Abstract
The excessive deposition of abdominal adipocytes in chickens is detrimental to poultry production. However, the regulatory factors that affect abdominal adipogenesis in chickens are still poorly understood. SLC22A16 is differentially expressed in abdominal preadipocytes and 10-day differentiated adipocytes in chickens, but its role in regulating chicken adipogenesis has not been reported. In this study, the function of SLC22A16 in chicken abdominal preadipocytes was investigated. SLC22A16 is significantly upregulated during abdominal adipocyte differentiation. The overexpression of SLC2A16 upregulated the expression of adipogenic marker genes and proliferation-related genes, and promoted the proliferation of adipocytes and the accumulation of triglycerides. The knockdown of SLC22A16 downregulated the expression of adipogenic marker genes and proliferation-related genes, inhibited the proliferation of adipocytes, and impaired the accumulation of triglycerides in adipocytes. In addition, LNC6302 was differentially expressed in abdominal preadipocytes and mature adipocytes, and was significantly positively correlated with the expression of SLC22A16. Interference with LNC6302 inhibits the expression of adipogenic marker genes and proliferation-related genes. The data supported the notion that LNC6302 promotes the differentiation of chicken abdominal adipocytes by cis-regulating the expression of SLC22A16. This study identified the role of SLC22A16 in the differentiation and proliferation of chicken adipocytes, providing a potential target for improving abdominal adipogenesis in chickens.
1. Introduction
In the poultry industry, abdominal fat is a component of the chicken body, and the excessive accumulation of abdominal fat can affect feed efficiency, meat quality, and consumer preferences [,]. Therefore, the accumulation of fat in the chicken abdomen is a disadvantageous factor for both merchants and consumers [,,]. It was reported that the heritability of abdominal fat (0.82) was significantly higher than that of chest muscle (0.55) and body weight (0.55) [], and that chicken abdominal preadipocytes have higher adipogenic differentiation ability than intramuscular preadipocytes []. Lipogenesis is regulated by a series of key transcription factors, such as peroxisome proliferator-activated receptor γ (PPARγ), the CCAAT/enhancer-binding protein α (C/EBPα), and fatty acid binding protein 4 (FABP4) [,]. Although there are currently many research reports on the deposition of abdominal fat in chickens, adipocyte differentiation is a complex and delicate process, and there are still many potential targets that have not been discovered. Therefore, it is important to study the molecular mechanisms of adipogenesis, which can accelerate the improvement of excessive abdominal fat in chicken.
At present, multiple genomic approaches can offer methods for identifying the genes or molecules responsible for fat deposition. For example, mRNA transcriptomics was used to identify 1700 differentially expressed mRNAs in the intramuscular fat of 24-month-old bulls and calves in Qinchuan cattle []. Moreover, 6814 differentially expressed genes were screened in goat subcutaneous preadipocytes and mature adipocytes using RNA-seq []. Long noncoding RNAs (lncRNAs) are a class of RNA with a length greater than 200nt and no or weak protein-coding potential, which regulates many levels of gene regulation from transcription to translation []. An increasing number of lncRNAs are being identified in fat metabolism and differentiation processes; for example, 283 differentially expressed lncRNAs were identified in preadipocytes and 3T3L1 adipocytes differentiated for 12 days []. A total of 2135 lncRNAs were identified during the differentiation of intramuscular adipocytes in the longissimus dorsi and semitendinosus of pigs []. The mechanism of lncRNAs’ function is related to their sequence, structure, and subcellular localization. lncRNAs in the nucleus regulate transcription and modify newly formed RNA through chromatin interactions and remodeling [,]. lncRNAs in the cytoplasm can regulate translational programs and assist in protein processing [,,]. Recently, some lncRNAs related to fat formation have been identified. The knockdown of lncRNA NR_015556 upregulates PPARγ and C/EBPα to promote C3H10T1/2 cell differentiation []. The knockdown of lncRNA MIR1HG reduces the enrichment of acetylation (AcH3) and histone H3 lysine 4 trimethylation (H3K4me3) in the FABP4 promoter, thereby inhibiting the adipocyte differentiation of human adipose-derived stem cells []. AdipoQ antisense lncRNA (AdipoQ AS lncRNA) inhibits mouse adipogenesis by forming an AdipoQ AS lncRNA/AdipoQ mRNA duplex []. LncPLAAT3-AS upregulates PLAAT3 expression by absorbing miR-503-5p to promote the differentiation of porcine preadipocytes []. These reports support the importance of both coding genes or lncRNAs in the process of adipogenesis.
This study found the differential expression of SLC22A16 in chicken abdominal preadipocyte and differentiated adipocytes for 10 days [], predicting its involvement in regulating the generation of chicken adipocytes. SLC22A16 is a member of the Solute Carrier protein (SLC) family, which includes membrane-bound transport proteins that manage multiple substrates and regulate the homeostasis of endogenous metabolites. At present, the reports on SLC22A16 only involve the pharmacokinetics of doxorubicin and carnitine transport, and there are no studies reporting the role of SLC22A16 in adipose differentiation [,,,]. To analyze the functionality of SLC22A16, we investigated the effect of SLC22A16 on the proliferation and differentiation of chicken abdominal adipocytes through overexpression and interference techniques. The results showed that SLC22A16 promotes the proliferation and differentiation of chicken abdominal adipocytes. Through the joint analysis of lncRNA data differentially expressed in chicken abdominal preadipocytes and adipocytes, a significant correlation was found between LNC6302 and SLC22A16 expression. LNC6302 promotes the proliferation and differentiation of chicken abdominal adipocytes by regulating the expression of SLC22A16. In summary, our study provides a potential target for improving abdominal fat deposition in chickens.
2. Materials and Methods
2.1. Ethics Statement
All animal experiments were approved by the Animal Care Committee of the College of Animal Science and Technology, Henan Agricultural University (approval code HNND2022030840; 7 March 2022), and were performed following the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of China. All efforts were made to minimize animal suffering.
2.2. Cell Culture
Primary chicken abdominal preadipocytes were isolated from the abdominal adipose tissue of two-week-old chickens following the methods described previously []. The IPC1 (immortal chicken abdominal preadipocytes infected with TERT retrovirus) preadipocyte strain was donated by Northeast Agricultural University []. IPC1 preadipocytes or primary abdominal preadipocytes were seeded in 12-well plates and maintained with basic medium (DMEM/F12, 10% FBS, and 1% penicillin/streptomycin). Once the cells reached 90% confluence, the differentiation medium (0.5 mM 3-isobutyl-1-methylxanthine, 1 µM dexamethasone, 10 µg/mL insulin, and 300 µM oleic acid) was used to replace the basic medium for 48 h. The differentiation medium was then replaced with maintenance medium (10 µg/mL insulin and 300 µM oleic acid) and maintained for 6 days.
