Comprehensive Identification and Expression Profiling of Epidermal Pattern Factor (EPF) Gene Family in Oilseed Rape (Brassica napus L.) under Salt Stress
Abstract
:1. Introduction
2. Methods
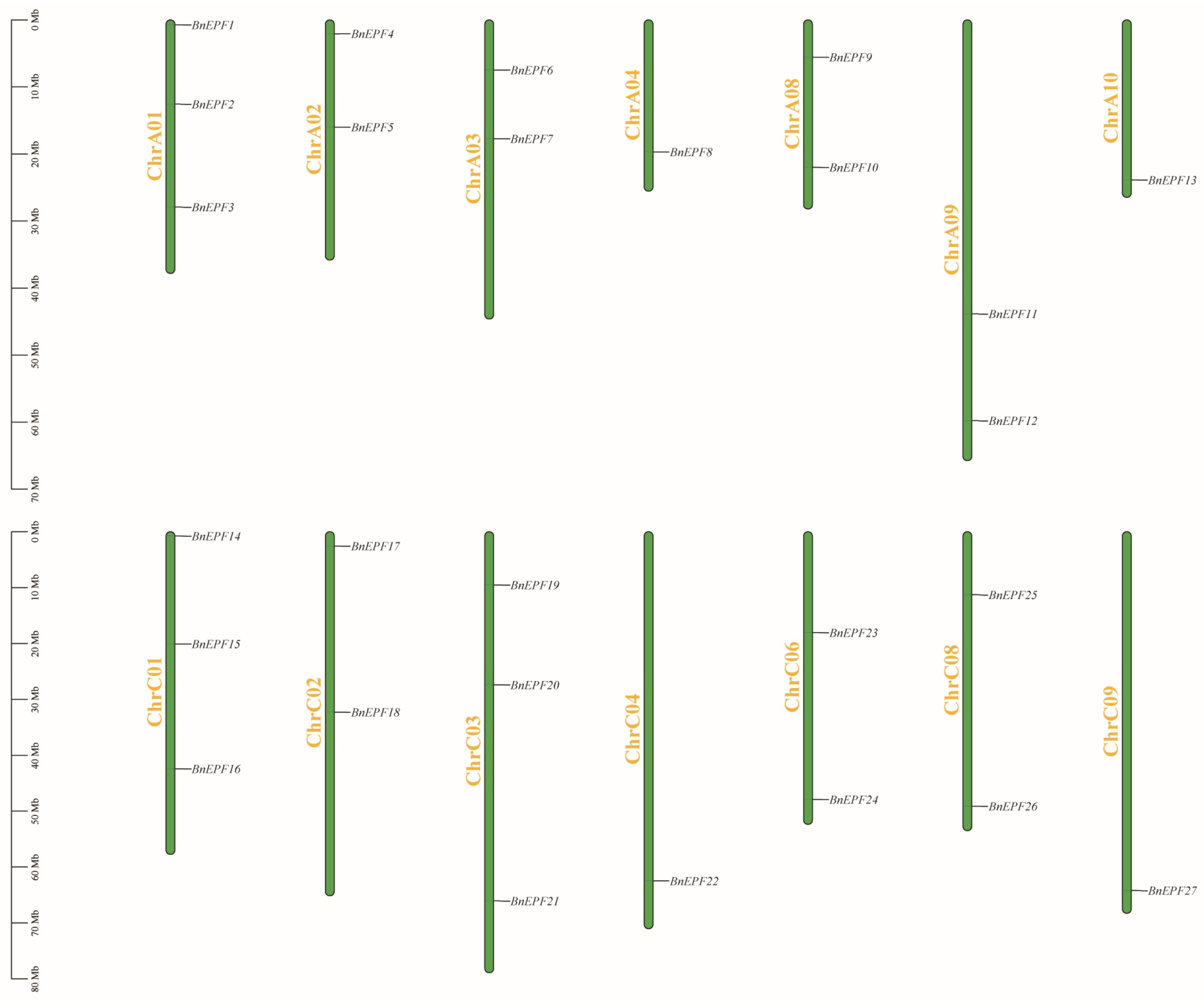
