Identifying Regions of the Genome Associated with Conception Rate to the First Service in Holstein Heifers Bred by Artificial Insemination and as Embryo Transfer Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Animals and Phenotype
2.2. DNA Extraction and Genotyping
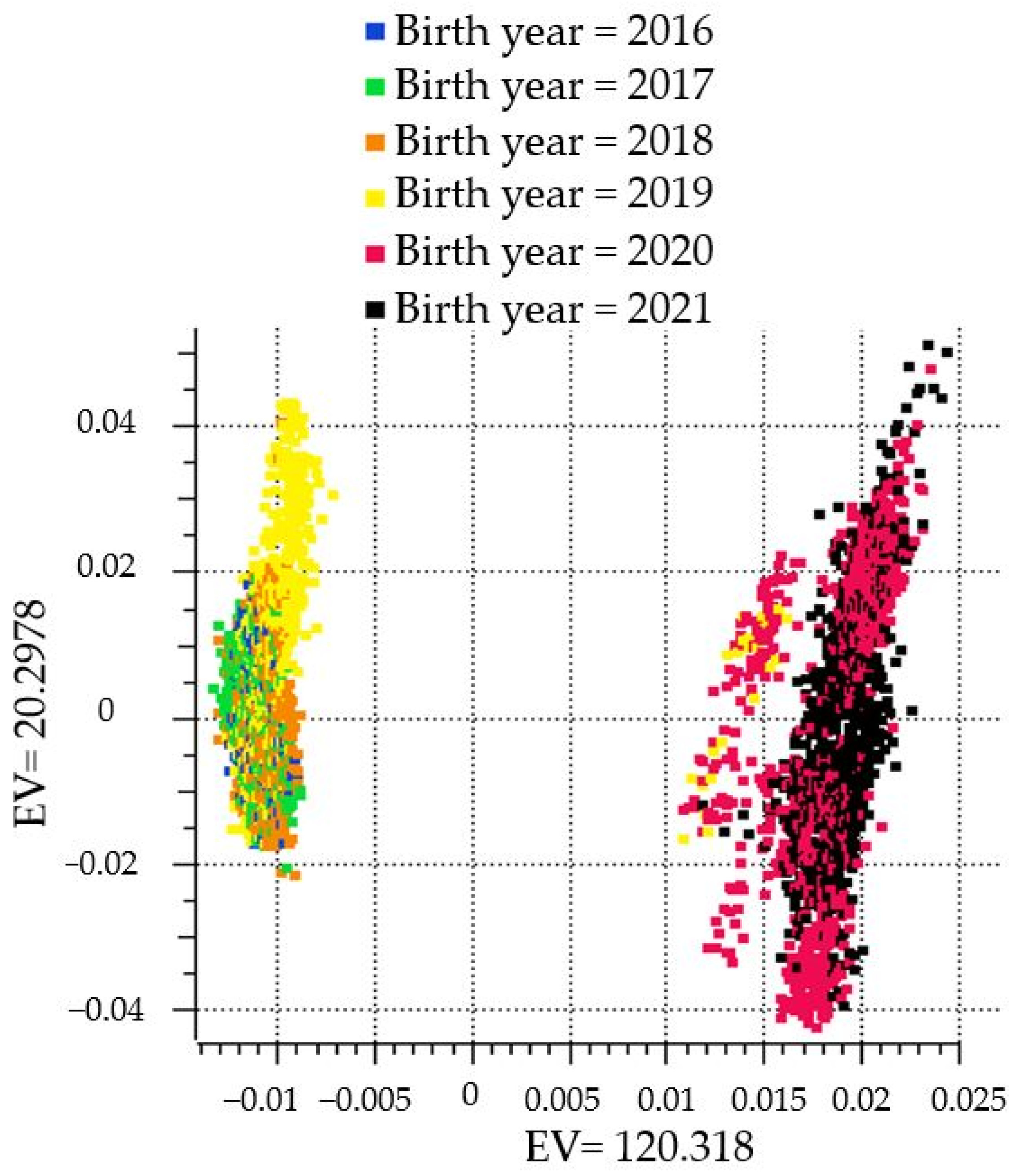
2.3. Quality Control
2.4. Genome-Wide Association Study (GWAS)
2.5. Gene Set Enrichment Analysis–Single Nucleotide Polymorphism
3. Results
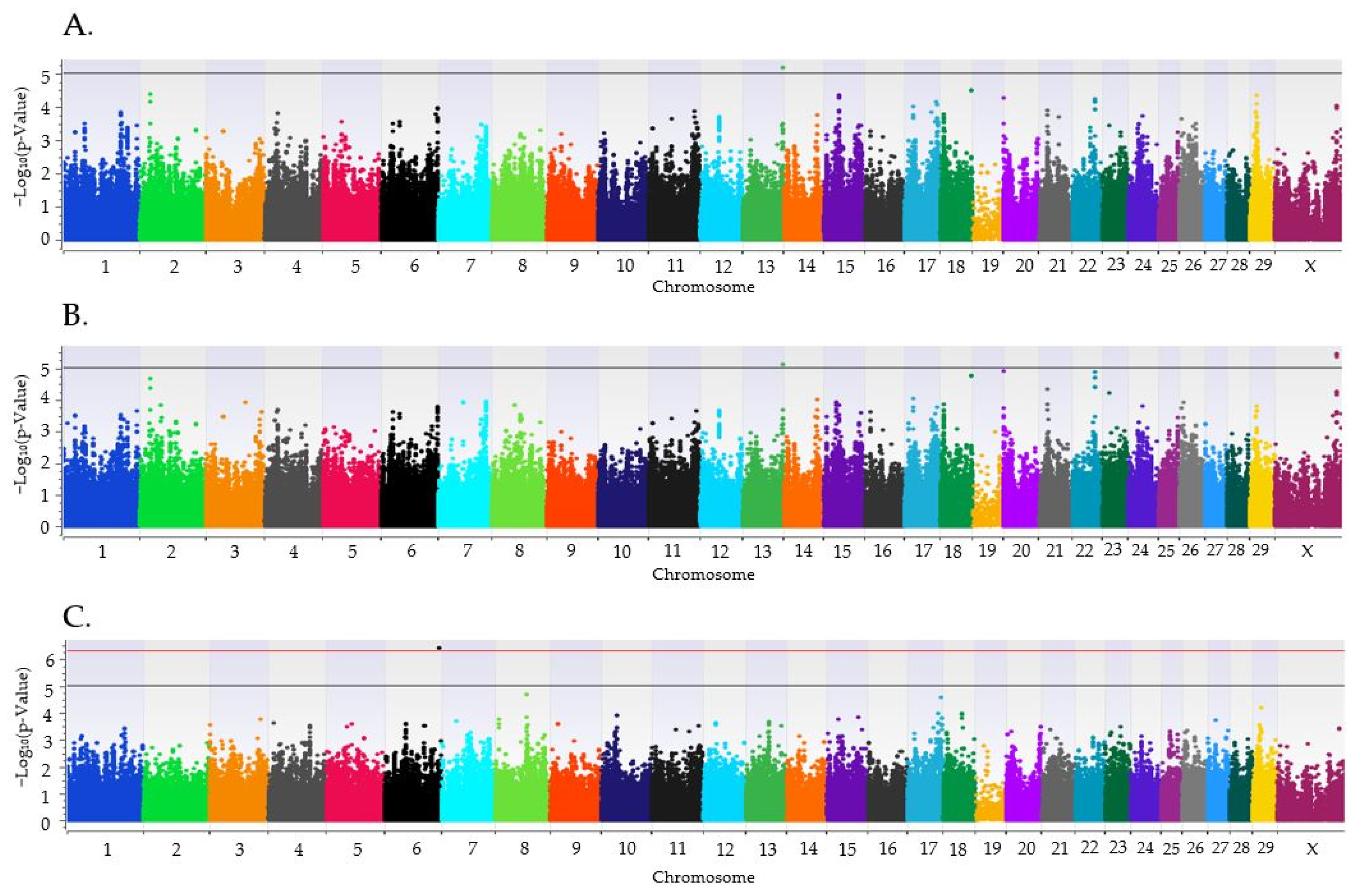
3.1. Genome-Wide Association Analysis
3.2. GSEA-SNP Results
4. Discussion
4.1. Comparison of Positional Candidate Genes with Other Studies
4.2. Comparison of Leading Edge Genes with Other Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari-Lari, M.; Mohebbi-Fani, M.; Rowshan-Ghasrodashti, A. Causes of culling in dairy cows and its relation to age at culling and interval from calving in Shiraz, Southern Iran. Vet. Res. Forum. 2012, 3, 233–237. [Google Scholar] [PubMed] [PubMed Central]
- Reece, W.O.; Erickson, H.H.; Goff, J.P.; Uemura, E.E. Dukes’ Physiology of Domestic Animals, 13th ed.; Wiley Blackwell.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Inskeep, E.K.; Dailey, R.A. Embryonic death in cattle. Vet. Clin. N. Am. Food Anim. Pract. 2005, 21, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Dunne, L.D.; Diskin, M.G.; Sreenan, J.M. Embryo and foetal loss in beef heifers between day 14 of gestation and full term. Anim. Reprod. Sci. 2000, 58, 39–44. Available online: https://www.sciencedirect.com/science/article/pii/S0378432099000883 (accessed on 7 August 2023). [CrossRef] [PubMed]
- Diskin, M.G.; Sreenan, J.M. Fertilization and embryonic mortality rates in beef heifers after artificial insemination. Reproduction 1980, 59, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Diskin, M.G.; Parr, M.H.; Morris, D.G. Embryo death in cattle: An update. Reprod. Fertil. Dev. 2012, 24, 244. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.T.; Franco, G.A.; Poole, R.K.; Hood, R.; Fernadez Montero, L.; Oliveira Filho, R.V.; Cooke, R.F.; Pohler, K.G. Pregnancy loss in beef cattle: A meta-analysis. Anim. Reprod. Sci. 2020, 212, 106251. [Google Scholar] [CrossRef] [PubMed]