2.3. Oil Red O Staining and Cellular TG Content Measurement
The cells to be tested were collected, washed with PBS three times, and fixed with 4% paraformaldehyde for 30 min. After that, the cells were washed twice with PBS and then stained with Oil Red O solution (60% Oil Red O, 40% deionized water) for 20 min. Before imaging, the cells were washed with PBS three times. All procedures were performed at room temperature. TG content was measured using a triglyceride content detection kit (APPLYGEN, Beijing, China) according to the manufacturer’s instructions. Briefly, lysis buffer was added to the cell precipitate, which was incubated at room temperature for 10 min; the cell supernatant was heated at 70 °C for 10 min, and then centrifuged at 2000 rpm for 5 min, with the supernatant then used for the assay.
2.4. Rapid Amplification of cDNA Ends (RACE)
In order to obtain the full-length sequence of LNC6302, we performed a RACE assay using the SMARTer RACE cDNA 5′/3′Kit (Clontech, Palo Alto, CA, USA) according to the manufacturer’s instructions. The total RNA isolated from preadipocytes and adipocytes was mixed and used for the RACE experiment. The primers used for 5′ and 3′ RACE were 5′-AAGTGTTAGTCAAAGGTTTTCCAA-3′ and 5′-CAGCATTAGAAAAGATAGAGATGT-3′, respectively.
2.5. Vector Construction
To construct the overexpression vectors, the chicken SLC22A16 coding region was cloned using SLC22A16 CDS primer. Then, the CDS fragment of SLC22A16 was inserted into the pcDNA3.1-EGFP vector using Hind III and EcoR I sites. To verify whether LNC6302 has the ability to encode proteins, we used a prokaryotic expression system in vitro and inserted the largest open reading frame fragment of LNC6302 into the pET-30a vector and induced translation using an IPTG inducer. pET-30a was used as a negative control, and pET-30a-EGFP as a positive control. Then, 5× loading buffer was added to the precipitate of the induced product and incubated at 100 °C for 10 min. Finally, electrophoresis detection was performed using SDS-PAGE. The primers used are shown in Table S1. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used for plasmid transfection according to the manufacturer’s instructions.
2.6. RNA Interference
siRNAs specifically target SLC22A16, and negative control siRNAs were ordered from GenePharma (Shanghai, China). The sequences of siRNAs for SLC22A16 were 5′-CCAGGCACACAGAACAATT-3′, and for negative control were UUCUCCGAACGUGUCACGUTT-3′. The lncRNA Smart Silencer for LNC6302 was used for RNA interference (RiboBio, Guangzhou, China). The primary abdominal adipocytes were transfected with 50 nM lncRNA Smart Silencer supplemented with reagent buffer following the manufacturer’s instructions. The medium was changed after 4–6 h of transfection. Then, cells were collected for downstream experiments.
2.7. Propidium Iodide Staining and Flow Cytometry Analysis
The treated cells were digested with trypsin, washed with PBS, and then fixed in 70% ethanol at −20 °C overnight. The DNA was incubated with propidium iodide (Solarbio, Beijing, China) staining solution at 4 °C for 30 min. The cells were measured using a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA).
2.8. Cell Proliferation Assays
The cell proliferation analysis and cell viability assays were performed using a CCK-8 kit and EdU proliferation assay, respectively. The cells were cultured in 96-well plates and, after transfection, 10 µL of CCK-8 solution was added to the cells every 12 h according to the manufacturer’s instructions (Dojindo, Kumamoto, Japan). After incubation in a 5% CO2 37 °C incubator for 2 h, the cell viability was detected at 450 nm using a Microplate Reader (Thermo, Waltham, MA, USA). The cells were cultured in 12-well plates, transfected for 48 h, and tested according to the instructions provided by the Cell-Light EdU Apollo 567 in vitro kit manufacturer (RiboBio, Guangzhou, China). Finally, the cells were photographed and recorded under a fluorescence microscope (Olympus, Tokyo, Japan) and counted using Image J software (version number: Ij53-win-java8) (NIH, Bethesda, MD, USA).
2.9. RNA Fluorescence In Situ Hybridization (RNA FISH) and Cytoplasmic and Nuclear RNA Extraction
FITC-labeled LNC6302 probes for the FISH assay were synthesized from RiboBio and the FISH kit was used according to its instructions (RiboBio, Guangzhou, China). The cytoplasmic and nuclear RNA were extracted using the PARISTM Kit (Life, Boston, MA, USA) based on the manufacturer’s instructions.
2.10. Total RNA Isolation, cDNA Synthesis, and Real-Time Quantitative PCR (RT-qPCR)
The total RNA was extracted from the cells using TRIzol reagent according to the manufacturer’s instructions. The extracted RNA (1 µg) was converted into cDNA using the PrimeScriptTM RT reagent kit with Gdna Eraser (TaKaRa, Tokyo, Japan). Quantitative RT-PCR was performed using a LightCycler®96 system (Roche, Basel, Switzerland) and SYBR Premix Ex Taq II kit (Vazyme, Nanjing, China). β-actin was selected as a reference gene, and the relative quantification of genes was performed using the 2−ΔΔCt method [].
2.11. Statistical Analysis
All assays were performed in triplicate. For statistical analysis, all data are presented as mean ± SEM. Before applying Student’s t-tests using GraphPad Prism 7.0 software (San Diego, CA, USA), we used the Shapiro–Wilk method to test whether the data followed a normal distribution. A p-value > 0.05 indicated that the data conformed to a normal distribution. A p-value < 0.05 was considered statistically significant in the Student’s t-test.
3. Results
3.1. Differential Expression of SLC22A16 in Chicken Adipocyte Differentiation
We found the differential expression of SLC22A16 in RNA-seq between the primary chicken abdominal preadipocytes and 10-day differentiated adipocytes. The qPCR identification of the expression of SLC22A16 showed that it had a higher expression level than preadipocytes and showed significantly upregulated expression in 10-day differentiated adipocytes (Figure 1A) (p < 0.01). During the differentiation process of chicken abdominal adipocytes, SLC22A16 showed an overall upward trend after differentiation, with the highest expression level at 4d (Figure 1B) (p < 0.01), suggesting that SLC22A16 plays a catalytic role in chicken adipogenesis.
