ROS-Induced Autophagy of Skeletal Muscle Confers Resistance of Rice Flower Carp (Cyprinus carpio) to Short-Term Fasting
Abstract
:1. Introduction
2. Methods and Materials
2.1. Fish Sampling and Tissue Preparation
2.2. RNA Extraction and Complementary DNA Synthesis
2.3. Quantitative Real-Time PCR (qPCR)
2.4. ROS, MDA, and Enzyme Activity
2.5. Transmission Electron Microscopy (TEM)
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. ROS Content of Muscle after Fasting
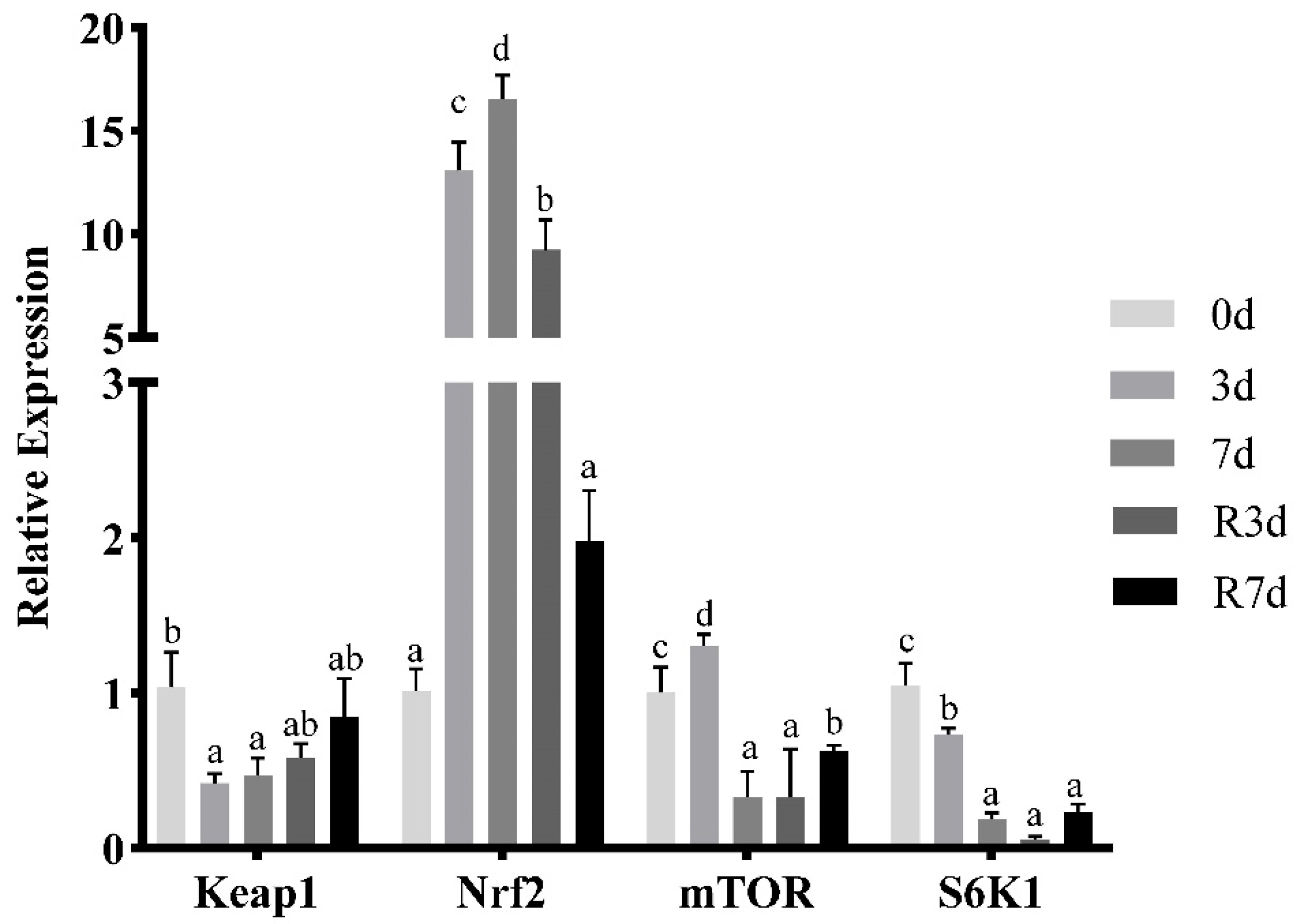
3.2. mRNA Expression of Genes Linked to Antioxidant-Related Signaling in Rice Flower Carp Muscles after Fasting
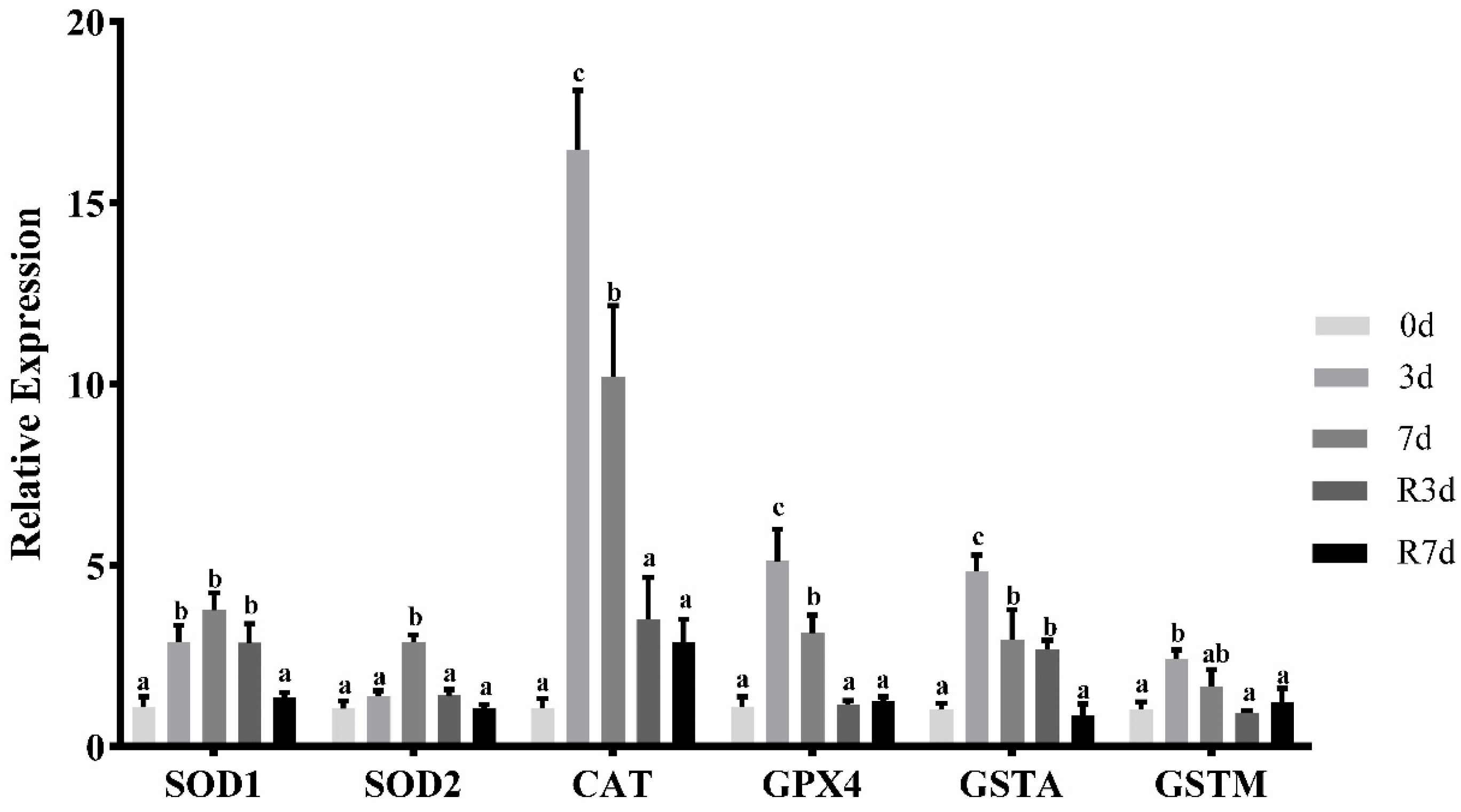
3.3. Antioxidant Parameters after Fasting in Rice Flower Carp Muscles
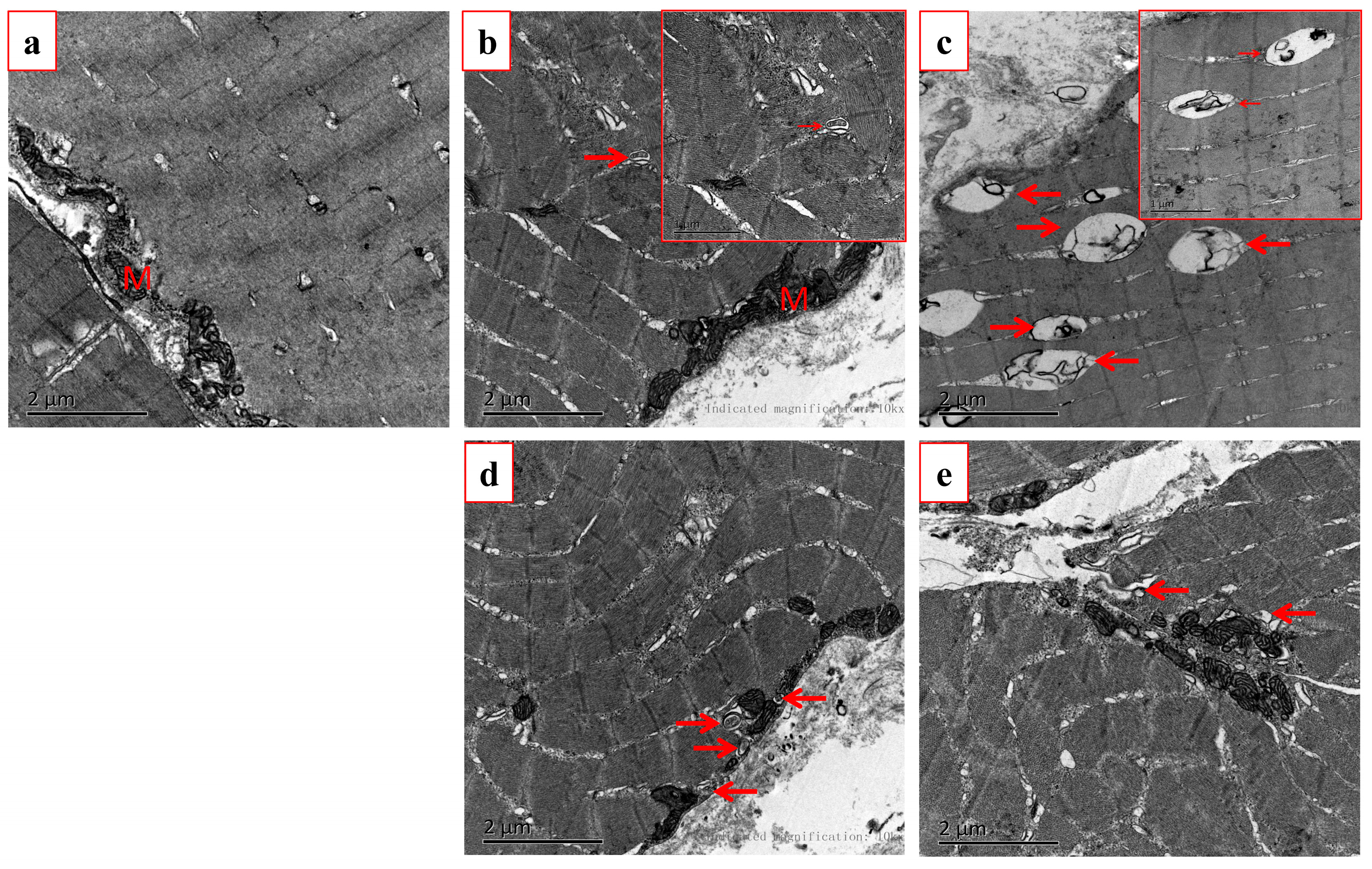
3.4. Effects of Fasting on Autophagosome Formation in Rice Flower Carp Muscle
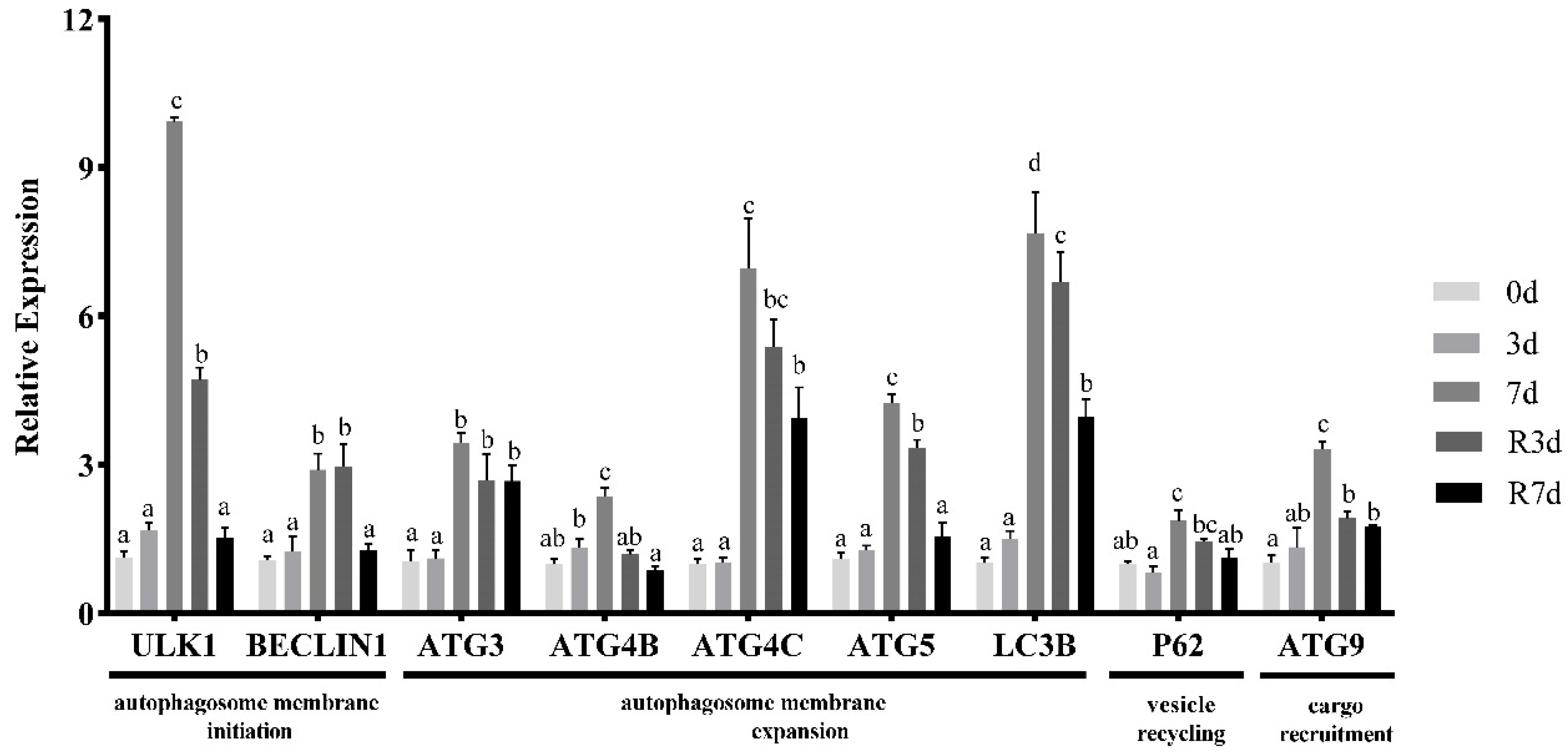
3.5. mRNA Expression of Autophagy-Related Genes
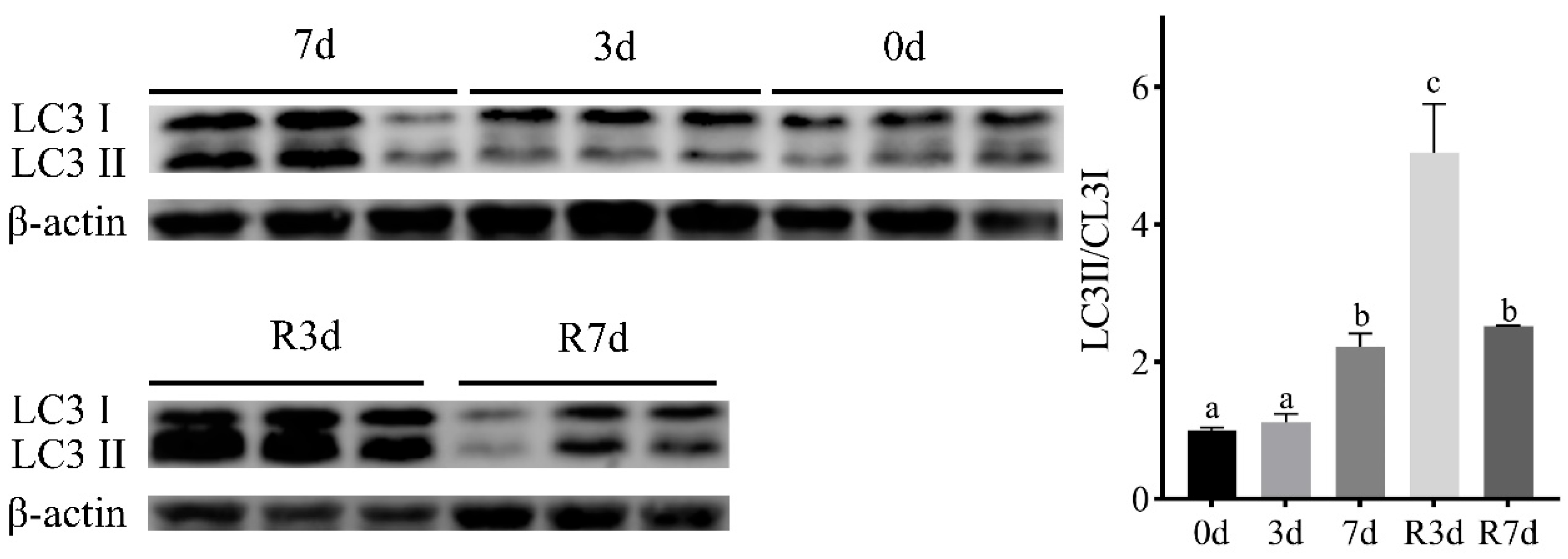
3.6. Conversion of LC3II during Fasting and Refeeding
