Does Sunlight Affect the Quality for Purposes of DNA Analysis of Blood Stain Evidence Collected from Different Surfaces?
Highlights
- DNA degradation is correlated to the length of sunlight exposure.
- DNA degradation is correlated to the type of surface the samples are collected from.
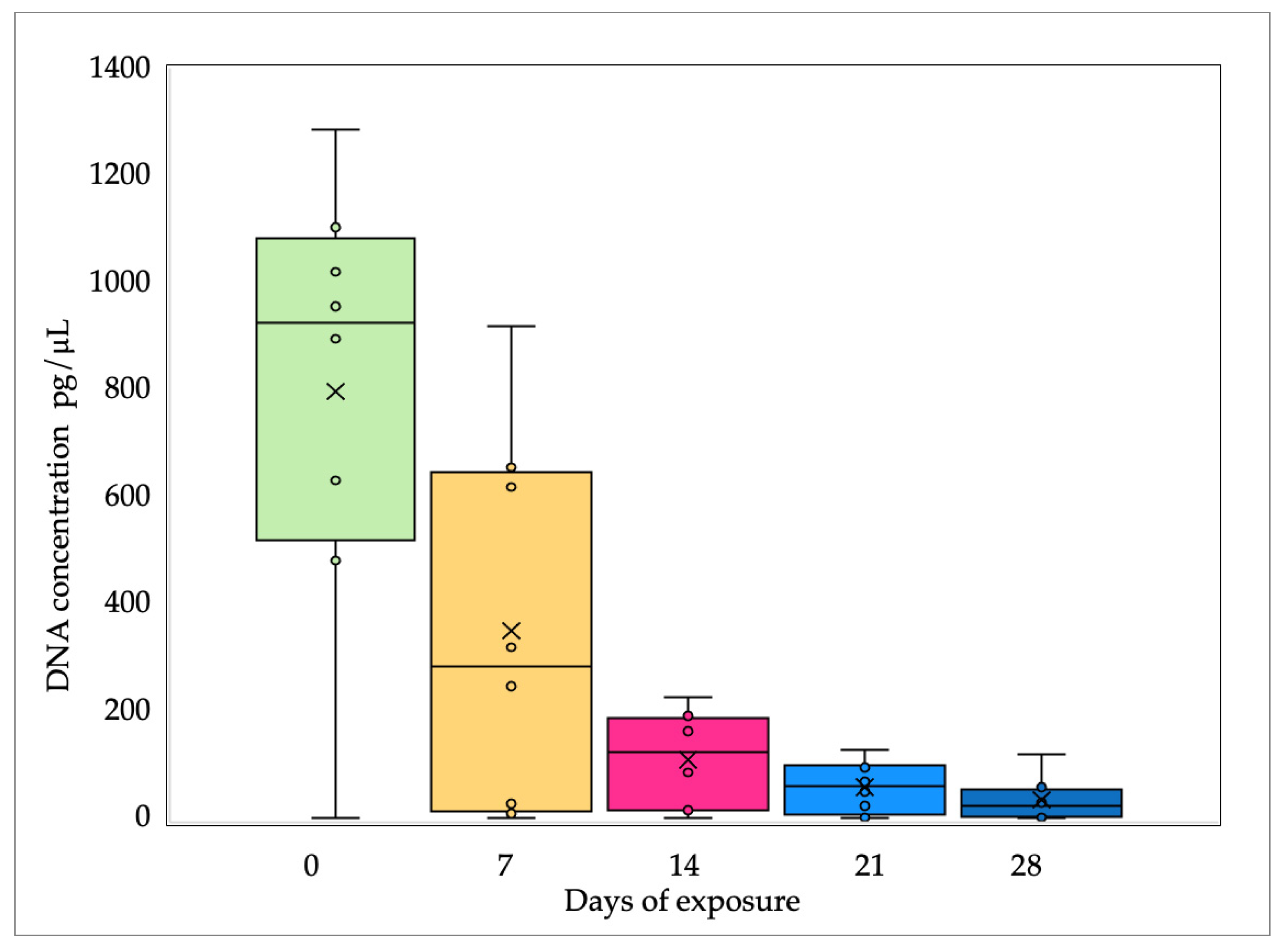
- DNA concentration from all samples decreased as the sunlight exposure time progressed.