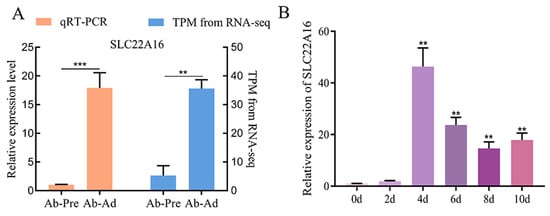
Figure 1.
Differential expression of SLC22A16 in chicken adipocyte differentiation. (A) Verification of the accuracy of sequencing results by qRT-PCR. TPM: transcripts per million. (B) Dynamic expression levels of SLC22A16 during adipogenic differentiation. These data are expressed as mean ± SEM (n = 3), ** p < 0.01, *** p < 0.001.
3.2. SLC22A16 Promotes the Differentiation of IPC1 Preadipocytes
To determine the role of SLC22A16 in preadipocyte differentiation, we used the overexpression and knockdown of SLC22A16 in ICP1 preadipocytes. First, the overexpression of SLC22A16 promotes the differentiation of ICP1 preadipocytes and significantly upregulates the mRNA expression of LPL and PPARG (Figure 2A) (p < 0.05). The intracellular triglyceride content and Oil Red O staining results showed that the overexpression of SLC22A16 significantly increased lipid droplet accumulation (Figure 2B,E) (p < 0.05). Second, knockdown of SLC22A16 significantly downregulated the mRNA levels of lipid marker genes CEBPA, LPL, FABP4, and PPARG (Figure 2C) (p < 0.05). Furthermore, evidence from the intracellular triglyceride assay and Oil Red O staining demonstrated that lipid droplet accumulation significantly declined in the SLC22A16 knockdown group (Figure 2B,E) (p < 0.05).
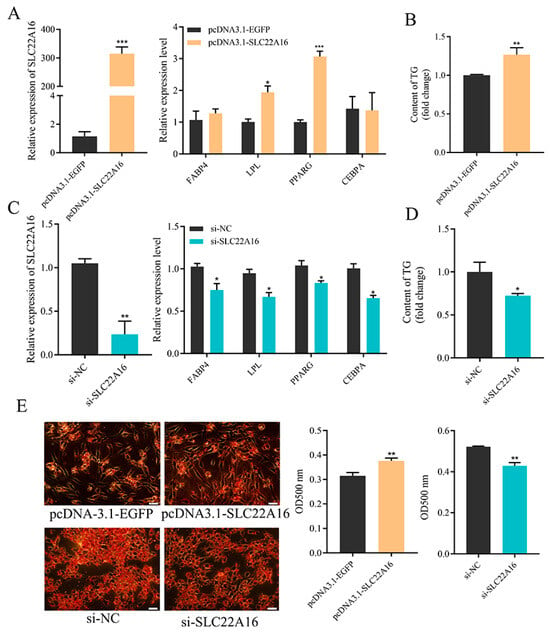
Figure 2.
SLC22A16 promotes the differentiation of chicken preadipocyte. (A) Overexpression efficiency of SLC22A16 and changes in adipocyte differentiation marker genes after overexpression of SLC22A16. (B) Changes in intracellular triglyceride content after overexpression of SLC22A16. (C) Interference efficiency of SLC22A16 and changes in adipocyte differentiation marker genes after interference with SLC22A16. (D) Changes in intracellular triglyceride content after interference with SLC22A16. (E) Number of lipid droplets in adipocytes stained with Oil Red O after overexpression and interference with SLC22A16. Scale bar: 100 µm. (mean ± SEM, n = 3; * p < 0.05, ** p < 0.01, *** p < 0.001).
3.3. SLC22A16 Promotes the Proliferation of ICP1 Preadipocytes
To detect whether SLC22A16 is involved in chicken preadipocyte proliferation, we used the overexpression and knockdown of SLC22A16 in ICP1 preadipocytes. EdU staining and a CCK-8 cell viability assay showed that the overexpression of SLC22A16 significantly promoted the proliferation of ICP1 preadipocytes, while knocking out SLC22A16 significantly inhibited the proliferation of ICP1 preadipocytes (Figure 3A,B) (p < 0.05). After SLC22A16 was knocked out, the number of cells in G1 phase was significantly increased, and the number of cells in S phase and G2 phase was significantly reduced (Figure 3C) (p < 0.05). Then, G1/S-specific cyclin-D1 (CCND1) and proliferating cell nuclear antigen (PCNA) were significantly decreased after SLC22A16 overexpression. The knockdown of SLC22A16 significantly inhibited the mRNA expression levels of CCND1 and CDK1 (Figure 3D) (p < 0.05).
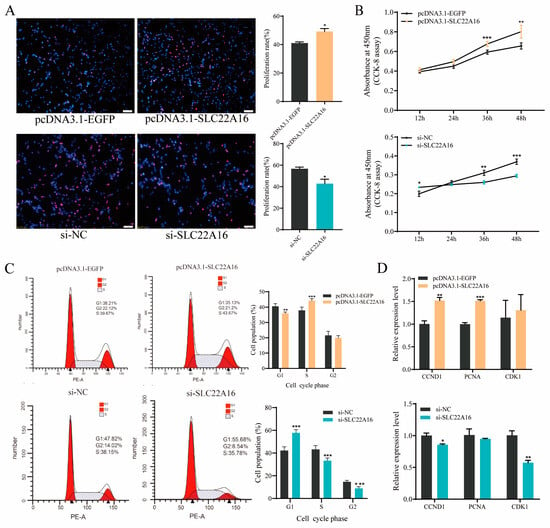
Figure 3.
SLC22A16 promotes the proliferation of chicken adipocytes. (A) EdU was used to detect the proliferation of chicken adipocytes after overexpression and interference with SLC22A16. Scale bar: 100 µm.(B) CCK-8 was used to detect cell growth after overexpression and interference with SLC22A16. (C) Cell cycle analysis of cells after overexpression and interference with SLC22A16. (D) Relative mRNA expression of proliferative genes 48 h after overexpression and interference with SLC22A16 (mean ± SEM, n = 3; * p < 0.05, ** p < 0.01, *** p < 0.001).
