Sinuous Is a Claudin Required for Locust Molt in Locusta migratoria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. The Sequence Analyses of Lmsinu
2.3. The Spatio-Temporal Expression Analysis of Lmsinu
2.4. The Functional Analysis of Lmsinu through RNA Interference (RNAi)
2.5. Microsection and Hematoxylin and Eosin (H&E) Staining of Integument
2.6. Microsection and Chitin Staining of Integument
2.7. Transmission Electron Microscopy of Integument
2.8. Data Analysis
3. Results
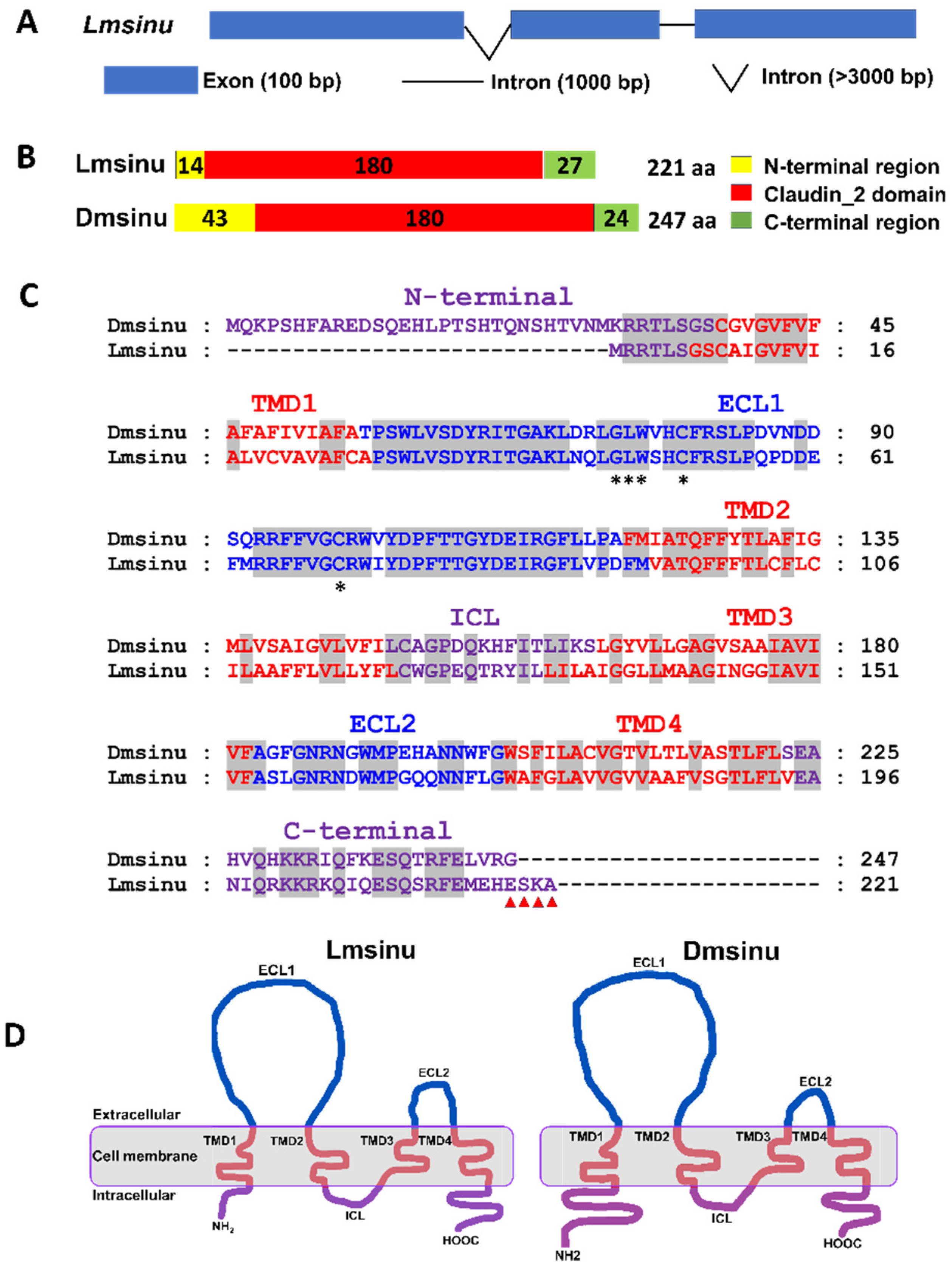
3.1. Bioinformatic Analysis of Lmsinu
3.2. Analysis of Spatiotemporal Expression Pattern of Lmsinu
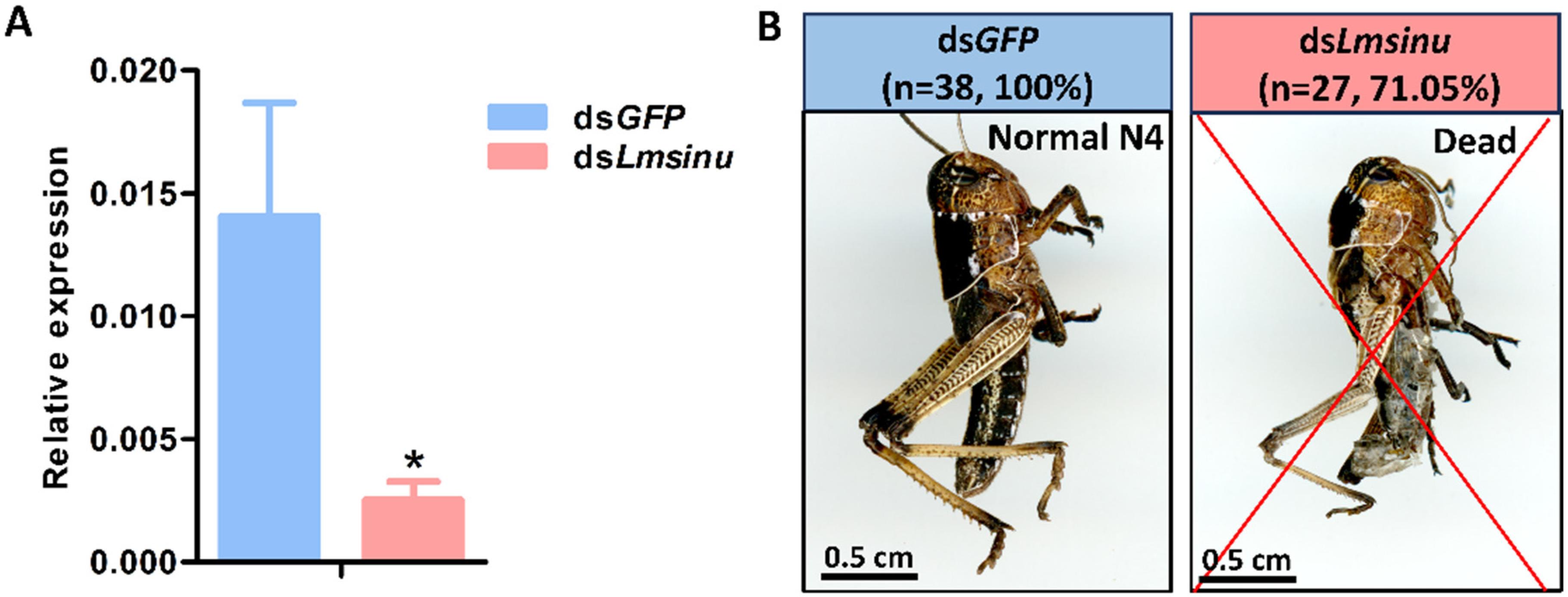
3.3. The Impact of Lmsinu Silencing on the Molting Process of Nymphs
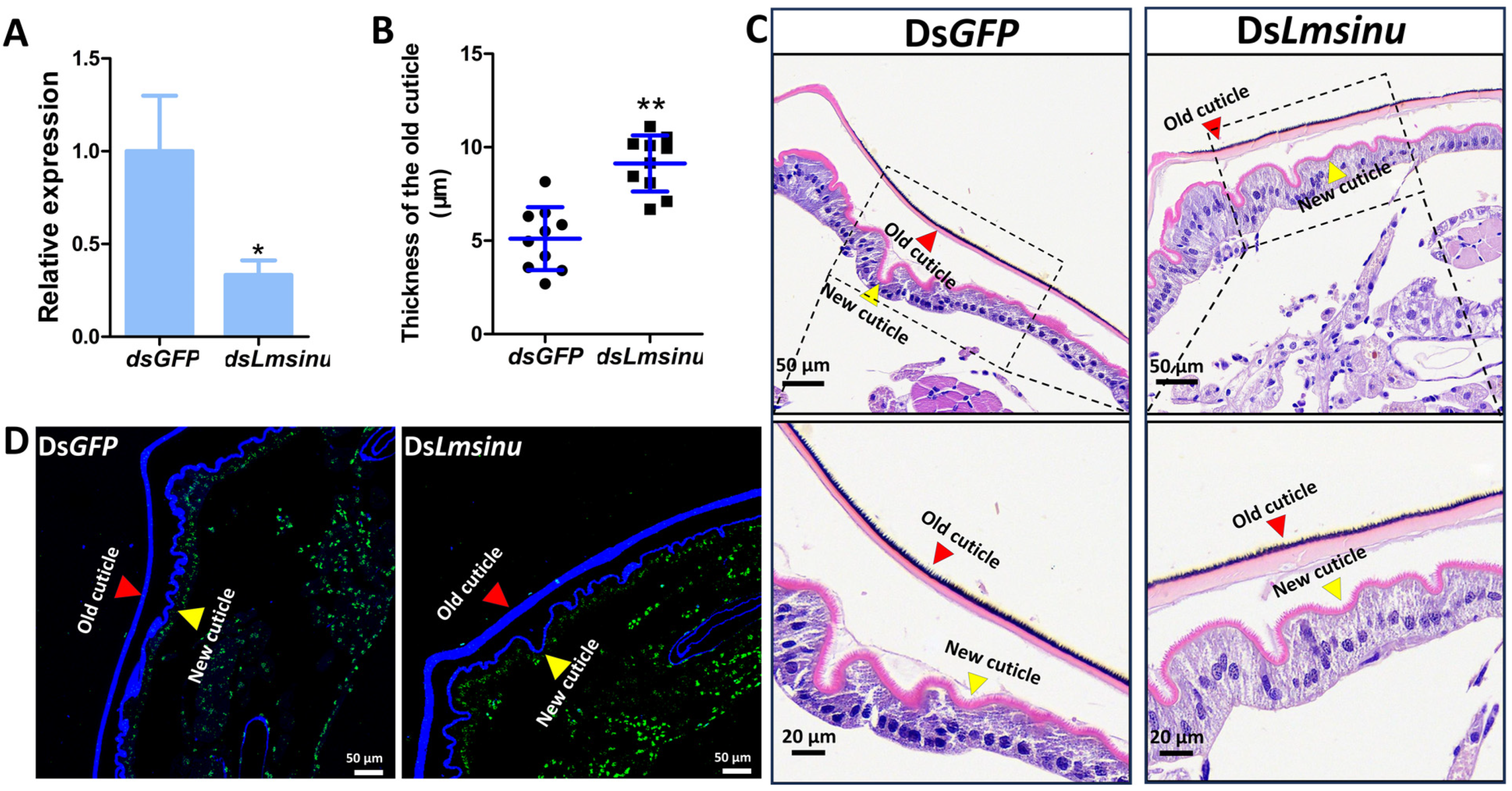
3.4. The Silencing Effects of Lmsinu on Nymphal Molting
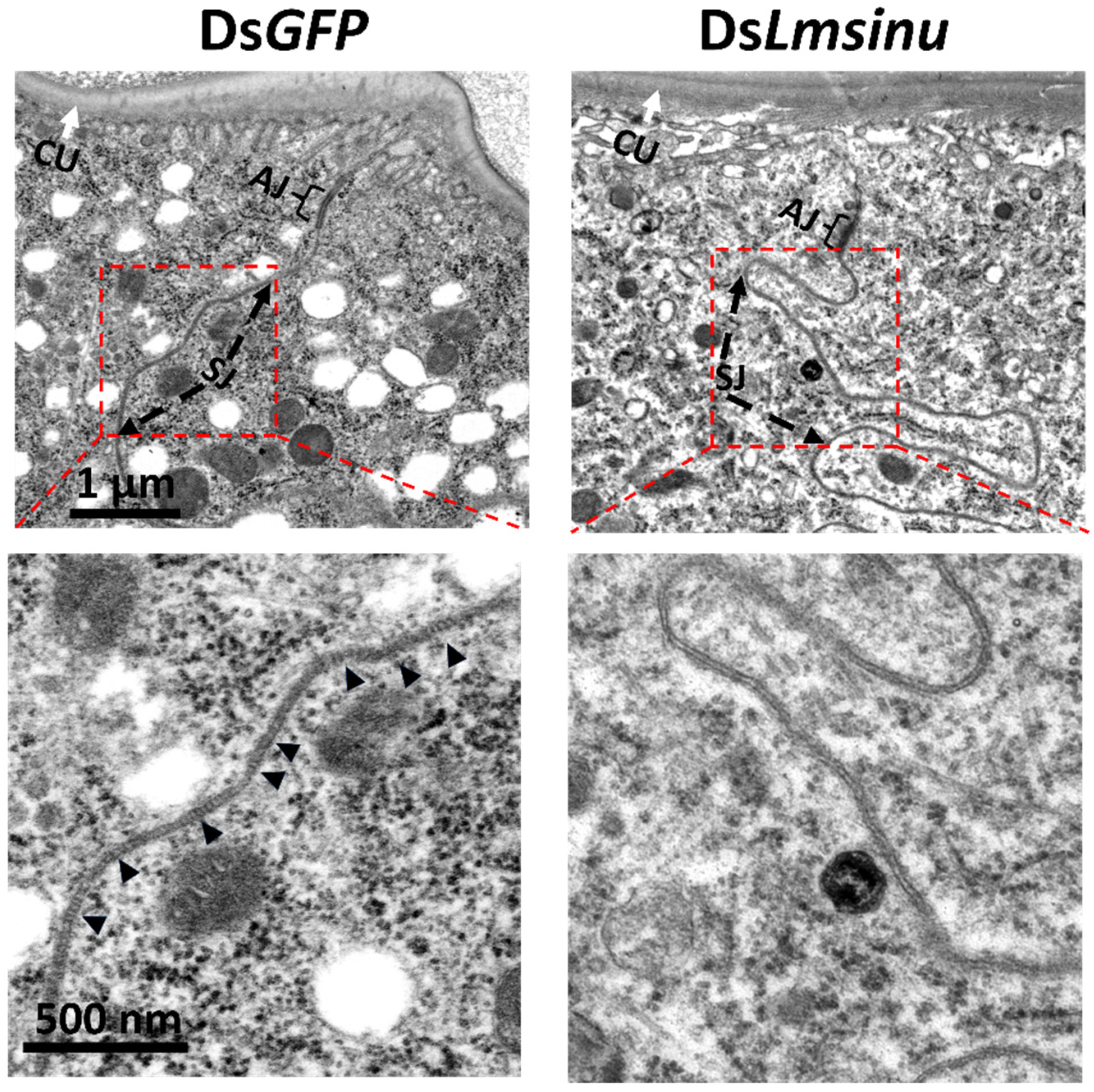
3.5. Effects of the Silencing Lmsinu on Cell Junctions in Epidermal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tepass, U.; Tanentzapf, G.; Ward, R.; Fehon, R. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 2001, 35, 747–784. [Google Scholar] [CrossRef] [PubMed]
- Edelblum, K.L.; Turner, J.R. Epithelial Cells: Structure, Transport, and Barrier Function. In Mucosal Immunology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–210. [Google Scholar]
- Whitsett, J.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Knust, E.; Bossinger, O. Composition and formation of intercellular junctions in epithelial cells. Science 2002, 298, 1955–1959. [Google Scholar] [CrossRef]