- After 28 days of sunlight exposure, it was not possible to amplify all 9 STR loci in any tested sample, regardless of the surface type.
- DNA samples of the highest quality and quantity were obtained from blood stain samples collected from glass, ceramics, galvanized steel, iron rods, and newsprint, respectively.
- Sunlight exposure (UV radiation) and surface type are environmental factors that affect DNA quality and quantity.
- Future studies should investigate the impact of additional factors such as humidity and temperature, as well as other types of surfaces (e.g., fabric) commonly encountered at crime scenes.
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation
2.3. DNA Extraction
2.4. DNA Quantification
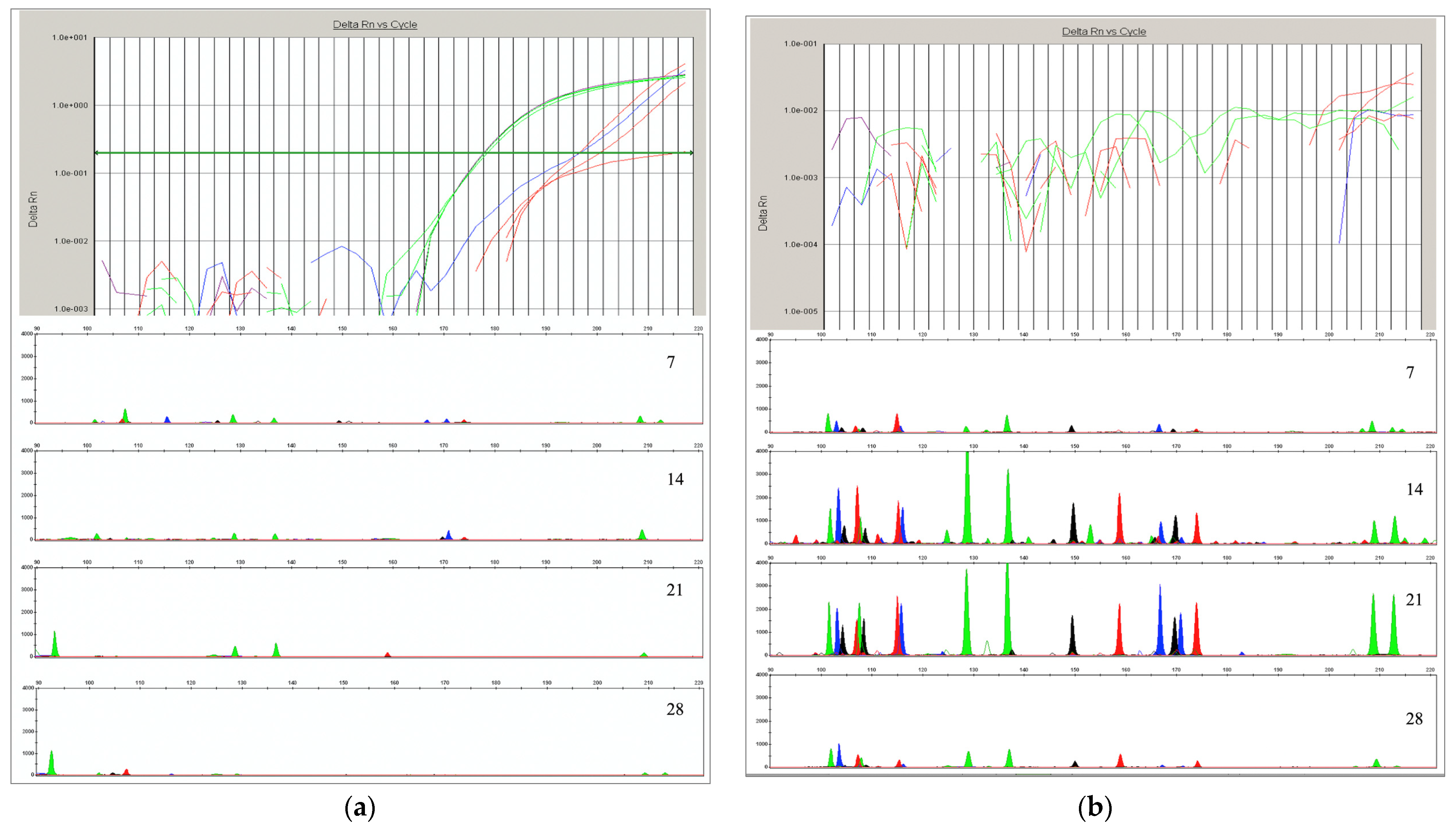
2.5. Autosomal STR Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. DNA Quantification
4.2. Autosomal STR Analysis
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krawczak, M.; Schmidtke, J. DNA Fingerprinting; Bios Scientific Publishers Ltd.: Oxford, UK, 1994. [Google Scholar]
- Crime Scene Investigator Network. Wildomar (CA): Crime Scene Resources Inc; c2000–2015. Collection and Preservation of Blood Evidence from Crime Scenes; [cited 10 June 2014]; [about 9 Screens]. Available online: http://www.crime-scene-investigator.net/blood.html (accessed on 20 March 2024).