- Albaaj, A.; Durocher, J.; LeBlanc, S.J.; Dufour, S. Meta-analysis of the incidence of pregnancy losses in dairy cows at different stages to 90 days of gestation. JDS Commun. 2022, 4, 144–148. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10039243/#:~:text=studies%20were%20included.-,Pregnancy%20losses%20averaged%2027%25%2C%2013%25%2C%207%25%2C,pregnancy%20losses%20in%20dairy%20cows (accessed on 15 August 2023). [CrossRef] [PubMed]
- Enting, H.; Kooij, D.; Dijkhuizen, A.A.; Huirne, R.B.M.; Noordhuizen-Stassen, E.N. Economic losses due to clinical lameness in dairy cattle. Livest. Prod. Sci. 1998, 49, 259–267. Available online: https://www.sciencedirect.com/science/article/pii/S0301622697000511 (accessed on 3 September 2023). [CrossRef]
- Norman, H.D.; Wright, J.R.; Hubbard, S.M.; Miller, R.H.; Hutchison, J.L. Reproductive status of Holstein and Jersey cows in the United States. J. Dairy Sci. 2010, 92, 3517–3528. Available online: https://www.sciencedirect.com/science/article/pii/S002203020970671X (accessed on 18 September 2023). [CrossRef] [PubMed]
- Chebel, R.C.; Santos, J.E.P.; Reynolds, J.P.; Cerri, R.L.A.; Juchem, S.O.; Overton, M. Factors affecting conception rate after artificial insemination and pregnancy loss in lactating dairy cows. Anim. Reprod. Sci. 2004, 84, 239–255. Available online: https://www.sciencedirect.com/science/article/pii/S0378432004000193 (accessed on 15 August 2023). [CrossRef]
- De Vries, A. Economic value of pregnancy in dairy cattle. J. Dairy Sci. 2006, 89, 3876–3885. [Google Scholar] [CrossRef]
- CDCB Cattle on Dairy Cattle Breeding. The Council of Dairy Cattle Breeding. 2024. Available online: https://webconnect.uscdcb.com/#/summary-stats/genetic-trend (accessed on 1 October 2023).