3.4. LNC6302 Is Associated with SLC22A16 Expression and Characterization of the LNC6302 Sequence
In order to understand the potential mechanism of the SLC22A16 gene functions in chicken abdominal adipocytes, we conducted a correlation analysis between the differentially expressed mRNA and lncRNA sequencing data of abdominal preadipocytes and 10-day differentiated adipocytes in chickens and found that differentially expressed LNC6302 was co-localized with SLC22A16 [,]. To further investigate LNC6302, we first characterized the sequence of LNC6302. The RACE experiment showed that LNC6302 had a polyadenylated transcript length of 3713bp (Figure S1). Using the BLAT tool in the UCSC Genome Browser (https://genome.ucsc.edu/ accessed on May 26, 2024) revealed that LNC6302 is located within the SLC22A16 gene (Figure 4A). In addition, the expression levels of LNC6302 and SLC22A16 were analyzed using CT and FPKM values, and the results showed a significant positive correlation between the expression levels of LNC6302 and SLC22A16 (Figure 4B); moreover, LNC6302 was also significantly expressed after abdominal adipocyte differentiation (Figure 4C). The analysis results of CPC online software (https://cpc.gao-lab.org/docs/quick_guide.jsp) accessed on 18 July 2023)showed that the coding ability of LNC6302 is similar to that of lncDC reported in previous studies [], and its coding ability is much lower than that of the coding gene GAPDH (Figure 4D). Meanwhile, the LNC6302 expression vector failed to produce protein through a translation assay in vitro, indicating that LNC6302 does not have coding potential (Figure 4E). The qPCR analysis of fractionated nuclear and cytoplasmic RNA showed that LNC6302 was expressed in both the nucleus and cytoplasm (Figure 4F). This result was confirmed by FISH (Figure 4G).
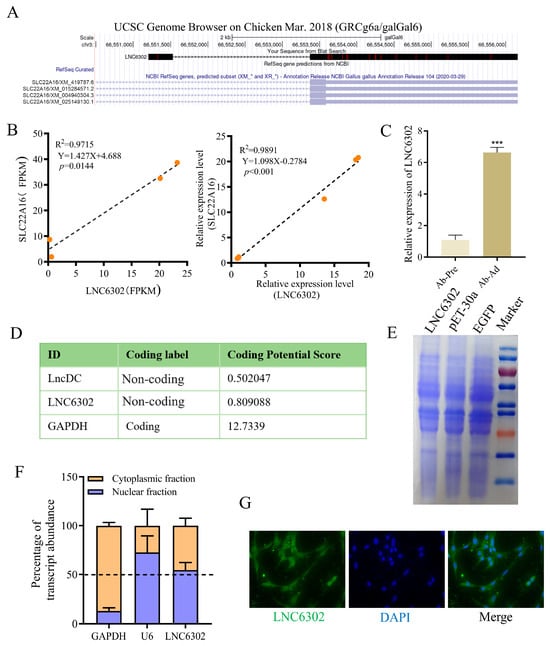
Figure 4.
Characterization of the LNC6302 sequence. (A) Genome location of chicken LNC6302 and SLC22A16. (B) Correlation analysis of the expression of LNC6302 and SLC22A16 in RNA-seq (FPKM) and RT-qPCR (Ct values) data. (C) Relative expression levels of LNC6302 in Ab-pre and Ab-Ad. (D) Coding potential of RNA sequences LNC6302, lncDC, and GAPDH using the Coding Potential Calculator (CPC) program. (E) In vitro translation assay using LNC6302 and EGFP constructs. Shown is Coomassie Blue staining. (F) Expression level of LNC6302 in nuclear and cytoplasm detected by RT-qPCR. (G) Location of LNC6302 in chicken preadipocytes detected by FISH (mean ± SEM, n = 3; *** p < 0.001).
3.5. Interference with LNC6302 Inhibits the Differentiation and Proliferation of Abdominal Preadipocytes
To further verify the role of LNC6302 in adipocyte differentiation, we knocked down LNC6302 in primary abdominal preadipocytes. After knocking out LNC6302, the expression levels of FABP4, LPL, PPARA, and PPARD were significantly inhibited (p < 0.05). PPARG showed a downward trend (p = 0.0772) (Figure 5A), the intracellular triglyceride content was significantly reduced (Figure 5B) (p < 0.05), and the Oil Red O staining results also showed a significant decrease in lipid droplet accumulation (Figure 5C) (p < 0.05). Furthermore, EdU staining and the CCK8 assay suggested that the knockdown of LNC6302 dramatically inhibited the proliferation of primary abdominal preadipocytes (Figure 5D,E) (p < 0.05). The flow cytometry detection results showed that knocking down LNC6302 inhibited the proportion of S-phase cells (Figure 5F) (p < 0.05), and the qPCR results showed that the proliferation marker genes CCND1, CDK1, and PCNA were also significantly inhibited (Figure 5G) (p < 0.05).
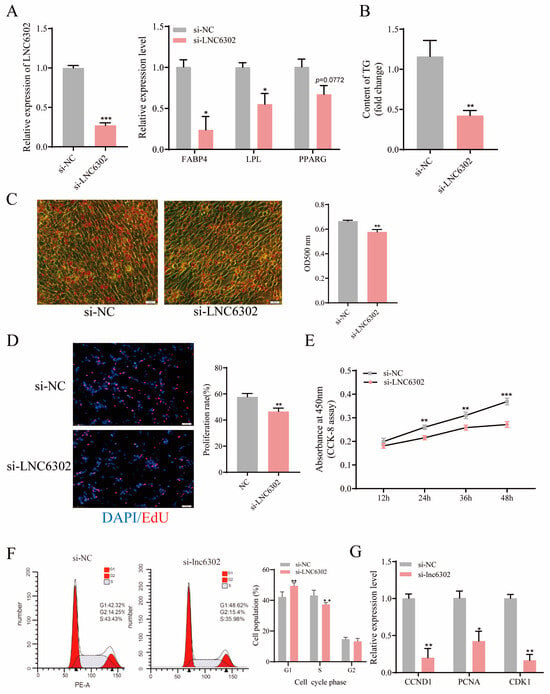
Figure 5.