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Caruso, G.; Maricchiolo, G.; Micale, V.; Genovese, L.; Caruso, R.; Denaro, M.G. Physiological responses to starvation in the European eel (Anguilla anguilla): Effects on haematological, biochemical, non-specific immune parameters and skin structures. Fish Physiol. Biochem. 2010, 36, 71–83. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Bakke-McKellep, A.M. Fasting and refeeding cause rapid changes in intestinal tissue mass and digestive enzyme capacities of Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 450–460. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Cardenete, G.; Hidalgo, M.C.; García-Alcázar, A.; Abellán, E.; Morales, A.E. Metabolic adjustments of Dentex dentex to prolonged starvation and refeeding. Fish Physiol. Biochem. 2012, 38, 1145–1157. [Google Scholar] [CrossRef]
- Mehner, T.; Wieser, W. Energetics and metabolic correlates of starvation in juvenile perch (Perca fluviatilis). J. Fish Biol. 2010, 45, 325–333. [Google Scholar] [CrossRef]
- Preez, H.H.D.; Strydom, W.; Winter, P.E.D. Oxygen consumption of two marine teleosts, Lithognathus mormyrus (linnaeus, 1758) and Lihognathus lithognathus (cuvier, 1830) (Teleosti: Sparidae). Comp. Biochem. Physiol. Part A Physiol. 1986, 85, 313–331. [Google Scholar] [CrossRef]