- CDCB Cattle on Dairy Cattle Breeding. Merit Selection Indices. 28 October 2022. Available online: https://uscdcb.com/merit-selection/ (accessed on 1 October 2023).
- Cole, J.B.; VanRaden, P. Symposium review: Possibilities in an age of genomics: The future of selection indices. J. Dairy Sci. 2017, 101, 3686–3701. Available online: https://www.sciencedirect.com/science/article/pii/S0022030217309694 (accessed on 18 September 2023). [CrossRef]
- Morrell, J.M. Artificial insemination: Current and future trends. In Artificial Insemination in Farm Animals; INTECH Open Access Publisher: London, UK, 2011. [Google Scholar]
- Selk, G. Embryonic Transfer in Cattle. Oklahoma Cooperative Extension Service, ANSI-3158. 2002. Available online: https://shareok.org/bitstream/handle/11244/49938/oksd_ansi_3158_2002-09.pdf?sequence=1 (accessed on 30 January 2024).
- Rutledge, J.J. Use of embryo transfer and IVF to bypass effects of heat stress. Theriogenology 2001, 55, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J. The incompletely fulfilled promise of embryo transfer in cattle-why aren’t pregnancy rates greater and what can we do about it? J. Anim. Sci. 2020, 98, skaa288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goossens, K.; Van Soom, A.; Van Poucke, M.; Vandaele, L.; Vandesompele, J.; Van Zeveren, A.; Peelman, L.J. Identification and expression analysis of genes associated with bovine blastocyst formation. BMC Dev. Biol. 2007, 7, 64. [Google Scholar] [CrossRef]
- Lee, Y.S.; Latham, K.; Sapienza, C.; Shore, S.K.; Litvin, J. Maternal Determinants of Oocyte and Embryo Quality. Ph.D. Dissertation, Temple University, Philadelphia, PA, USA, 2011. Available online: https://scholarshare.temple.edu/bitstream/handle/20.500.12613/577/Lee_temple_0225E_10588.pdf?sequence=1&isAllowed=y (accessed on 10 April 2024).
- Zhao, L.; Zheng, X.; Liu, J.; Zheng, R.; Yang, R.; Wang, Y.; Sun, L. PPAR signaling pathway in the first trimester placenta from in vitro fertilization and embryo transfer. Biomed. Pharmacother. 2019, 118, 109251. [Google Scholar] [CrossRef] [PubMed]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. Board invited review: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Lonergan, P.; Woods, A.; Fair, T.; Carter, F.; Rizos, D.; Ward, F.; Quinn, K.; Evans, A. Effect of embryo source and recipient progesterone environment on embryo development in cattle. Reprod. Fertil. Dev. 2007, 19, 861–868. [Google Scholar] [CrossRef]
- Mess, A.M.; Carreira, A.C.; Marinovic de Oliveira, C.; Fratini, P.; Favaron, P.O.; da Barreto, R.; Pfarrer, C.; Meirelles, F.V.; Miglino, M.A. Vascularization and VEGF expression altered in bovine yolk sacs from IVF and NT technologies. Theriogenology 2017, 87, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Browning, B.L.; Browning, S.R. Genotype imputation with millions of reference samples. Am. J. Hum. Genet. 2016, 98, 116–126. [Google Scholar] [CrossRef]
- Kang, H.M.; Sul, J.H.; Service, S.K.; Zaitlen, N.A.; Kong, S.; Freimer, N.B.; Sabatti, C.; Eskin, E. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 2010, 42, 348–354. [Google Scholar] [CrossRef] [PubMed]
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Galliou, J.M.; Kiser, J.N.; Oliver, K.F.; Seabury, C.M.; Moraes, J.G.N.; Burns, G.W.; Spencer, T.E.; Dalton, J.; Neibergs, H.L. Identification of Loci and Pathways Associated with Heifer Conception Rate in U.S. Holsteins. Genes 2020, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.M.; Clark, A.G. Linkage disequilibrium and the mapping of complex human traits. Trends Genet. 2002, 18, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Devlin, B.; Roeder, K. Genomic Control for Association Studies. Biometrics 1999, 55, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. Analysing biological pathways in genome-wide association studies. Nat. Rev. Genet. 2010, 11, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Charmpi, K.; Ycart, B. Weighted Kolmogorov Smirnov testing: An alternative for Gene Set Enrichment Analysis. Stat. Appl. Genet. Mol. Biol. 2015, 14, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Aulchenko, Y.S.; Ripke, S.; Isaacs, A.; van Duijn, C.M. Genabel: An R library for genome-wide association analysis. Bioinformatics 2007, 23, 1294–1296. [Google Scholar] [CrossRef]
- Karssen, L.C.; van Duijn, C.M.; Aulchenko, Y.S. The GenABEL Project for statistical genomics. F1000Research 2016, 5, 914. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neupane, M.; Geary, T.W.; Kiser, J.N.; Burns, G.W.; Hansen, P.J.; Spencer, T.E.; Neibergs, H.L. Loci and pathways associated with uterine capacity for pregnancy and fertility in beef cattle. PLoS ONE 2017, 12, e0188997. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.F.; Wahl, A.M.; Dick, M.; Toenges, J.A.; Kiser, J.N.; Galliou, J.M.; Moraes, J.G.N.; Burns, G.W.; Dalton, J.; Spencer, T.E.; et al. Genomic Analysis of Spontaneous Abortion in Holstein Heifers and Primiparous Cows. Genes 2019, 10, 954. [Google Scholar] [CrossRef]