- Kawabe, H.; Nakanishi, H.; Asada, M.; Fukuhara, A.; Morimoto, K.; Takeuchi, M.; Takai, Y. Pilt, a novel peripheral membrane protein at tight junctions in epithelial cells. J. Biol. Chem. 2001, 276, 48350–48355. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell–cell junctions organize structural and signaling networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, B.; Manning, S.A.; Fonseka, Y.; Oorschot, V.; Crawford, S.A.; Ramm, G.; Harvey, K.F. Basal spot junctions of Drosophila epithelial tissues respond to morphogenetic forces and regulate Hippo signaling. Dev. Cell 2024, 59, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Izumi, Y. Molecular dissection of smooth septate junctions: Understanding their roles in arthropod physiology: Smooth septate junction-associated proteins. Ann. N. Y. Acad. Sci. 2017, 1397, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Negi, N.; Thakur, H.; Battu, J.R.; Turnbull, M. Insect gap junctions could be a potential target for pest management. Ann. Entomol. Soc. Am. 2022, 115, 449–460. [Google Scholar] [CrossRef]
- Rice, C.; De, O.; Alhadyian, H.; Hall, S.; Ward, R.E. Expanding the Junction: New Insights into Non-Occluding Roles for Septate Junction Proteins during Development. J. Dev. Biol. 2021, 9, 11. [Google Scholar] [CrossRef]
- Jonusaite, S.; Kelly, S.P.; Donini, A. Identification of the septate junction protein gliotactin in the mosquito Aedes aegypti: Evidence for a role in increased paracellular permeability in larvae. J. Exp. Biol. 2017, 220, 2354–2363. [Google Scholar] [CrossRef]
- Hu, X.; Boeckman, C.J.; Cong, B.; Steimel, J.P.; Richtman, N.M.; Sturtz, K.; Wang, Y.; Walker, C.L.; Yin, J.; Unger, A.; et al. Characterization of DvSSJ1 transcripts targeting the smooth septate junction (SSJ) of western corn rootworm (Diabrotica virgifera virgifera). Sci. Rep. 2020, 10, 11139. [Google Scholar] [CrossRef] [PubMed]