- Sohal, R.S.; Allen, R.G. Oxidative stress as a causal factor in differentiation and aging: A unifying hypothesis. Exp. Gerontol. 1990, 25, 499–522. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: A personal view. Nutr. Rev. 1994, 52, 253–265. [Google Scholar] [CrossRef]
- Morales, A.E.; Pérez-Jiménez, A.; Hidalgo, M.C.; Abellán, E.; Cardenete, G. Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 139, 153–161. [Google Scholar] [CrossRef]
- Guan, L.; Zhang, L.; Gong, Z.C.; Hou, X.N.; Xu, Y.X.; Feng, X.H.; Wang, H.Y.; You, H. FoxO3 inactivation promotes human cholangiocarcinoma tumorigenesis and chemoresistance through Keap1-Nrf2 signaling. Hepatology 2016, 63, 1914–1927. [Google Scholar] [CrossRef]
- Rahman, M.; Mofarrahi, M.; Kristof, A.S.; Nkengfac, B.; Harel, S.; Hussain, S.N. Reactive oxygen species regulation of autophagy in skeletal muscles. Antioxid. Redox Signal. 2014, 20, 443–459. [Google Scholar] [CrossRef]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef]
- Eisenberg-Lerner, A.; Bialik, S.; Simon, H.U.; Kimchi, A. Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009, 16, 966–975. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abeliovich, H.; Agostinis, P.; Agrawal, D.K.; Aliev, G.; Askew, D.S.; Paglin, S. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008, 4, 151–175. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Yabu, T.; Imamura, S.; Mizusawa, N.; Touhata, K.; Yamashita, M. Induction of autophagy by amino acid starvation in fish cells. Mar. Biotechnol. 2012, 14, 491–501. [Google Scholar] [CrossRef]
- Xia, G.; Zhu, T.; Li, X.; Jin, Y.; Zhou, J.; Xiao, J. ROS-mediated autophagy through the AMPK signaling pathway protects INS-1 cells from human islet amyloid polypeptide-induced cytotoxicity. Mol. Med. Rep. 2018, 18, 2744–2752. [Google Scholar] [CrossRef]
- Yang, M.; Chuan, Y.; Guo, C.; Liao, J.; Xu, Y.; Mei, X.; Liu, Y.X.; Huang, H.C.; He, X.H.; Zhu, S.S. Panax notoginseng root cell death caused by the autotoxic ginsenoside Rg1 is due to over-accumulation of ROS, as revealed by transcriptomic and cellular approaches. Front. Plant Sci. 2018, 9, 264. [Google Scholar] [CrossRef]
- Periasamy, M.; Herrera, J.L.; Reis, F.C. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab. J. 2017, 41, 327–336. [Google Scholar] [CrossRef]
- Robinson, M.K.; Rustum, R.R.; Chambers, E.A.; Rounds, J.D.; Wilmore, D.W.; Danny, O.; Jacobs, M.D. Starvation enhances hepatic free radical release following endotoxemia. J. Surg. Res. 1997, 69, 325–330. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2019, 38, e101812. [Google Scholar] [CrossRef]