- Thacker, C.R.; Oguzturun, C.; Ball, K.M.; Syndercombe Court, D. An investigation into methods to produce artificially degraded DNA. Int. Congr. Ser. 2006, 1288, 592–594. [Google Scholar] [CrossRef]
- Goodsell, D.S. The molecular perspective: Ultraviolet light and pyrimidine dimers. Oncologist 2001, 63, 298–299. [Google Scholar] [CrossRef]
- Hall, A.; Sims, L.M.; Ballantyne, J. Assessment of DNA damage induced by terrestrial UV irradiation of dried bloodstains: Forensic implications. Forensic Sci. Int. Genet. 2014, 8, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Miyamura, Y.; Bowen, M.P.; Correa, G.; Ono, Y.; Goodarzi, H. Light, including ultraviolet. J. Autoimmun. 2010, 34, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Khorwal, D.; Mathur, G.K.; Ahmed, U.; Daga, S.S. Environmental Factors Affecting the Concentration of DNA in Blood and Saliva Stains: A Review. J. Forensic Sci. Res. 2024, 8, 009–015. [Google Scholar]
- El-Yazbi, A.F.; Loppnow, G.R. Detecting UV-induced nucleic-acid damage. TrAC Trends Anal. Chem. 2014, 61, 83–91. [Google Scholar] [CrossRef]
- Rahi, G.S.; Adams, J.L.; Yuan, J.; Devone, D.-J.; Lodhi, K.M. Whole human blood DNA degradation associated with artificial ultraviolet and solar radiations as a function of exposure time. Forensic Sci. Int. 2021, 319, 110674. [Google Scholar] [CrossRef] [PubMed]
- Hrycay, E.G.; Bandiera, S.M. Involvement of Cytochrome P450 in Reactive Oxygen Species Formation and Cancer. Adv. Pharmacol. 2015, 74, 35–84. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J. Singlet oxygen induces oxidation of cellular DNA. J. Biol. Chem. 2000, 275, 40601–40604. [Google Scholar]
- Hall, A.; Ballantyne, J. Characterization of UVC-induced DNA damage in bloodstains: Forensic implications. Anal. Bioanal. Chem. 2004, 380, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Medina-Paz, F.; Kuba, B.; Kryvorutsky, E.; Roca, G.; Zapico, S.C. Assessment of Blood and Semen Detection and DNA Collection from Swabs up to Three Months after Deposition on Five Different Cloth Materials. Int. J. Mol. Sci. 2024, 25, 3522. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R. New Perspectives for Whole Genome Amplification in Forensic STR Analysis. Int. J. Mol. Sci. 2022, 23, 7090. [Google Scholar] [CrossRef]
- Chierto, E.; Aneli, S.; Nocco, N.; Riem, A.; Onofri, M.; Carnevali, E.; Robino, C. Assessing DNA Degradation through Differential Amplification Efficiency of Total Human and Human Male DNA in a Forensic qPCR Assay. Genes 2024, 15, 622. [Google Scholar] [CrossRef] [PubMed]
- Tuchinda, C.; Srivannaboon, S.; Lim, H.W. Photoprotection by window glass, automobile glass, and sunglasses. J. Am. Acad. Dermatol. 2006, 54, 845–854. [Google Scholar] [CrossRef]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 1991, 10, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Applied Biosystems. Quantifiler® Human DNA Quantification Kit and Quantifiler® Y Human Male DNA Quantification Kit: User Manual. Foster City (CA): Life Technologies Corporation; 2012 March [cited 10 June 2014], 216p. Available online: http://www3.appliedbiosystems.com/cms/groups/applied_markets_support/documents/generaldocuments/cms_041395.pdf (accessed on 20 March 2024).