- Beitel, G.J.; Krasnow, M.A. Genetic control of epithelial tube size in the Drosophila tracheal system. Development 2000, 127, 3271–3282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, L.; Cheng, X.; Yang, R.; Li, D.; Zhang, C.; Bao, Y. A CYP380C10 gene is required for waterproofing and water retention in the insect integument. J. Insect Physiol. 2022, 138, 104380. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W. The evolution of insect metamorphosis. Curr. Biol. 2019, 29, R1252–R1268. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Yang, Q. Chitin Organizing and Modifying Enzymes and Proteins Involved in Remodeling of the Insect Cuticle. Adv. Exp. Med. Biol. 2019, 1142, 83–114. [Google Scholar] [PubMed]
- Nestel, D.; Tolmasky, D.; Rabossi, A.; Quesada-Allué, L.A. Lipid, carbohydrates and protein patterns during metamorphosis of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2003, 96, 237–244. [Google Scholar] [CrossRef]
- Willis, J.H.; Papandreou, N.C.; Iconomidou, V.A.; Smith, R.F.; Hamodrakas, S.J. Cuticular proteins. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 134–166. [Google Scholar]
- Wigglesworth, V.B. The epidermal cell and the metamorphosis of insects. Nature 1960, 188, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, X.; Yang, P.; Jiang, X.; Jiang, F.; Zhao, D.; Li, B.; Cui, F.; Wei, J.; Ma, C.; et al. The locust genome provides insight into swarm formation and long-distance flight. Nat. Commun. 2014, 5, 2957. [Google Scholar] [CrossRef]
- Long, G.; Gong, M.; Yang, H.; Yang, X.; Zhou, C.; Jin, D. Buprofezin affects the molting process by regulating nuclear receptors SfHR3 and SfHR4 in Sogatella furcifera. Pestic. Biochem. Physiol. 2023, 197, 105695. [Google Scholar] [CrossRef]