- Hosoki, A.; Yonekura, S.I.; Zhao, Q.L.; Wei, Z.L.; Takasaki, I.; Tabuchi, Y.; Wang, L.L.; Hasuike, S.; Nomura, T.; Tachibana, A.; et al. Mitochondria-targeted superoxide dismutase (SOD2) regulates radiation resistance and radiation stress response in HeLa cells. J. Radiat. Res. 2012, 53, 58–71. [Google Scholar] [CrossRef]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, X.; Chen, Y.; Dong, X.; Luo, S.; Liu, H.; Zhang, D. Effects of L-carnitine against H2O2-induced oxidative stress in grass carp ovary cells (Ctenopharyngodon idellus). Fish Physiol. Biochem. 2016, 42, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, H.; Ming, L. Multiple roles of autophagy in the sorafenib resistance of hepatocellular carcinoma. Cell. Physiol. Biochem. 2017, 44, 716–727. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological functions of autophagy genes: A disease perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Fan, X.; Hou, T.; Zhang, S.; Guan, Y.; Jia, J.; Wang, Z. The cellular responses of autophagy, apoptosis, and 5-methylcytosine level in zebrafish cells upon nutrient deprivation stress. Chemosphere 2020, 241, 124989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Harder, B.; De La Vega, M.R.