Interference with LNC6302 inhibits the differentiation and proliferation of abdominal preadipocytes. (A) Relative expression level after interference with LNC6302 and the changes in adipocyte differentiation marker genes after interference with LNC6302. (B) Changes in intracellular triglyceride content after interference with LNC6302. (C) Number of lipid droplets in adipocytes stained with Oil Red O after interference with LNC6302. Scale bar: 100 μm. (D) EdU was used to detect the proliferation of chicken adipocytes after interference with LNC6302. Scale bar: 100 μm. (E) CCK-8 was used to detect cell growth after interference with LNC6302. (F) Cell cycle analysis of cells after interference with LNC6302. (G) Relative mRNA expression of proliferative genes 48 h after interference with LNC6302 (mean ± SEM, n = 3; * p < 0.05, ** p < 0.01, *** p < 0.001).
3.6. LNC6302 Promotes Abdominal Preadipocyte Differentiation by Activating SLC22A16 in Cis-Regulating Manner
To test whether LNC6302 inhibits adipogenesis in abdominal adipocytes in a SLC22A16-dependent manner, si-LNC6302 with pcDNA3.1-SLC22A16 vector was transfected into the cells. After the overexpression of SLC22A16, the expression of SLC22A16, PPARG, and FABP4 was significantly upregulated, and Oil Red O staining showed a significant increase in lipid droplet content (p < 0.05). When si-LNC6302 and pcDNA3.1-SLC22A16 co-transfection was conducted, the promoting effect of SLC22A16 on adipocyte differentiation was inhibited by si-LNC6302 (Figure 6A,B) (p < 0.05). As shown in Figure 6C, LNC6302 promotes the differentiation of chicken abdominal adipocytes by cis-regulating the expression of SLC22A16.
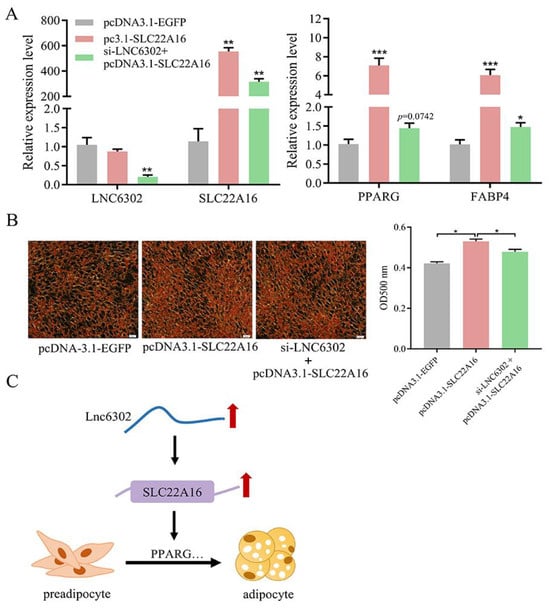
Figure 6.
(A) Expression level of LNC6302, SLC22A16, and adipogenic markers PPARG and FABP4 evaluated by RT-qPCR. (B) Oil Red O staining of abdominal preadipocytes transfected with si-NC, si-LNC6302, or pcDNA3.1-SLC22A16 and si-LNC6302. Scale bar: 100 μm. (C) Proposed model of LNC6302 regulation on adipocyte differentiation. LNC6302 carries out cis-activiting regulation of SLC22A16, which promotes targets such as PPARG, thus regulating adipogenesis. Red arrow: Upregulated expression. Black arrow: Regulatory relationship. (mean ± SEM, n = 3; * p < 0.05, ** p < 0.01, *** p < 0.001).
4. Discussion
Excessive fat deposition has always been a major problem faced by the poultry industry, as it poses certain obstacles to profitable agricultural economies. So far, various methods have been explored to reduce fat deposition, including feeding methods, feed formulas, and genetic selection [,,]. However, adipogenesis is a delicate and complex process closely linked with the proliferation and differentiation of adipocytes, as well as the accumulation of lipids within mature adipocytes []. In recent years, there have been many reports about adipocyte differentiation [,,]. In our previous research, we used chicken preadipocytes differentiated for 0 and 10 days to carry out RNA seq transcriptome sequencing []. SLC22A16 is one of the differential genes, and SLC22A16 is upregulated in the process of chicken adipocyte differentiation, suggesting that it may participate in adipocyte differentiation. However, the impact of SLC22A16 on adipocyte differentiation and adipogenesis has not been reported.
The SLC family is a member of the Major Facilitator Superfamily (MFS), which is mainly responsible for the transport of various carbohydrates, organic alcohols, and other substances in organisms []. Although more than 100 transporter families have been identified and classified, the SLC family is the second largest membrane protein family after G-protein-coupled receptors []. Therefore, the importance of the SLC transporter family to organisms is self-evident. There are reports that SLC22A16 is mainly responsible for the transport of organic cations and carnitine []. Knocking down SLC22A16 reduces the viability and cell cycle progression of acute myeloid leukemia cells, and can lead to pupal arrest in fruit flies [,]. The expression of SLC22A16 in gastric cancer tissue is higher than that in normal tissue. In patients with recurrent treatment, the expression of SLC22A16 is also significantly higher than that in patients without recurrence []. Iron is one of the indispensable factors in DNA synthesis in organisms [], and iron depletion in melanoma SK-Mel-28 cells can lead to the downregulation of the polyamine import gene SLC22A16 []. Atsushi Enomoto found that SLC22A16-mediated L-carnitine transport is essential during spermatogenesis [], indicating that SLC22A16 plays an important role in maintaining cell viability.