- Zhao, X.M.; Qin, Z.Y.; Zhang, J.; Yang, Y.; Jia, P.; Yang, Q.; Ma, E.B.; Zhang, J.Z. Nuclear receptor hormone receptor 39 is required for locust moulting by regulating the chitinase and carboxypeptidase genes. Insect Mol. Biol. 2019, 28, 537–549. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts α and β transmembrane proteins using deep neural networks. bioRxiv 2022, 2004–2022. [Google Scholar] [CrossRef]
- Nicholas, K.B. GeneDoc, analysis and visualization of genetic variation. Embnew. News 1997, 4, 14. [Google Scholar]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic. Acids. Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Cao, J.; Zhang, S.; Zhang, H.; Wu, X.; Zhang, Q.; Liu, X. Selection and Assessment of Reference Genes for Quantitative PCR Normalization in Migratory Locust Locusta migratoria (Orthoptera: Acrididae). PLoS ONE 2014, 9, e98164. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Manickam, A.; Shahin, R.; Ote, M.; Iwanaga, M. Expression of matrix metalloproteinase genes during basement membrane degradation in the metamorphosis of Bombyx mori. Gene 2018, 638, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Q.; Yang, M.L.; Wang, Y.L.; Liu, Q.; Wang, H.M.; Zhang, J.; Li, T. Cuticular protein LmTwdl1 is involved in molt development of the Migratory locust. Insect Sci. 2016, 23, 520–530. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Liu, W.M.; Zhao, X.M.; Yu, Z.T.; Guo, H.F.; Yang, Y.; Moussian, B.; Zhu, K.Y.; Zhang, J.Z. Lipophorin receptor is required for the accumulations of cuticular hydrocarbons and ovarian neutral lipids in Locusta migratoria. Int. J. Biol. Macromol. 2023, 236, 123746. [Google Scholar] [CrossRef]
- Zhao, X.M.; Qin, Z.Y.; Liu, W.M.; Liu, X.J.; Moussian, B.; Ma, E.B.; Li, S.; Zhang, J.Z. Nuclear receptor HR3 controls locust molt by regulating chitin synthesis and degradation genes of Locusta migratoria. Insect Biochem. Mol. Biol. 2018, 92, 1–11. [Google Scholar] [CrossRef]