- Tinkanen, H.; Bläuer, M.; Laippala, P.; Tuohimaa, P.; Kujansuu, E. Correlation between serum inhibin B and other indicators of the ovarian function. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 94, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Magon, N.; Agrawal, S.; Malik, S.; Babu, K. Growth hormone in the management of female infertility. Indian J. Endocrinol. Metab. 2011, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Mansouri-Attia, N.; Oliveira, L.J.; Forde, N.; Fahey, A.G.; Browne, J.A.; Roche, J.F.; Sandra, O.; Reinaud, P.; Lonergan, P.; Fair, T. Pivotal role for monocytes/macrophages and dendritic cells in maternal immune response to the developing embryo in cattle1. Biol. Reprod. 2012, 87, 121. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, U.; Eggert, A.O.; Kapp, M.; McLellan, A.D.; Geijtenbeek, T.B.H.; Dietl, J.; van Kooyk, Y.; Kämpgen, E. Unique appearance of proliferating antigen-presenting cells expressing DC-sign (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 2003, 162, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Nakashima, A.; Shima, T.; Zhou, X.; Saito, S. Toll-like receptor signaling in uterine natural killer cells—Role in embryonic loss. J. Reprod. Immunol. 2009, 83, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Demetrio, D.G.B.; Santos, R.M.; Demetrio, C.G.B.; Vasconcelos, J.L.M. Factors affecting conception rates following artificial insemination or embryo transfer in lactating Holstein cows. J. Dairy Sci. 2007, 90, 5073–5082. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Gümen, A.; Guenther, J.N.; Souza, A.H.; Caraviello, D.Z.; Wiltbank, M.C. Comparison of artificial insemination versus embryo transfer in lactating dairy cows. Theriogenology 2006, 65, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Ensembl, E.-E. Gene: AFAP1. Bos_taurus—Ensembl Genome Browser 111. 2024. Available online: http://useast.ensembl.org/Bos_taurus/Gene/Summary?db=core%3Bg (accessed on 14 March 2024).
- Zhang, S.; Zou, Y.; Tang, X.; Zhang, Y.; Yang, N.; Xu, K.; Xu, Y. Silencing of AFAP1-AS1 lncrna impairs cell proliferation and migration by epigenetically promoting DUSP5 expression in pre-eclampsia. J. Cell. Biochem. 2021, 122, 1506–1516. [Google Scholar] [CrossRef]
- Davenport, K.M.; Ortega, M.S.; Liu, H.; O’Neil, E.V.; Kelleher, A.M.; Warren, W.C.; Spencer, T.E. Single-nuclei RNA sequencing (snrna-seq) uncovers trophoblast cell types and lineages in the mature bovine placenta. Proc. Natl. Acad. Sci. USA 2023, 120, e2221526120. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.G.; Littlejohn, M.D.; Mitchell, M.D.; Roche, J.R.; Meier, S. Endometrial gene expression during early pregnancy differs between fertile and subfertile dairy cow strains. Physiol. Genom. 2012, 44, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Barreta, M.H.; Gasperin, B.G.; Ferreira, R.; Rovani, M.; Pereira, G.R.; Bohrer, R.C.; de Oliveira, J.F.; Goncalves, P.B. The components of the angiotensin-(1-7) system are differentially expressed during follicular wave in cattle. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Ferreira, R.; Gasperin, B.G.; Siqueira, L.C.; de Oliveira, J.F.; Santos, R.A.; Reis, A.M.; Goncalves, P.B. Molecular characterization and regulation of the angiotensin-converting enzyme type 2/angiotensin-(1-7)/MAS receptor axis during the ovulation process in cattle. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Valdes, G.; Neves, L.A.; Anton, L.; Corthorn, J.; Chacon, C.; Germain, A.M.; Merrill, D.C.; Ferrario, C.M.; Sarao, R.; Penninger, J.; et al. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 2006, 27, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Yagil, Y.; Bursztyn, M.; Barkalifa, R.; Scharf, S.; Yagil, C. ACE2 expression and activity are enhanced during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1953–R1961. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.A.; Stovall, K.; Joyner, J.; Valdes, G.; Gallagher, P.E.; Ferrario, C.M.; Merrill, D.C.; Brosnihan, K.B. ACE2 and ANG-(1-7) in the rat uterus during early and late gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R151–R161. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, M.S.; Strawn, W.B.; Groban, L.; Yamaleyeva, L.M.; Chappell, M.C.; Horta, C.; Atkins, K.; Firmes, L.; Gurley, S.B.; Brosnihan, K.B. Angiotensin-converting enzyme 2 deficiency is associated with impaired gestational weight gain and fetal growth restriction. Hypertension 2011, 58, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Azinheira Nobrega Cruz, N.; Stoll, D.; Casarini, D.E.; Bertagnolli, M. Role of ace2 in pregnancy and potential implications for COVID-19 susceptibility. Clin. Sci. 2021, 135, 1805–1824. [Google Scholar] [CrossRef]
- Ghadhanfar, E.; Alsalem, A.; Al-Kandari, S.; Naser, J.; Babiker, F.; Al-Bader, M. The role of ACE2, angiotensin-(1–7) and MAS1 receptor axis in glucocorticoid-induced intrauterine growth restriction. Reprod. Biol. Endocrinol. 2017, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Yamaleyeva, L.M.; Pulgar, V.M.; Lindsey, S.H.; Yamane, L.; Varagic, J.; McGee, C.; daSilva, M.; Lopes Bonfa, P.; Gurley, S.B.; Brosnihan, K.B. Uterine artery dysfunction in pregnant Ace2 knockout mice is associated with placental hypoxia and reduced umbilical blood flow velocity. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E84–E94. [Google Scholar] [CrossRef] [PubMed]
- Izadyar, F.; Colenbrander, B.; Bevers, M.M. In vitro maturation of bovine oocytes in the presence of growth hormone accelerates nuclear maturation and promotes subsequent embryonic development. Mol. Reprod. Dev. 1996, 45, 372–377. [Google Scholar] [CrossRef]