- Mulero, J.J.; Chang, C.W.; Lagace, R.E.; Wang, D.Y.; Bas, J.L.; McMahon, T.P.; Hennessy, L.K. Development and validation of the AmpFISTR MiniFiler PCR amplification kit: A MiniSTR multiplex for the analysis of degraded and/or PCR inhibited DNA. J. Forensic Sci. 2008, 53, 838–852. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumawat, R.K.; Kushwaha, P.; Rana, M. Biological Sources of DNA: The Target Materials for Forensic DNA Typing. In Handbook of DNA Profiling; Dash, H.R., Shrivastava, P., Lorente, J.A., Eds.; Springer: Cham, Szwitzerland, 2022. [Google Scholar]
- Poetsch, M.; Markwerth, P.; Konrad, H.; Bajanowski, T.; Helmus, J. About the influence of environmental factors on the persistence of DNA—A long-term study. Int. J. Leg. Med. 2022, 136, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.J.; Walsh, S.J.; van Oorschot, R.A.; Gunn, P.R.; Evans, L.; Roux, C. Assessing trace DNA evidence from a residential burglary: Abundance, transfer and persistence. Forensic Sci. Int. Genet. Suppl. Ser. 2008, 1, 442–443. [Google Scholar] [CrossRef]
- Poetsch, M.; Pfeifer, M.; Konrad, H.; Bajanowski, T.; Helmus, J. Impact of several wearers on the persistence of DNA on clothes—A study with experimental scenarios. Int. J. Leg. Med. 2017, 132, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Szkuta, B.; Ballantyne, K.N.; van Oorschot, R.A.H. Transfer and persistence of DNA on the hands and the influence of activities performed. Forensic Sci. Int. Genet. 2017, 28, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.C.; Wong, H.Y.; Lee, J.Y.; Waffa, Z.B.M.; Aw, Z.Q.; Fauzi, S.N.A.B.M.; Hoe, S.Y.; Lim, M.L.; Syn, C.K.C. Persistence of DNA in the Singapore context. Int. J. Leg. Med. 2019, 133, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, J.M.; Gomaa, R.; Attalla, S.M.; Nader, L.M. Investigation of DNA degradation in forensic blood samples after exposure to different environmental condi-tions. Int. J. Med. Toxicol. Leg. Med. 2021, 24, 66–74. [Google Scholar]
- Abdel Hady, R.H.; Thabet, H.Z.; Ebrahem, N.E.; Yassa, H.A. Thermal Effects on DNA Degradation in Blood and Seminal Stains: Forensic View. Acad. Forensic Pathol. 2021, 11, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pistono, A.; Ryan, S.; Szkuta, B.; Meakin, G.E. The effect of climatic simulations on DNA persistence on glass, cotton and polyester. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 274–276. [Google Scholar] [CrossRef]
- Wikipedia. San Francisco: Wikipedia, The Free Encyclopedia; c2001-2015. Bleaching of Wood Pulp; [Updated 2014 November 23; Cited 2014 August 26]; [about 6 Screens]. Available online: http://en.wikipedia.org/wiki/Bleaching_of_wood_pulp#Chlorine_dioxide (accessed on 24 March 2024).