- Wu, V.M.; Schulte, J.; Hirschi, A.; Tepass, U.; Beitel, G.J. Sinuous is a drosophila claudin required for septate junction organization and epithelial tube size control. J. Cell Biol. 2004, 164, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and Function of Claudins. Biochim. Biophys. Acta 2008, 1778, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006, 68, 403–429. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Riedel, D.; Schuh, R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev. Cell 2003, 5, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.S.; Furuse, M.; Beitel, G.J. The Drosophila claudin kune-kune is required for septate junction organization and tracheal tube size control. Genetics 2010, 185, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Jonusaite, S.; Kelly, S.P.; Donini, A. The response of claudin-like transmembrane septate junction proteins to altered environmental ion levels in the larval mosquito Aedes aegypti. J. Comp. Physiol. B 2016, 186, 589–602. [Google Scholar] [CrossRef]
- Banerjee, S.; Sousa, A.D.; Bhat, M.A. Organization and function of septate junctions: An evolutionary perspective. Cell Biochem. Biophys. 2006, 46, 65–77. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhang, S.; Kong, X.; Liu, F.; Zhang, Z. RNAi-Mediated Silencing of the Chitinase 5 Gene for Fall Webworm (Hyphantria cunea) Can Inhibit Larval Molting Depending on the Timing of dsRNA Injection. Insects 2021, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Zhou, C.; Long, G.Y.; Yang, H.; Chen, C.; Jin, D. Characterization and functional analysis of chitinase family genes involved in nymph-adult transition of Sogatella furcifera. Insect Sci. 2021, 28, 901–916. [Google Scholar] [CrossRef]