- Seifer, D.B.; Lambert-Messerlian, G.; Hogan, J.W.; Gárdiner, A.C.; Blazar, A.S.; Berk, C.A. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil. Steril. 1997, 67, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Vermeulen, N.; Mocanu, E.; Gianaroli, L.; Lundin, K.; Rautakallio-Hokkanen, S.; Tapanainen, J.S.; Veiga, A. SARS-CoV-2, fertility and assisted reproduction. Hum. Reprod. Update 2022, 29, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Weatherbee, B.A.; Glover, D.M.; Zernicka-Goetz, M. Expression of SARS-CoV-2 receptor ACE2 and the protease TMPRSS2 suggests susceptibility of the human embryo in the first trimester. Open Biol. 2020, 10, 200162. [Google Scholar] [CrossRef]
- Hull, K.; Harvey, S. Growth hormone: Roles in female reproduction. J. Endocrinol. 2001, 168, 1–23. [Google Scholar] [CrossRef]
- Poretsky, L.; Cataldo, N.A.; Rosenwaks, Z.; Giudice, L.C. The insulin-related ovarian regulatory system in health and disease. Endocr. Rev. 1999, 20, 535–582. [Google Scholar] [CrossRef]
- Hall, J.E.; Weltand, C.K.; Cramer, D.W. Inhibin A and inhibin B reflect ovarian function in assisted reproduction but are less useful at predicting outcome. Hum. Reprod. 1999, 14, 409–415. [Google Scholar] [CrossRef]
- Chauvet, V.; Qian, F.; Boute, N.; Cai, Y.; Phakdeekitacharoen, B.; Onuchic, L.F.; Attié-Bitach, T.; Guicharnaud, L.; Devuyst, O.; Germino, G.G.; et al. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am. J. Pathol. 2002, 160, 973–983. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, M.A.; Outeda, P.; Zhou, Q.; Zhou, F.; Menezes, L.F.; Qian, F.; Huso, D.L.; Germino, G.G.; Piontek, K.B.; Watnick, T. PKD1 and Pkd2 are required for normal placental development. PLoS ONE 2010, 5, e0012821. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, F.; Aflatoonian, R.; Javanmard, S.H.; Saifi, B.; Ashrafi, M.; Mehdizadeh, M. Apolipoprotein A1 as a novel anti-implantation biomarker in polycystic ovary syndrome: A case-control study. J. Res. Med. Sci. 2015, 20, 1039–1045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.; Wang, H.; Wang, W.; Zeng, S.; Zhong, Y.; Li, D.-J. Prevention of embryo loss in non-obese diabetic mice using adoptive ITGA2+ISG20+ natural killer-cell transfer. Reproduction 2009, 137, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Löb, S.; Amann, N.; Kuhn, C.; Schmoeckel, E.; Wöckel, A.; Zatizehni, A.; Kaltofen, T.; Keckstein, S.; Mumm, J.-N.; Meister, S.; et al. Interleukin-1 β is significantly upregulated in the decidua of spontaneous and recurrent miscarriage placentas. J. Reprod. Immunol. 2021, 144, 103283. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.; Das, S.K.; Paria, B.C.; Lim, H.; Song, H.; Matsumoto, H.; Knudtson, K.L.; DuBois, R.N.; Dey, S.K. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J. Biol. Chem. 2001, 276, 44137–44145. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Bose, H.S. Early steps in steroidogenesis: Intracellular Cholesterol trafficking. J. Lipid Res. 2011, 52, 2111–2135. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, D.; Ferreira-Dias, G.; Okuda, K. Regulation of luteal function and corpus luteum regression in cows: Hormonal control, immune mechanisms and intercellular communication. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 57–65. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Yu, W.; Yang, Y.; Wang, F.; Yu, X.; Wu, H.; Ma, Y.; Cao, B.; Wang, Y.-L. The mystery of the life tree: The Placentas. Biol. Reprod. 2022, 107, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; López-Martín, S.; Monguió-Tortajada, M.; Borràs, F.E.; Yáñez-Mó, M.; Ramírez, M.Á. Bovine endometrial MSC: Mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation. Stem Cell Res. Ther. 2019, 10, 23. [Google Scholar] [CrossRef]
- Hugo, H.; Ackland, M.L.; Blick, T.; Lawrence, M.G.; Clements, J.A.; Williams, E.D.; Thompson, E.W. Epithelial—Mesenchymal and mesenchymal—Epithelial transitions in carcinoma progression. J. Cell. Physiol. 2007, 213, 374–383. [Google Scholar] [CrossRef]
- Brosens, J.J.; Hodgetts, A.; Feroze-Zaidi, F.; Sherwin, J.R.; Fusi, L.; Salker, M.S.; Higham, J.; Rose, G.L.; Kajihara, T.; Young, S.L.; et al. Proteomic analysis of endometrium from fertile and infertile patients suggests a role for apolipoprotein A-I in embryo implantation failure and endometriosis. Mol. Hum. Reprod. 2009, 16, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O.; Stein, T.P.; Steer, R.A.; Williams, K.P. Maternal Circulating Lipid Profile during Early Pregnancy: Racial/Ethnic Differences and Association with Spontaneous Preterm Delivery. Nutrients 2017, 9, 19. [Google Scholar] [CrossRef]
- Rødgaard, T.; Heegaard, P.M.H.; Callesen, H. Non-invasive assessment of in-vitro embryo quality to improve transfer success. Reprod. BioMed. Online 2015, 31, 585–592. [Google Scholar] [CrossRef]
- Verma, P.; Nair, R.R.; Singh, S.; Rajender, S.; Khanna, A.; Jha, R.K.; Singh, K. High Level of APOA1 in Blood and Maternal Fetal Interface Is Associated with Early Miscarriage. Reprod. Sci. 2019, 26, 649–656. [Google Scholar] [CrossRef]
- Guzel, E.; Basar, M.; Ocak, N.; Arici, A.; Kayisli, U.A. Bidirectional interaction between unfolded-protein-response key protein HSPA5 and estrogen signaling in human endometrium1. Biol. Reprod. 2011, 85, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Koshi, K.; Suzuki, Y.; Nakaya, Y.; Imai, K.; Hosoe, M.; Takahashi, T.; Kizaki, K.; Miyazawa, T.; Hashizume, K. Bovine trophoblastic cell differentiation and binucleation involves enhanced endogenous retrovirus element expression. Reprod. Biol. Endocrinol. 2012, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Wooding, F.B.P. The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta 1992, 13, 101–113. [Google Scholar] [CrossRef]