In this study, we first investigated the function of SLC22A16 on the proliferation of chicken abdominal adipocytes by the overexpression and interference with SLC22A16. The EdU assay and cell cycle assay showed that the overexpression of SLC22A16 can significantly promote the proliferation of chicken abdominal preadipocytes and significantly promote the expression of CCND1 and PCNA mRNA. These two genes are used as marker genes for proliferation. CCND1, one of three unlinked proteins, regulates the transition between G1 and S phases of the cell cycle []. PCNA plays an essential role in the process of DNA replication and repair []. In addition, this study indicates that SLC22A16 promotes the differentiation of abdominal preadipocytes in chickens. The results of the Oil Red O staining and triglyceride content assay showed a significant increase in lipid droplet accumulation in chicken abdominal adipocytes. The expression levels of PPARG and CEBPA were significantly altered by SLC22A16, and there is ample evidence to suggest that PPARG and CEBPA are the main transcription factors involved in adipocyte differentiation [,,]. lncRNAs are important regulatory molecules and actively participate in various biological processes [,,,]. The role of lncRNAs in chicken adipocyte differentiation has also attracted the attention of researchers [,]. In order to understand the upstream regulatory mechanism of SLC22A16, this study conducted a joint analysis with our published lncRNA sequencing data and found a positive correlation between LNC6302 and SLC22A16 expression during chicken adipocyte differentiation [], with LNC6302 being located adjacent to SLC22A16. The mechanism of action of lncRNA is correlated with its expression distribution, and there are different regulatory mechanisms in the nucleus or cytoplasm [,,]. RNA FISH indicates that LNC6302 is expressed in both the nucleus and cytoplasm, suggesting that the mechanisms by which LNC6302 regulates chicken adipocyte differentiation may be diverse. One of the mechanisms of action of lncRNA is the cis regulation of the expression of neighboring protein coding genes []. Studies have reported that lncRNA AC092159.2 can cis-regulate the transcription of TMEM18 and thereby affect adipocyte differentiation []. The lncRNA BADLNCR1 inhibits GLRX5 transcription activity and inhibits bovine adipocyte differentiation []. Therefore, we focused on the relationship between LNC6302 and SLC22A16. As shown in the results, the downregulation of LNC6302 inhibited the expression of SLC22A16, and the overexpression of SLC22A16 also restored adipocyte differentiation after the downregulation of LNC6302.
5. Conclusions
In summary, our data indicate that SLC22A16 is a positive regulatory factor for the proliferation and differentiation of chicken adipocytes, and reveal that LNC6302 promotes the differentiation of abdominal fat by regulating the expression of SLC22A16 in chickens. This study enriches the regulatory network of chicken adipocyte differentiation, and it provides a potential target for molecular breeding.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes15060758/s1, Figure S1: The characteristic of chicken LNC6302 sequence, Table S1: Primer sequences for RT-qPCR.
Author Contributions
The individual contributions are shown as follows: Conceptualization, G.S. and X.K.; methodology, G.S.; software, W.L. and T.Z.; validation, X.M., Y.H. and C.L.; formal analysis, X.M.; investigation, C.L.; resources, D.L.; data curation, Y.H.; writing—original draft preparation, X.M.; writing—review and editing, X.M.; visualization, X.M. and Y.H.; supervision, G.S.; project administration, G.S.; funding acquisition, G.S. and X.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32072710), the Shennong Laboratory (SN01-2022-05), and the Scientific Studio of Zhongyuan Scholars (234400510023).
Institutional Review Board Statement
All animal experiments were approved by the Animal Care Committee of the College of Animal Science and Technology, Henan Agricultural University (approval code HNND2022030840; 7 March 2022), and were performed following the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of China. All efforts were made to minimize animal suffering.
Informed Consent Statement
Not applicable.
Data Availability Statement
All RNA-seq datasets supporting the results of this article have been submitted to the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO). The accession numbers are SRR7067303, SRR7067304, SRR7067305, SRR7067306, SRR6459507, SRR6459508, SRR6459509, and SRR6459510.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Zhang, X.Y.; Wu, M.Q.; Wang, S.Z.; Zhang, H.; Du, Z.Q.; Li, Y.M.; Cao, Z.P.; Luan, P.; Leng, L.; Li, H. Genetic selection on abdominal fat content alters the reproductive performance of broilers. Animal 2018, 12, 1232–1241. [Google Scholar] [CrossRef]
- Abdalla, B.A.; Chen, J.; Nie, Q.; Zhang, X. Genomic Insights Into the Multiple Factors Controlling Abdominal Fat Deposition in a Chicken Model. Front. Genet. 2018, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, T.; Zhang, S.; Huang, J.; Zhang, G.; Xie, K.; Wang, J.; Wu, H.; Dai, G. Identification of Long Non-Coding RNA-Associated Competing Endogenous RNA Network in the Differentiation of Chicken Preadipocytes. Genes 2019, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Demeure, O.; Duclos, M.J.; Bacciu, N.; Le Mignon, G.; Filangi, O.; Pitel, F.; Boland, A.; Lagarrigue, S.; Cogburn, L.A.; Simon, J. Genome-wide interval mapping using SNPs identifies new QTL for growth, body composition and several physiological variables in an F2 intercross between fat and lean chicken lines. Genet. Sel. Evol. Gse 2013, 45, 36. [Google Scholar] [CrossRef] [PubMed]
- Ramiah, S.K.; Meng, G.Y.; Sheau Wei, T.; Swee Keong, Y.; Ebrahimi, M. Dietary Conjugated Linoleic Acid Supplementation Leads to Downregulation of PPAR Transcription in Broiler Chickens and Reduction of Adipocyte Cellularity. Ppar Res. 2014, 2014, e137652. [Google Scholar] [CrossRef] [PubMed]