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, F.; Ye, Q.; Wang, H. The circadian clock regulates autophagy directly through the nuclear hormone receptor Nr1d1/Rev-erbα and indirectly via Cebpb/(C/ebpβ) in zebrafish. Autophagy 2016, 12, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer (5′-3′) | Product (bp) |
|---|---|---|
| ATG3 F | CGCCAGTTTTGAAGGAATCT | 142 |
| ATG3 R | AGATAGGGCTTCACCTTTGCT | |
| ATG4B F | TAGGTGGGAAGCCGAACA | 202 |
| ATG4B R | CAGAAGAAGCCAGCAGCAA | |
| ATG4C F | CCGAGGCATTAGATGACGATA | 119 |
| ATG4C R | GATGGTGGAGCCAGGAAGA | |
| ATG5 F | CGTTGGGAGACCTGCTGAA | 180 |
| ATG5 R | TTGGTGCTGGGATGATGCT | |
| CAT F | AGCAGACACCGTCCGAGAT | 200 |
| CAT R | GCAAACTCCAGAAGTCCCAA | |
| GPX4 F | AACCAGTTTGGAAAGCAGGAG | 227 |
| GPX4 R | TTCACCACCTGACCTTCTCG | |
| GSTA F | AGACGACCCGACTGAACAAG | 188 |
| GSTA R | TCTCCAAGTATCCATCCCACA | |
| GSTM F | CTTGCTCAACCAATCCGTCT | 188 |
| GSTM R | GCATTGCTTTGGACCACTTT | |
| ULK1 F | AGGGAGGAAGCCGTAGAAACA | 161 |
| ULK1 R | ACAGTGGACAAAAGCCAAGGT | |
| BECLIN1 F | AGGAGGTGAAGAGCGATAAGG | 123 |