- Mané-Padrós, D.; Borràs-Castells, F.; Belles, X.; Martín, D. Nuclear receptor HR4 plays an essential role in the ecdysteroid-triggered gene cascade in the development of the hemimetabolous insect Blattella germanica. Mol. Cell Endocrinol. 2012, 348, 322–330. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Meng, Q.W.; Deng, P.; Guo, W.C.; Li, G.Q. Leptinotarsa hormone receptor 4 (HR4) tunes ecdysteroidogenesis and mediates 20-hydroxyecdysone signaling during larval-pupal metamorphosis. Insect Biochem. Mol. Biol. 2018, 94, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, K.; Wang, X.; Ma, M.; Luo, X.; Chen, W.; Chen, A.; Peng, Z.; Zhang, D. Role of nuclear receptors NlHR3 and NlFTZ-F1 in regulating molting and reproduction in Nilaparvata lugens (stål). Front. Physiol. 2023, 14, 1123583. [Google Scholar] [CrossRef] [PubMed]
- Marlena, H.; Jennifer, L.; Christian, L.; Susanne, D. Knockdown of Na,K-ATPase β-subunits in Oncopeltus fasciatus induces molting problems and alterations in tracheal morphology. Insect Sci. 2023, 30, 375–397. [Google Scholar]
- Zhang, X.Q.; Rui Yang, R.; Jin, L.; Li, G.Q. Requirement of Snakeskin for normal functions of midgut and Malpighian tubules in Henosepilachna vigintioctopunctata. Arch. Insect Biochem. Physiol. 2023, 114, e22033. [Google Scholar] [CrossRef] [PubMed]
- Tiklova, K.; Senti, K.A.; Wang, S.; Graslund, A.; Samakovlis, C. Epithelial septate junction assembly relies on melanotransferrin iron binding and endocytosis in Drosophila. Nat. Cell Biol. 2010, 12, 1071–1077. [Google Scholar] [CrossRef]