- Jee, B.; Dhar, R.; Singh, S.; Karmakar, S. Heat shock proteins and their role in pregnancy: Redefining the function of “Old rum in a new bottle”. Front. Cell Dev. Biol. 2021, 9, 648463. [Google Scholar] [CrossRef]
- Löb, S.; Ochmann, B.; Ma, Z.; Vilsmaier, T.; Kuhn, C.; Schmoeckel, E.; Herbert, S.-L.; Kolben, T.; Wöckel, A.; Mahner, S.; et al. The role of interleukin-18 in recurrent early pregnancy loss. J. Reprod. Immunol. 2021, 148, 103432. [Google Scholar] [CrossRef]
- Yoshino, O.; Osuga, Y.; Yano, T.; Koga, K.; Tsutsumi, O.; Taketani, Y. Evidence for the presence of interleukin (IL)-18, IL-18 receptor and IL-18 binding protein expression in the human endometrium. Fertil. Steril. 2001, 76, S63. [Google Scholar] [CrossRef]
- Lédée-Bataille, N.; Dubanchet, S.; Coulomb-L’hermine, A.; Durand-Gasselin, I.; Frydman, R.; Chaouat, G. A new role for Natural Killer Cells, interleukin (IL)-12, and il-18 in repeated implantation failure after in vitro fertilization. Fertil. Steril. 2004, 81, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Haufe, T.; Scholz, C.; Kuhn, C.; Friese, K.; Karamouti, M.; Makrigiannakis, A.; Jeschke, U. Placental interleukin-15 expression in recurrent miscarriage. Am. J. Reprod. Immunol. 2010, 64, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Searle, R.F.; Robson, S.C.; Innes, B.A.; Bulmer, J.N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Okun, E.; Griffioen, K.J.; Gen Son, T.; Lee, J.; Roberts, N.J.; Mughal, M.R.; Hutchison, E.; Cheng, A.; Arumugam, T.V.; Lathia, J.D.; et al. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J. Neurochem. 2010, 114, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Polei, M.; Günther, J.; Koczan, D.; Fürbass, R. Gene expression profiles of bovine uninucleate trophoblast cells and trophoblast giant cells: A data note. BMC Res. Notes 2020, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Senger, P.L. Pathways to Pregnancy & Parturition; Current Conceptions: Redmond, OR, USA, 2012. [Google Scholar]
- Su, Y.; Li, Q.; Zhang, Q.; Li, Z.; Yao, X.; Guo, Y.; Xiao, L.; Wang, X.; Ni, H. Exosomes derived from placental trophoblast cells regulate endometrial epithelial receptivity in dairy cows during pregnancy. J. Reprod. Dev. 2022, 68, 21–29. [Google Scholar] [CrossRef]
- Wooding, F.B. The ruminant placental trophoblast binucleate cell: An evolutionary breakthrough. Biol. Reprod. 2022, 107, 705–716. [Google Scholar] [CrossRef]
- Abdollahi-Arpanahi, R.; Carvalho, M.R.; Ribeiro, E.S.; Peñagaricano, F. Association of lipid-related genes implicated in conceptus elongation with female fertility traits in dairy cattle. J. Dairy Sci. 2019, 102, 10020–10029. [Google Scholar] [CrossRef]
- Mullen, M.P.; Forde, N.; Parr, M.H.; Diskin, M.G.; Morris, D.G.; Nally, J.E.; Evans, A.C.; Crowe, M.A. Alterations in systemic concentrations of progesterone during the early luteal phase affect RBP4 expression in the bovine uterus. Reprod. Fertil. Dev. 2012, 24, 715. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Greco, L.F.; Bisinotto, R.S.; Lima, F.S.; Thatcher, W.W.; Santos, J.E. Biology of preimplantation conceptus at the onset of elongation in Dairy Cows1. Biol. Reprod. 2016, 94, 97. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.S. Symposium review: Lipids as regulators of conceptus development: Implications for metabolic regulation of reproduction in dairy cattle. J. Dairy Sci. 2018, 101, 3630–3641. [Google Scholar] [CrossRef]
- Ingman, W.V.; Robertson, S.A. Defining the actions of transforming growth factor β in reproduction. BioEssays 2002, 24, 904–914. [Google Scholar] [CrossRef]
- Monsivais, D.; Matzuk, M.M.; Pangas, S.A. The TGF-β family in the reproductive tract. Cold Spring Harb. Perspect. Biol. 2017, 9, a022251. [Google Scholar] [CrossRef]
- Kallapur, S.; Ormsby, I.; Doetschman, T. Strain dependency of TGF?1 function during embryogenesis. Mol. Reprod. Dev. 1999, 52, 341–349. [Google Scholar] [CrossRef]
- Ingman, W.V.; Robertson, S.A. The essential roles of TGFB1 in reproduction. Cytokine Growth Factor Rev. 2009, 20, 233–239. [Google Scholar] [CrossRef]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef] [PubMed]
- Akanno, E.C.; Plastow, G.; Fitzsimmons, C.; Miller, S.P.; Baron, V.; Ominski, K.; Basarab, J.A. Genome-wide association for heifer reproduction and calf performance traits in beef cattle. Genome 2015, 58, 549–557. [Google Scholar] [CrossRef]
- Blaschek, M.; Kaya, A.; Zwald, N.; Memili, E.; Kirkpatrick, B.W. A whole-genome assocation analysis of noncompensatory fertility in Holstein bulls. J. Dairy Sci. 2011, 94, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Cochran, S.D.; Cole, J.B.; Null, D.J.; Hansen, P.J. Discovery of single nucleotide polymorphisms in candidate genes associated with fertility and production traits in Holstein cattle. BMC Genet. 2013, 14, 49. [Google Scholar] [CrossRef]
- Cole, J.B.; Wiggans, G.R.; Ma, L.; Sonstegard, T.S.; Lawlor, T.J., Jr.; Crooker, B.A.; Van Tassell, C.P.; Yang, J.; Wang, S.; Matukumalli, L.; et al. Genome-wide association analysis of thirty one production, health, and reproduction and body conformation traits in contemporary US Holstein cows. BMC Genom. 2011, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; VanRaden, P.M.; Null, D.J.; Hutchison, J.L.; Hubbard, S.M. Haplotype Tests for Economically Important Traits of Dairy Cattle. AIP RESEARCH REPORT GENOMIC5 (12-20). 2020. Available online: https://www.ars.usda.gov/ARSUserFiles/80420530/Publications/ARR/Haplotype%20tests_ARR-Genomic5.pdf (accessed on 1 February 2024).
