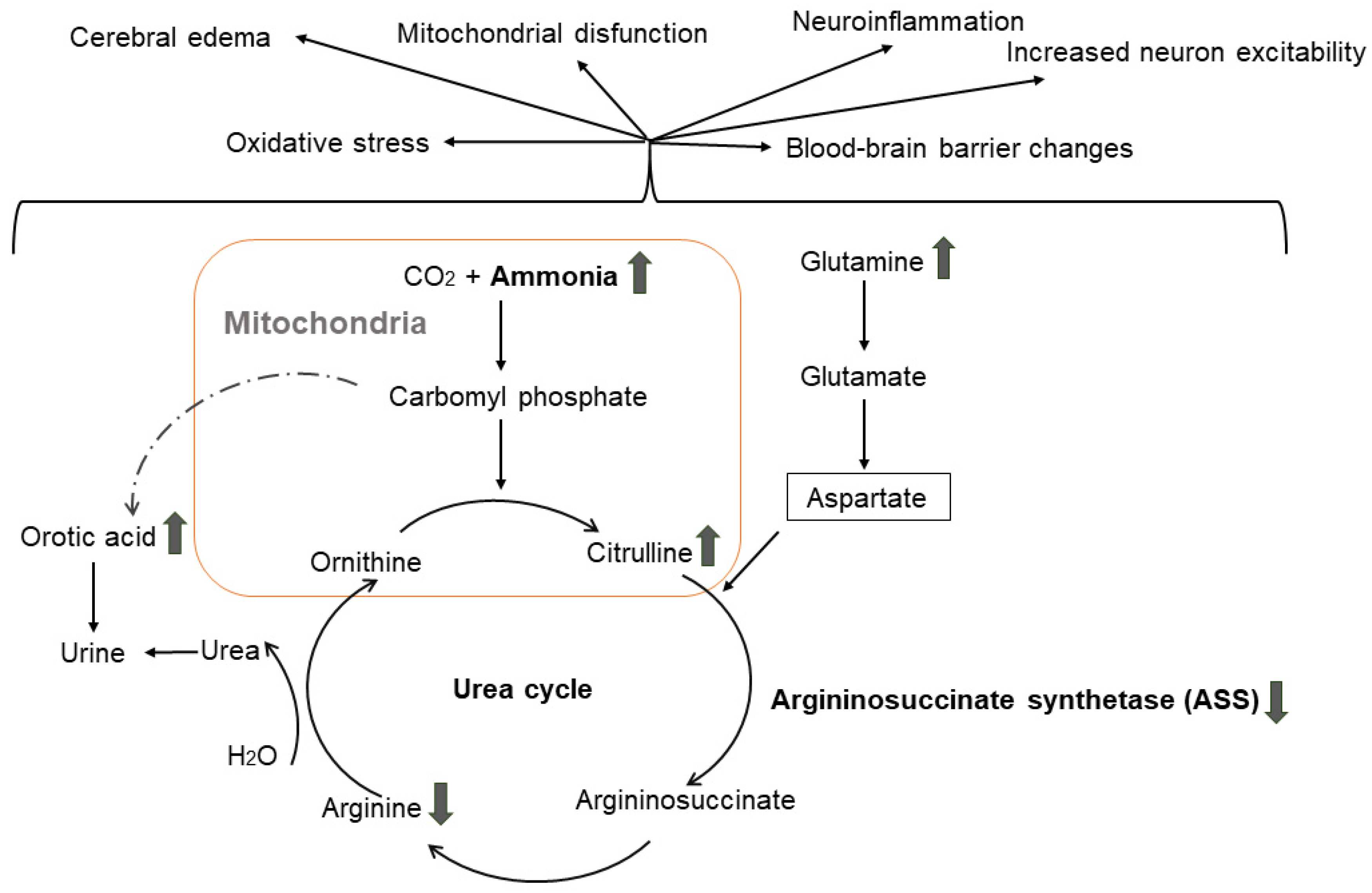
| Variant | Current Study | Bulgarian gnomAD Exomes, v.2.1.1 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Allele Count | Allele Number | MAF (%) | Allele Count | Allele Number | MAF (%) | ||
| rs148918985, ASS1, C>T, p.Arg265Cys | 4 | 807 | 0.50 | 0 | 2666 | 0 | 0.002457 |
| rs121908641, G>A, ASS1, p.Gly390Arg | 13 | 1032 | 1.26 | 2 | 2568 | 0.08 | 0.000002218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karachanak-Yankova, S.; Serbezov, D.; Antov, G.; Stancheva, M.; Mihaylova, M.; Hadjidekova, S.; Toncheva, D.; Pashov, A.; Belejanska, D.; Zhelev, Y.; et al. Rare Pathogenic Variants in Pooled Whole-Exome Sequencing Data Suggest Hyperammonemia as a Possible Cause of Dementia Not Classified as Alzheimer’s Disease or Frontotemporal Dementia. Genes 2024, 15, 753. https://doi.org/10.3390/genes15060753
Karachanak-Yankova S, Serbezov D, Antov G, Stancheva M, Mihaylova M, Hadjidekova S, Toncheva D, Pashov A, Belejanska D, Zhelev Y, et al. Rare Pathogenic Variants in Pooled Whole-Exome Sequencing Data Suggest Hyperammonemia as a Possible Cause of Dementia Not Classified as Alzheimer’s Disease or Frontotemporal Dementia. Genes. 2024; 15(6):753. https://doi.org/10.3390/genes15060753
Chicago/Turabian StyleKarachanak-Yankova, Sena, Dimitar Serbezov, Georgi Antov, Mikaela Stancheva, Marta Mihaylova, Savina Hadjidekova, Draga Toncheva, Anastas Pashov, Diyana Belejanska, Yavor Zhelev, and et al. 2024. "Rare Pathogenic Variants in Pooled Whole-Exome Sequencing Data Suggest Hyperammonemia as a Possible Cause of Dementia Not Classified as Alzheimer’s Disease or Frontotemporal Dementia" Genes 15, no. 6: 753. https://doi.org/10.3390/genes15060753