- Cahaner, A.; Nitsan, Z. Evaluation of simultaneous selection for live body weight and against abdominal fat in broilers. Poult. Sci. 1985, 64, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, X.; Zhai, Y.; Zhang, D.; Sui, L.; Li, W.; Jiang, R.; Han, R.; Li, G.; Li, Z. Comprehensive Transcriptome Analysis of lncRNAs Reveals the Role of lncAD in Chicken Intramuscular and Abdominal Adipogenesis. J. Agric. Food Chem. 2020, 68, 3678–3688. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.T.; Lane, M.D. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc. Natl. Acad. Sci. USA 1994, 91, 8757–8761. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, H.B.; Wang, Y.N.; Wang, H.C.; Zhang, S.; Hong, J.Y.; Guo, H.F.; Chen, D.; Yang, Y.; Zan, L.S. Transcriptome analysis of mRNA and microRNAs in intramuscular fat tissues of castrated and intact male Chinese Qinchuan cattle. PLoS ONE 2017, 12, e0185961. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; He, C.; Zhu, J.; Xiong, Y.; Lin, Y. RNA-seq analysis reveals the positive role of KLF5 in the differentiation of subcutaneous adipocyte in goats. Gene 2022, 808, 145969. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Vaughn, I.W.; Mcmanus, M.T. Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs. PLoS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, J.; Pan, X.; Zhang, M.; Huang, W.; Liu, Y.; Yang, H.; Cheng, Z.; Zhang, G.; Qie, M.; et al. LncRNA MIR99AHG enhances adipocyte differentiation by targeting miR-29b-3p to upregulate PPARγ. Mol. Cell. Endocrinol. 2022, 550, 111648. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Ge, Z.; Guo, Z.; Wang, Y.; Yang, G.; Sun, S.; Li, X. Screening of lncRNA profiles during intramuscular adipogenic differentiation in longissimus dorsi and semitendinosus muscles in pigs. Anim. Biotechnol. 2023, 34, 4616–4626. [Google Scholar] [CrossRef] [PubMed]
- Melé, M.; Rinn, J.L. “Cat’s Cradling” the 3D Genome by the Act of LncRNA Transcription. Mol. Cell 2016, 62, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Engreitz, J.M.; Sirokman, K.; Mcdonel, P.; Shishkin, A.A.; Surka, C.; Russell, P.; Grossman, S.R.; Chow, A.Y.; Guttman, M.; Lander, E.S. RNA-RNA Interactions Enable Specific Targeting of Noncoding RNAs to Nascent Pre-mRNAs and Chromatin Sites. Cell 2014, 159, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Grelet, S.; Link, L.A.; Howley, B.; Obellianne, C.; Palanisamy, V.; Gangaraju, V.K.; Diehl, J.A.; Howe, P.H. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat. Cell Biol. 2017, 19, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wang, L.; Zhan, H.; Dai, J.; Song, X. A long noncoding RNA distributed in both nucleus and cytoplasm operates in the PYCARD-regulated apoptosis by coordinating the epigenetic and translational regulation. PLoS Genet. 2019, 15, e1008144. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Ma, X.K.; Xing, Y.H.; Zheng, C.C.; Chen, L.L. Distinct Processing of lncRNAs Contributes to Non-conserved Functions in Stem Cells. Cell 2020, 181, 621–636.e22. [Google Scholar] [CrossRef]
- Zuo, C.; Pan, Y.; Leng, D.; Chen, X.; Dong, F.; Lin, Z.; Dai, Z.; Wang, Z. Transcriptome analysis of long non-coding RNAs reveals NR_015556 lncRNA is a novel regulator for adipocyte differentiation. Biochem. Biophys. Res. Commun. 2022, 601, 79–85. [Google Scholar] [CrossRef]
- Huang, Y.; Jin, C.; Zheng, Y.; Li, X.; Zhang, S.; Zhang, Y.; Jia, L.; Li, W. Knockdown of lncRNA MIR31HG inhibits adipocyte differentiation of human adipose-derived stem cells via histone modification of FABP4. Sci. Rep. 2017, 7, 8080. [Google Scholar] [CrossRef]
- Cai, R.; Sun, Y.; Qimuge, N.; Wang, G.; Wang, Y.; Chu, G.; Yu, T.; Yang, G.; Pang, W. Adiponectin AS lncRNA inhibits adipogenesis by transferring from nucleus to cytoplasm and attenuating Adiponectin mRNA translation. Biochim. Biophys. Acta 2018, 1863, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chai, J.; Wang, Y.; Gu, Y.; Long, K.; Li, M.; Jin, L. LncPLAAT3-AS Regulates PLAAT3-Mediated Adipocyte Differentiation and Lipogenesis in Pigs through miR-503-5p. Genes 2023, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, J.; Zhu, S.; Du, Z.; Li, D.; Li, W.; Li, Z.; Tian, Y.; Kang, X.; Sun, G. MiRNAs and mRNAs Analysis during Abdominal Preadipocyte Differentiation in Chickens. Animals 2020, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R.; Nordstrm, K.J.V.; Stephansson, O.; Hgglund, M.G.A.; Schith, H.B. The solute carrier (SLC) complement of the human genome: Phylogenetic classification reveals four major families. FEBS Lett. 2008, 582, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific Organic Cation Transporters: Structure, Function, Physiological Roles, and Biopharmaceutical Implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, A.N.; Burckhardt, G. Organic Anion Transporters of the SLC22 Family: Biopharmaceutical, Physiological, and Pathological Roles. Pharm. Res. 2007, 24, 450–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.; Han, K.; Zhang, G.; Wang, J.; Xie, K.; Xue, Q. Genome-Wide Analysis of lncRNA and mRNA Expression During Differentiation of Abdominal Preadipocytes in the Chicken. G3 Genes Genomes Genet. 2017, 7, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Tianmu, Z.; Chunyan, W.; Shanshan, W.; Yuxiang, W.; Hui, L.; Ning, W.; Ouellette, M.M. Immortalization of chicken preadipocytes by retroviral transduction of chicken TERT and TR. PLoS ONE 2017, 12, e0177348. [Google Scholar]
- Li, X.; Wang, Y.; Li, J.; Mei, X.; Liu, Y.; Huang, H. qPCRtools: An R package for qPCR data processing and visualization. Front. Genet. 2022, 13, 1002704. [Google Scholar] [CrossRef]
- Wang, P.; Xue, Y.; Han, Y.; Lin, L.; Wu, C.; Xu, S.; Jiang, Z.; Xu, J.; Liu, Q.; Cao, X. The STAT3-Binding Long Noncoding RNA lnc-DC Controls Human Dendritic Cell Differentiation. Science 2014, 344, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Nakaue, H.S.; Hu, C.Y.; Mirosh, L.W. Effect of full feed and early feed restriction on broiler performance, abdominal fat level, cellularity, and fat metabolism in broiler chickens. Poult. Sci. 1995, 74, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, L.; Liu, S.; Zhang, Z.; Wang, X.; Lin, H. Propionate inhibits fat deposition via affecting feed intake and modulating gut microbiota in broilers. Poult. Sci. 2021, 100, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Nematbakhsh, S.; Pei Pei, C.; Selamat, J.; Nordin, N.; Idris, L.H.; Abdull Razis, A.F. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Singh, A.K.; Srivastava, S.; Upadhyay, V.; Sethi, A.; Siddiqui, S.; Trivedi, A.K. AIP4 regulates adipocyte differentiation by targeting C/EBPα for ubiquitin-mediated proteasomal degradation. J. Cell. Biochem. 2023, 124, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ivoa, H.; Stéphanie, L.; Tao, Y.; Lucía, R.-A.; Aurélie, N.; Guroflor, L.; Fatemeh, G.; Arne, K.; Bernard, J. Waves of sumoylation support transcription dynamics during adipocyte differentiation. Nucleic Acids Res. 2022, 3, 1351–1369. [Google Scholar]