- Guillaume, F.; Gautier, M.; Ben Jemaa, S.; Fritz, S.; Eggen, A.; Boichard, D.; Druet, T. Refinement of two female fertility qtl using alternative phenotypes in French Holstein Dairy Cattle. Anim. Genet. 2007, 38, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Häfliger, I.M.; Spengeler, M.; Seefried, F.R.; Drögemüller, C. Four novel candidate causal variants for deficient homozygous haplotypes in Holstein cattle. Sci. Rep. 2022, 12, 5435. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.K.; Guldbrandsten, B.; Su, G.; Thomsen, B.; Lund, M.S. Genome scan detects quantitative trait loci affecting female fertility traits in Danish and Swedish Holstein cattle. J. Dairy Sci. 2009, 92, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.K.; Sahana, G.; Guldbrandtsen, B.; Lund, M.S. Validation of associations for female fertility traits in Nordic Holstein, Nordic Red, and Jersey dairy cattle. BMC Genet. 2014, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.K.; Buitenhuis, B.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Genome-wide association study for female fertility in Nordic Red cattle. BMC Genet. 2015, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kirkpatrick, B.W.; Rosa, G.J.M.; Khatib, H. A genome-wide assocation study using selective DNA pooling identifies candidate markers for feritliy in Holstein cattle. Anim. Genet. 2010, 41, 570–578. [Google Scholar] [CrossRef]
- Iso-Touru, T.; Sahana, G.; Guldbrandtsen, B.; Lund, M.S.; Vikki, J. Genome-wide association analysis of milk yield traits in Nordic Red cattle using imputed whole genome sequence variants. BMC Genet. 2016, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, Y.; Sahana, G.; Zhang, Q.; Liu, L.; Lund, M.S.; Su, G. Genome-wide association studies for female fertility traits in Chinese and Nordic Holsteins. Sci. Rep. 2017, 7, 8487. [Google Scholar] [CrossRef]
- Minozzi, G.; Nicolazzi, E.L.; Stella, A.; Biffani, S.; Negrini, R.; Lazzari, B.; Ajmone-Marsan, P.; Williams, J.L. Genome wide analysis of fertility and production traits in Italian Holstein cattle. PLoS ONE 2013, 8, e80219. [Google Scholar] [CrossRef]
- Minten, M.A.; Bilby, T.R.; Bruno, R.G.S.; Allen, C.C.; Madsen, C.A.; Wang, Z.; Sawyer, J.E.; Tibary, A.; Neibergs, H.L.; Geary, T.W.; et al. Effects of fertility on gene expression and function of the bovine endometrium. PLoS ONE 2013, 8, e69444. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.G.; Behura, S.K.; Geary, T.W.; Hansen, P.J.; Neibergs, H.L.; Spencer, T.E. Uterine influences on conceptus development in fertility-classified animals. Proc. Natl. Acad. Sci. USA 2018, 115, E1749–E1758. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.P.; Rothammer, S.; Seichter, D.; Russ, I.; Hinrichs, D.; Tetens, J.; Thaller, G.; Medugorac, I. Genome-wide mapping of 10 calving and fertility traits in Holstein dairy cattle with special regard to chromosome 18. J. Dairy Sci. 2017, 100, 1987–2006. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Sargolzaei, M.; Abo-Ismail, M.K.; May, N.; Miller, S.P.; Schenkel, F.; Moore, S.S.; Stothard, P. Genome-wide assocation for milk production and female fertility traits in Canadian dairy Holstein cattle. BMC Genet. 2016, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.S.; Denicol, A.C.; Cole, J.B.; Null, D.J.; Hansen, P.J. Use of single nucleotide polymorphisms in candidate genes associated with daughter pregnancy rate for prediction of genetic merit for reprodiction in Holstein cows. Anim. Genet. 2016, 47, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.S.; Denicol, A.C.; Cole, J.B.; Null, D.J.; Taylor, J.F.; Schnabel, R.D.; Hansen, P.J. Association of single nucleotide polymorphisms in candidate genes previously related to genetic variation in fertility with phenotypic measurements of reproductive function in Holstein cows. J. Dairy Sci. 2017, 100, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Sahana, G.; Guldbrandtsen, B.; Bendixen, C.; Lund, M.S. Genome-wide association mapping for female fertility traits in Danish and Swedish Holstein cattle. Anim. Genet. 2010, 41, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Mesbah-Uddin, M.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Haplotypes responsible for early embryonic lethality detected in Nordic Holsteins. J. Dairy Sci. 2019, 102, 11116–11123. [Google Scholar] [CrossRef]