- Hayatsu, H.; Pan, S.K.; Ukita, T. Reaction of sodium hypochlorite with nucleic acids and their constituents. Chem. Pharm. Bull. 1971, 19, 2189–2192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alaeddini, R.; Walsh, S.J.; Abbas, A. Forensic implications of genetic analyses from degraded DNA—A review. Forensic Sci. Int. Genet. 2010, 4, 148–157. [Google Scholar] [CrossRef]
- Sutlovic, D.; Definis-Gojanovic, M.; Andjelinovic, S.; Gugic, D.; Primorac, D. Taq polymerase reverses inhibition of quantitative real time polymerase chain reaction by humic acid. Croat. Med. J. 2005, 46, 556–562. [Google Scholar] [PubMed]
- Sutlovic, D.; Gamulin, S.; Definis-Gojanovic, M.; Gugic, D.; Andjelinovic, S. Interaction of humic acids with human DNA: Proposed mechanisms and kinetics. Electrophoresis 2008, 29, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Bruns, M.A.; Tiedje, J.M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996, 62, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, C.C.; Vahjen, W. Interference of humic acids and DNA exreacted directly from soil in detection and transformation of recombinant DNA from bacteria and yeast. Appl. Environ. Microbiol. 1993, 59, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Olson, B.H. Rapid method for separation of bacterial DNA from humic substances in sedimentes for polymerase chain reaction. Appl. Environ. Microbiol. 1992, 58, 2292–2295. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Swaroop, S.S.; Sankhla, M.S.; Rajeev, K. Health Risks of Newspaper Ink when Used as Food Packaging Material. Lett. Appl. NanoBioScience 2021, 10, 2614–2623. [Google Scholar]
- Sutlovic, D.; Andjelinovic, S.; Drmic, I.; Definis-Gojanovic, M.; Primorac, D. Heavy Metals from Mass Graves Bones and Identification by Genomic DNA. Final Program and Abstracts. 3rd European—American Intensive Course in Forensic Genetics and Mayo Clinic Course in Advanced Molecular and Cellular Medicine; 2003 September 1–September 5; Zagreb, Croatia. Zagreb: Studio Hrg; 2003 September [cited 2014 August 10]. 145p. Available online: http://isabs.hr/publications/3rd_conference_Book_of_Abstracts.pdf (accessed on 25 March 2024).
- Dalecka, B.; Mezule, L. Study of potential PCR inhibitors in drinking water for Escherichia coli identification. Agron. Res. 2018, 16, 1351–1359. [Google Scholar]