| BECLIN1 R | CCAGGCGACGGTTGTGA | |
| LC3B F | ACATTTGAGCAGCGGGTG | 208 |
| LC3B R | GGAAGAAAGCCTGATTGGAGT | |
| SOD1 F | ATGGTGGACCGACGGATAGT | 187 |
| SOD1 R | TCTTCATTGCCTCCTTTACCC | |
| SOD2 F | ACTACAGGTCTCGTCCCACT | 123 |
| SOD2 R | GACATTCTCCCAGTTCACAA | |
| Nrf2 F | TGCCCAATGAGAATCCCTT | 166 |
| Nrf2 R | ATGGGACTTTACTACGGTGG | |
| Keap1 F | TTTGCGGAGGAGATTGGC | 202 |
| Keap1 R | TGACCCAAGCGACCCTAC | |
| mTOR F | AGGATGGCACCTGACTATGA | 252 |
| mTOR R | ATTTGAGCCCTGAGATGAAG | |
| S6K1 F | CTGCTCCACATTAGACACCT | 233 |
| S6K1 R | TCTTCCTGGGCTTTACATAC | |
| P62 F | CTCTTTACCCTCACCTGCCTCA | 101 |
| P62 R | AGTCAACCAGCCGCCTTCAT | |
| ATG9 F | TCAATGAGTTGGACCACGAGC | 151 |
| ATG9 R | GCGATGAGGACAGCCAGAAGA | |
| RpL13 F | CAGAGGCTGAAGGAGTACCG | 122 |
| RpL13 R | ATGACGGGTCCAGTAAGCTG |
| Name | 0 Day | 3 Day | 7 Day | R3 | R7 |
|---|---|---|---|---|---|
| ROS | 100.03 ± 0.67 a | 119.42 ± 5.11 bc | 130.56 ± 4.29 c | 106.93 ± 3.52 ab | 107.98 ± 3.95 ab |
| MDA | 1.63 ± 0.28 b | 2.51 ± 0.20 c | 3.44 ± 0.14 d | 1.56 ± 0.29 b | 0.96 ± 0.086 a |
| SOD | 5.97 ± 0.45 a | 16.37 ± 0.34 c | 24.05 ± 2.08 d | 18.66 ± 0.97 c | 11.41 ± 0.73 b |
| CAT | 1.65 ± 0.54 a | 3.56 ± 0.96 e | 3.03 ± 0.07 d | 2.45 ± 0.13 c | 2.14 ± 0.10 b |