| Breeding Type | No. Pregnant to the First Service | No. Not Pregnant to the First Service | Total |
|---|---|---|---|
| Artificial insemination | 1575 | 1254 | 2829 |
| Embryo Transfer Recipients | 988 | 1098 | 2086 |
| BTA 1 | Position (bp) 2 | p-Value 3 | SNP 4 | Minor Allele | Freq. Cases 5 | Freq. Controls |
|---|---|---|---|---|---|---|
| 6 | 114,186,062 | 3.81 × 10−7 | rs136769131 | A | 0.379 | 0.433 |
| 13 | 83,298,964 | 6.63 × 10−6 | rs43097654 | T | 0.050 | 0.027 |
| X | 127,944,565 | 2.47 × 10−6 | rs133237751 | T | 0.392 | 0.373 |
| X | 127,962,670 | 1.78 × 10−6 | rs110097934 | A | 0.391 | 0.373 |
| X | 127,976,137 | 1.78 × 10−6 | rs110705866 | T | 0.475 | 0.409 |
| Gene Set (Database ID) 1 | # Genes 2 (# LEG) 3 | NES 4 | Leading Edge Genes 5 |
|---|---|---|---|
| Heifer Conception Rate to the First AI Service | |||
| Sodium Ion Transmembrane Transporter Activity (GO:0015081) | 25 (13) | 3.0 | SCN4B, ATP1B3, SLC1A1, SCNN1A, SLC6A3, PKD2, ATP1A1, SLC24A1, SLC6A17, SLC6A15, SLC8A1, SLC6A13, SLC6A6 |
| Heifer Conception Rate to the First ET Service | |||
| Regulation of Protein Transport (GO:0051223) | 121 (49) | 3.4 | F2RL1, F2R, DRD2, KDELR1, KDELR2, IL5, HSPA5, HMGN3, PDE2A, PIK3R1, GCG, TGFB2, TRIP6, FIS1, GAPVD1, ZPR1, TLR2, OAZ1, DNM1L, YWHAB, CASP4, TLR10, TLR6, CDK5, CABP1, PKD2, CYLD, CLEC6A, SLC51B, PBLD, TGFBR1, ASPH, ARRB1, IL18, NOD2, GPLD1, SIRT4, ANG, PKIA, ANKRD1, PRDX1, IL1B, DMTN, SERGEF, APOA1, IFNG, TGFB1, RBM22, APOA2 |
| Regulation of Establishment of Protein Localization (GO:0070201) | 125 (53) | 3.23 | F2RL1, F2R, DRD2, KDELR1, KDELR2, IL5, HSPA5, HMGN3, PDE2A, PIK3R1, GCG, TGFB2, TRIP6, FIS1, GAPVD1, ZPR1, TLR2, OAZ1, DNM1L, YWHAB, CASP4, TLR10, TLR6, CDK5, CABP1, PKD2, CYLD, CLEC6A, SLC51B, PBLD, TGFBR1, ASPH, ARRB1, IL18, NOD2, GPLD1, SIRT4, ANG, PKIA, ANKRD1, PRDX1, IL1B, DMTN, SERGEF, APOA1, IFNG, TGFB1, RBM22, APOA2, ANXA4, APOD, SNAP25, RBP4 |
| Liposaccharide Metabolic Process and Glycolipid Metabolic Process (GO:1903509) | 18 (5) | 3.05 | DPM3, ST6GALNAC6, BAX, PIGY, GPLD1 |
| Regulation of Protein Secretion (GO:0050708) | 69 (35) | 3.03 | F2RL1, F2R, DRD2, IL5, HMGN3, GCG, TGFB2, TLR2, DNM1L, CASP4, TLR10, TLR6, CLEC6A, ARRB1, NOD2, GPLD1, SIRT4, ANG, ANKRD1, IL1B, SERGEF, APOA1, IFNG, TGFB1, APOA2, ANXA4, RBP4, FKBP1B, IL10, IL1A, NR1H3, IFNAR1, GHRH, GHRL, INHBB, CHGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelson, V.C.; Kiser, J.N.; Davenport, K.M.; Suarez, E.M.; Murdoch, B.M.; Neibergs, H.L. Identifying Regions of the Genome Associated with Conception Rate to the First Service in Holstein Heifers Bred by Artificial Insemination and as Embryo Transfer Recipients. Genes 2024, 15, 765. https://doi.org/10.3390/genes15060765
Kelson VC, Kiser JN, Davenport KM, Suarez EM, Murdoch BM, Neibergs HL. Identifying Regions of the Genome Associated with Conception Rate to the First Service in Holstein Heifers Bred by Artificial Insemination and as Embryo Transfer Recipients. Genes. 2024; 15(6):765. https://doi.org/10.3390/genes15060765
Chicago/Turabian StyleKelson, Victoria C., Jennifer N. Kiser, Kimberly M. Davenport, Emaly M. Suarez, Brenda M. Murdoch, and Holly L. Neibergs. 2024. "Identifying Regions of the Genome Associated with Conception Rate to the First Service in Holstein Heifers Bred by Artificial Insemination and as Embryo Transfer Recipients" Genes 15, no. 6: 765. https://doi.org/10.3390/genes15060765
APA StyleKelson, V. C., Kiser, J. N., Davenport, K. M., Suarez, E. M., Murdoch, B. M., & Neibergs, H. L. (2024). Identifying Regions of the Genome Associated with Conception Rate to the First Service in Holstein Heifers Bred by Artificial Insemination and as Embryo Transfer Recipients. Genes, 15(6), 765. https://doi.org/10.3390/genes15060765