- Arsenault, H.; Kuffel, A.; Daeid, N.N.; Gray, A. Trace DNA and its persistence on various surfaces: A long term study investigating the influence of surface type and environmental conditions—Part one, metals. Forensic Sci. Int. Genet. 2024, 70, 103011. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Andjelinovic, S.; Martin, P.; Sutlovic, D.; Erceg, I.; Huffine, E.; Fernández de Simón, L.; Albarrán, C.; Definis-Gojanovic, M.; Fernández-Rodriguez, A.; et al. DNA typing from skeletal remains: Evaluation of multiplex and megaplex STR systems on DNA isolated from bone and teeth samples. Croat. Med. J. 2001, 42, 260–266. [Google Scholar] [PubMed]
- Senge, T.; Madea, B.; Junge, A.; Rothschild, M.A.; Schneider, P.M. STRs, mini STRs and SNPs—A comparative study for typing degraded DNA. Leg. Med. 2011, 13, 68–74. [Google Scholar] [CrossRef]
- Butler, J.M. Forensic DNA Typing: Biology, Technology, and Genetics of STR Markers, 4th ed.; Elsevier Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Alketbi, S.K.; Goodwin, W. The effect of surface type, collection and extraction methods on touch DNA. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 704–706. [Google Scholar] [CrossRef]
- Schulte, J.; Rittiner, N.; Kron, S.; Seiberle, I.; Schulz, I. Collecting touch DNA from glass surfaces using different sampling solutions and volumes: Immediate and storage effects on genetic STR analysis. J. Forensic Sci. 2023, 68, 1133–1147. [Google Scholar] [CrossRef]
- Ricci, U.; Nutini, A.L.; Gerundino, F.; Boschi, B.; Pelo, E. The best possible result from the minimum available. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 154–155. [Google Scholar] [CrossRef]
- Alonso, A.; Martin, P.; Albarran, C.; Garcia, P.; Primorac, D.; Garcia, O.L.; Fernandez de Simon, L.; Garcia-Hirschfeld, J.; Sancho, M.; Fernandez-Piqueras, J. Specific quantification of human genomes from low copy number DNA samples in forensic and ancient DNA studies. Croat. Med. J. 2003, 44, 273–280. [Google Scholar] [PubMed]
- Dissing, J.; Søndervang, A.; Lund, S. Exploring the limits for the survival of DNA in blood stains. J. Forensic Leg. Med. 2010, 17, 392–396. [Google Scholar] [CrossRef]

| Sunlight Days | Average of Daily (Day/Night) Indoor Temperatures (°C) | Average of Daily Relative Humidity (%) |
|---|---|---|
| 1–7 | 25.1 | 48.9 |
| 8–14 | 27.3 | 59.0 |
| 15–21 | 30.3 | 50.6 |
| 22–28 | 27.4 | 54.5 |
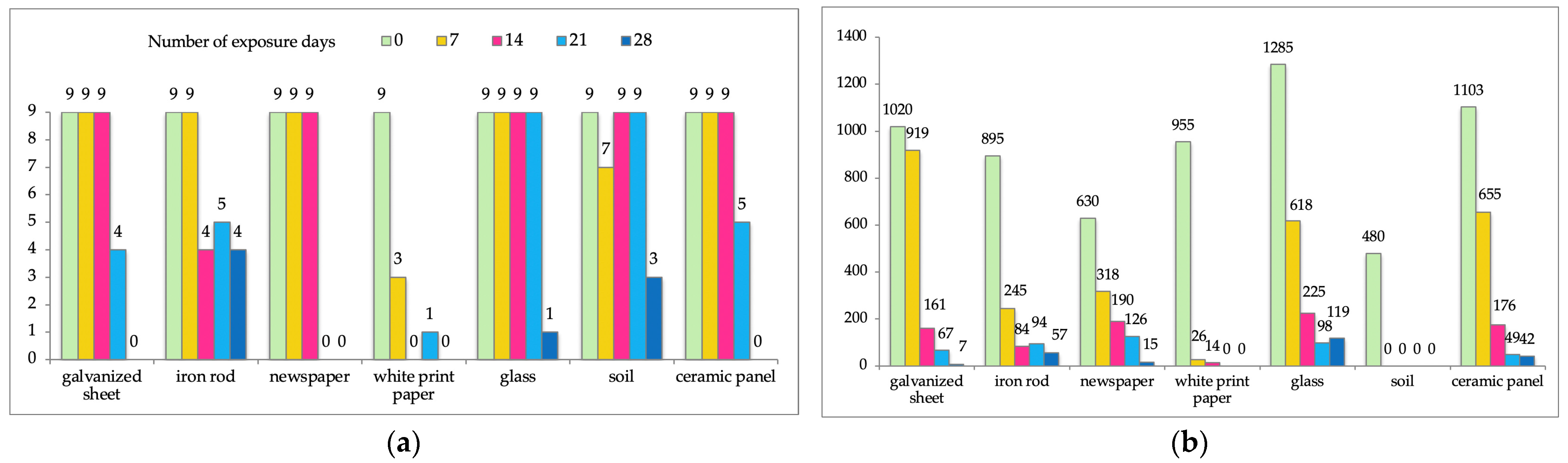
| Sample Number | Type of Surface | Number of Days of Sunlight | Ct | DNA Concentration (pg/μL) | Number of Successfully Amplified Loci (Including Amelogenin) | Spearman’s Rho Correlation Coefficient * |
|---|---|---|---|---|---|---|
| 1-0 | 1-Galvanized sheet | 0 | 27.10 | 1020 | 9/9 | rs = 0.894 p < 0.05 |