| GST | 10.91 ± 0.56 ab | 37.25 ± 8.51 d | 27.63 ± 1.93 cd | 17.28 ± 2.01 bc | 3.82 ± 0.85 a |
| GPX | 36.09 ± 1.55 a | 76.96 ± 3.22 d | 64.25 ± 2.11 c | 61.27 ± 2.15 bc | 54.20 ± 4.43 b |
| GR | 23.40 ± 2.23 a | 74.87 ± 2.74 c | 94.31 ± 6.04 d | 73.79 ± 7.82 c | 49.00 ± 4.55 b |
| GSH | 10.39 ± 0.15 ab | 25.17 ± 2.00 c | 52.55 ± 0.47 d | 13.73 ± 1.33 b | 7.55 ± 1.43 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Luo, J.; Xu, Z.; Liu, Z.; Bao, L.; Xue, L. ROS-Induced Autophagy of Skeletal Muscle Confers Resistance of Rice Flower Carp (Cyprinus carpio) to Short-Term Fasting. Genes 2024, 15, 840. https://doi.org/10.3390/genes15070840
Cheng J, Luo J, Xu Z, Liu Z, Bao L, Xue L. ROS-Induced Autophagy of Skeletal Muscle Confers Resistance of Rice Flower Carp (Cyprinus carpio) to Short-Term Fasting. Genes. 2024; 15(7):840. https://doi.org/10.3390/genes15070840
Chicago/Turabian StyleCheng, Jia, Junhan Luo, Ziyang Xu, Zhouying Liu, Lingsheng Bao, and Liangyi Xue. 2024. "ROS-Induced Autophagy of Skeletal Muscle Confers Resistance of Rice Flower Carp (Cyprinus carpio) to Short-Term Fasting" Genes 15, no. 7: 840. https://doi.org/10.3390/genes15070840