- Abbas, M.; Fan, Y.H.; Shi, X.K.; Gao, L.; Wang, Y.L.; Li, T.; Cooper, A.M.W.; Silver, K.; Zhu, K.Y.; Zhang, J.Z. Identification of Rab family genes and functional analyses of LmRab5 and LmRab11A in the development and RNA interference of Locusta migratoria. Insect Sci. 2022, 29, 320–332. [Google Scholar] [CrossRef]
- Laprise, P.; Lau, K.M.; Harris, K.P.; Silva-Gagliardi, N.F.; Paul, S.M.; Beronja, S.; Beitel, G.J.; McGlade, C.J.; Tepass, U. Yurt, Coracle, Neurexin IV and the Na+,K+-ATPase form a novel group of epithelial polarity proteins. Nature 2009, 459, 1141–1145. [Google Scholar] [CrossRef]
- Laprise, P.; Beronja, S.; Silva-Gagliardi, N.F.; Pellikka, M.; Jensen, A.M.; McGlade, C.J.; Tepass, U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev. Cell 2006, 11, 363–374. [Google Scholar] [CrossRef]
- Caveney, S.; Podgorski, C. Intercellular communication in a positional field. Ultrastructural correlates and tracer analysis of communication between insect epidermal cells. Tissue Cell 1975, 7, 559–574. [Google Scholar] [CrossRef]
- Schwarz, H.; Moussian, B. Electron-microscopic and genetic dissection of arthropod cuticle Differentiation. Mod. Res. Educ. Top. Microsc. 2007, 3, 316–325. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, H.; Lan, Q.; Liu, X.; Wu, H.; Zhang, J.; Zhao, X.; Wang, Y. Sinuous Is a Claudin Required for Locust Molt in Locusta migratoria. Genes 2024, 15, 850. https://doi.org/10.3390/genes15070850
Zhang Y, Li H, Lan Q, Liu X, Wu H, Zhang J, Zhao X, Wang Y. Sinuous Is a Claudin Required for Locust Molt in Locusta migratoria. Genes. 2024; 15(7):850. https://doi.org/10.3390/genes15070850
Chicago/Turabian StyleZhang, Yichao, Hongjing Li, Qiuyan Lan, Xiaoman Liu, Haihua Wu, Jianzhen Zhang, Xiaoming Zhao, and Yanli Wang. 2024. "Sinuous Is a Claudin Required for Locust Molt in Locusta migratoria" Genes 15, no. 7: 850. https://doi.org/10.3390/genes15070850