| 1-1 | 7 | 27.86 | 919 | 9/9 | ||
| 1-2 | 14 | 30.11 | 161 | 9/9 | ||
| 1-3 | 21 | 31.16 | 67 | 4/9 | ||
| 1-4 | 28 | 34.23 | 7 | 0/9 | ||
| 2-0 | 2-Iron rod | 0 | 27.45 | 895 | 9/9 | rs = 0.949 p < 0.05 |
| 2-1 | 7 | 29.82 | 245 | 9/9 | ||
| 2-2 | 14 | 30.47 | 84 | 4/9 | ||
| 2-3 | 21 | 30.33 | 94 | 5/9 | ||
| 2-4 | 28 | 30.93 | 57 | 4/9 | ||
| 3-0 | 3-Newspaper | 0 | 28.29 | 630 | 9/9 | rs = 0.866 p = 0.058 |
| 3-1 | 7 | 29.20 | 318 | 9/9 | ||
| 3-2 | 14 | 29.82 | 190 | 9/9 | ||
| 3-3 | 21 | 30.37 | 126 | 0/9 | ||
| 3-4 | 28 | 33.01 | 15 | 0/9 | ||
| 4-0 | 4-White printer paper (80 g) | 0 | 27.65 | 955 | 9/9 | rs = 0.821 p = 0.089 |
| 4-1 | 7 | 32.52 | 26 | 3/9 | ||
| 4-2 | 14 | 33.49 | 14 | 0/9 | ||
| 4-3 | 21 | 38.52 | 0.33 | 1/9 | ||
| 4-4 | 28 | Undetected ** | Undetected | 0/9 | ||
| 5-0 | 5-Glass | 0 | 27.05 | 1285 | 9/9 | rs = 0.353 p = 0.559 |
| 5-1 | 7 | 28.38 | 618 | 9/9 | ||
| 5-2 | 14 | 29.66 | 225 | 9/9 | ||
| 5-3 | 21 | 30.67 | 98 | 9/9 | ||
| 5-4 | 28 | 30.61 | 119 | 1/9 | ||
| 6-0 | 6-Soil | 0 | 29.20 | 480 | 9/9 | rs = 0.395 p = 0.510 |
| 6-1 | 7 | Undetected | Undetected | 7/9 | ||
| 6-2 | 14 | Undetected | Undetected | 9/9 | ||
| 6-3 | 21 | Undetected | Undetected | 9/9 | ||
| 6-4 | 28 | Undetected | Undetected | 3/9 | ||
| 7-0 | 7-Ceramic panel | 0 | 27.15 | 1103 | 9/9 | rs = 0.894 p < 0.05 |
| 7-1 | 7 | 28.31 | 655 | 9/9 | ||
| 7-2 | 14 | 29.91 | 176 | 9/9 | ||
| 7-3 | 21 | 31.51 | 49 | 5/9 | ||
| 7-4 | 28 | 31.70 | 42 | 0/9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sliskovic, L.; Milos, I.; Zecic, A.; Kuret, S.; Sutlovic, D. Does Sunlight Affect the Quality for Purposes of DNA Analysis of Blood Stain Evidence Collected from Different Surfaces? Genes 2024, 15, 888. https://doi.org/10.3390/genes15070888
Sliskovic L, Milos I, Zecic A, Kuret S, Sutlovic D. Does Sunlight Affect the Quality for Purposes of DNA Analysis of Blood Stain Evidence Collected from Different Surfaces? Genes. 2024; 15(7):888. https://doi.org/10.3390/genes15070888
Chicago/Turabian StyleSliskovic, Livia, Ivana Milos, Antonia Zecic, Sendi Kuret, and Davorka Sutlovic. 2024. "Does Sunlight Affect the Quality for Purposes of DNA Analysis of Blood Stain Evidence Collected from Different Surfaces?" Genes 15, no. 7: 888. https://doi.org/10.3390/genes15070888
APA StyleSliskovic, L., Milos, I., Zecic, A., Kuret, S., & Sutlovic, D. (2024). Does Sunlight Affect the Quality for Purposes of DNA Analysis of Blood Stain Evidence Collected from Different Surfaces? Genes, 15(7), 888. https://doi.org/10.3390/genes15070888