- Yue, J.; Sun, C.; Tang, J.; Zhang, Q.; Lou, M.; Sun, H.; Zhang, L. Downregulation of miRNA-155-5p contributes to the adipogenic activity of 2-ethylhexyl diphenyl phosphate in 3T3-L1 preadipocytes. Toxicology 2023, 487, 153452. [Google Scholar] [CrossRef]
- Olcese, C.; Bartoloni, L. The sugar phosphate/phosphate exchanger family SLC37. Wiley Interdiplinary Rev. Membr. Transp. Signal. 2013, 2, 255–264. [Google Scholar] [CrossRef]
- Haitina, T. Function, Pharmacology, Evolution and Anatomical Localization of G Protein-Coupled Receptors and Solute Carriers. PhD Thesis, Uppsala University, Uppsala, Sweden, 2009. [Google Scholar]
- Aouida, M.; Poulin, R.; Ramotar, D. The Human Carnitine Transporter SLC22A16 Mediates High Affinity Uptake of the Anticancer Polyamine Analogue Bleomycin-A5. J. Biol. Chem. 2010, 285, 6275. [Google Scholar] [CrossRef]
- Wu, Y.; Hurren, R.; Maclean, N.; Gronda, M.; Schimmer, A.D. Carnitine transporter CT2 (SLC22A16) is over-expressed in acute myeloid leukemia (AML) and target knockdown reduces growth and viability of AML cells. Apoptosis 2015, 20, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, D.C.; Azad, P.; Ali, S.; Granados, J.C.; Haddad, G.G.; Nigam, S.K. Drosophila SLC22 Orthologs Related to OATs, OCTs, and OCTNs Regulate Development and Responsiveness to Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 2002. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Yue, X. SLC22A16 upregulation is an independent unfavorable prognostic indicator in gastric cancer. Future Oncology 2018, 14, 2139–2148. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Lane, D.J.R.; Bae, D.H.; Siafakas, A.R.; Rahmanto, Y.S.; Al-Akra, L.; Jansson, P.J.; Casero, R.A.; Richardson, D.R.; Biophys, B. Coupling of the polyamine and iron metabolism pathways in the regulation of proliferation: Mechanistic links to alterations in key polyamine biosynthetic and catabolic enzymes hhs public access author manuscript. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1864, 2793–2813. [Google Scholar] [CrossRef]
- Enomoto, A.; Wempe, M.F.; Tsuchida, H.; Shin, H.J.; Cha, S.H.; Anzai, N.; Goto, A.; Sakamoto, A.; Niwa, T.; Kanai, Y.; et al. Molecular identification of a novel carnitine transporter specific to human testis. Insights into the mechanism of carnitine recognition. J. Biol. Chem. 2002, 277, 36262–36271. [Google Scholar] [CrossRef]
- Zang, Y.; Li, J.; Wan, B.; Tai, Y. circRNA circ-CCND1 promotes the proliferation of laryngeal squamous cell carcinoma through elevating CCND1 expression via interacting with HuR and miR-646. J. Cell. Mol. Med. 2020, 24, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- González-Magaña, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, T.; Li, Z.; Sun, Z.; Wang, S.; Shen, H.; Hou, L.; Li, S.; Wei, Y.; Zhuo, B.; et al. TET2 is recruited by CREB to promote Cebpb, Cebpa, and Pparg transcription by facilitating hydroxymethylation during adipocyte differentiation. iScience 2023, 26, 108312. [Google Scholar] [CrossRef]
- Kamble, P.G.; Hetty, S.; Vranic, M.; Almby, K.; Castillejo-López, C.; Abalo, X.M.; Pereira, M.J.; Eriksson, J.W. Proof-of-concept for CRISPR/Cas9 gene editing in human preadipocytes: Deletion of FKBP5 and PPARG and effects on adipocyte differentiation and metabolism. Sci. Rep. 2020, 10, 10565. [Google Scholar] [CrossRef]
- Li, X.; Zeng, S.; Chen, L.; Zhang, Y.; Li, X.; Zhang, B.; Su, D.; Du, Q.; Zhang, J.; Wang, H.; et al. An intronic enhancer of Cebpa regulates adipocyte differentiation and adipose tissue development via long-range loop formation. Cell Prolif. 2024, 57, e13552. [Google Scholar] [CrossRef] [PubMed]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Grammatikakis, I.; Lal, A. Significance of lncRNA abundance to function. Mamm. Genome 2022, 33, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Taheriazam, A.; Orouei, S.; Fallah, S.; Sanaei, A.; Hejazi, E.S.; Kakavand, A.; Rezaei, S.; Heidari, H.; Behroozaghdam, M.; et al. LncRNA-miRNA axis in tumor progression and therapy response: An emphasis on molecular interactions and therapeutic interventions. Biomed. Pharmacother. 2022, 154, 113609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Hu, C.; Yi, H. Shiny transcriptional junk: lncRNA-derived peptides in cancers and immune responses. Life Sci. 2023, 316, 121434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, S.; He, Y.; Li, X.; Zhu, Y.; Lin, X.; Chen, L.; Zhao, Y.; Niu, L.; Zhang, S.; et al. LncRNA-Mediated Adipogenesis in Different Adipocytes. Int. J. Mol. Sci. 2022, 23, 7488. [Google Scholar] [CrossRef] [PubMed]
- Ru, W.; Zhang, S.; Liu, J.; Liu, W.; Huang, B.; Chen, H. Non-Coding RNAs and Adipogenesis. Int. J. Mol. Sci. 2023, 24, 9978. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.B.; Tsitsipatis, D.; Gorospe, M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell 2022, 82, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Martínez, H.N.; Recillas-Targa, F. Emerging Functions of lncRNA Loci beyond the Transcript Itself. Int. J. Mol. Sci. 2022, 23, 6258. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Winkler, L.; Jimenez, M.; Zimmer, J.T.; Williams, A.; Simon, M.D.; Dimitrova, N. Functional elements of the cis-regulatory lincRNA-p21. Cell Rep. 2022, 39, 110687. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cao, X.G.; Hu, J.; Li, J.; Wen, J. The role and possible mechanism of lncRNA AC092159.2 in modulating adipocyte differentiation. J. Mol. Endocrinol. 2019, 62, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, M.; Jian, W.; Song, C.; Chen, H. A novel lncRNA BADLNCR1 inhibits bovine adipogenesis by repressing GLRX5 expression. J. Cell. Mol. Med. 2020, 24, 7175–7186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).