2.1. Identification and Chromosome Localization of BnEPF Family Members
2.2. Phylogenetic Assessment
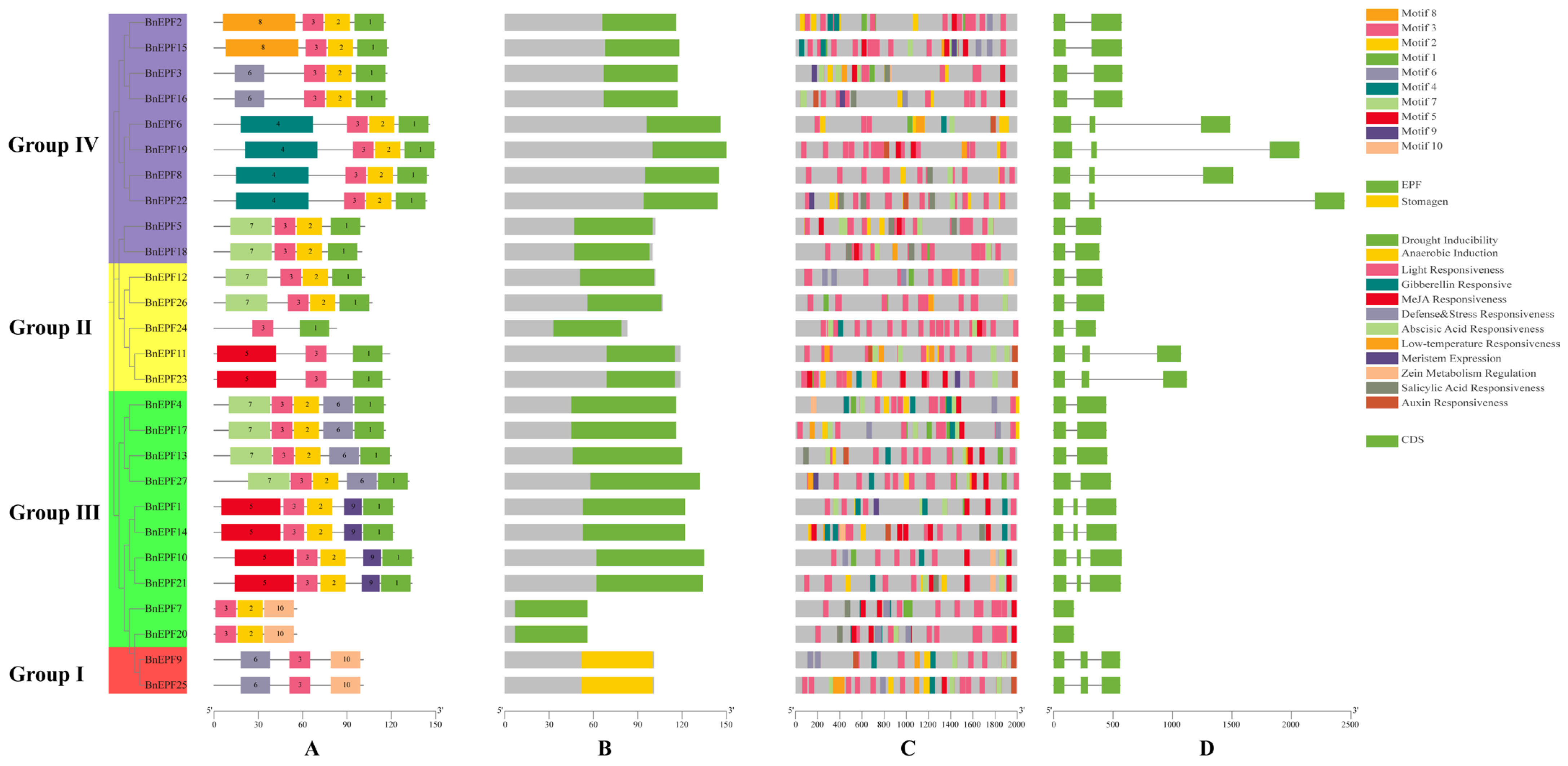
2.3. Prediction of Gene Structure, Conserved Motifs in prOTEins, and Cis-Regulatory Elements in Promoters
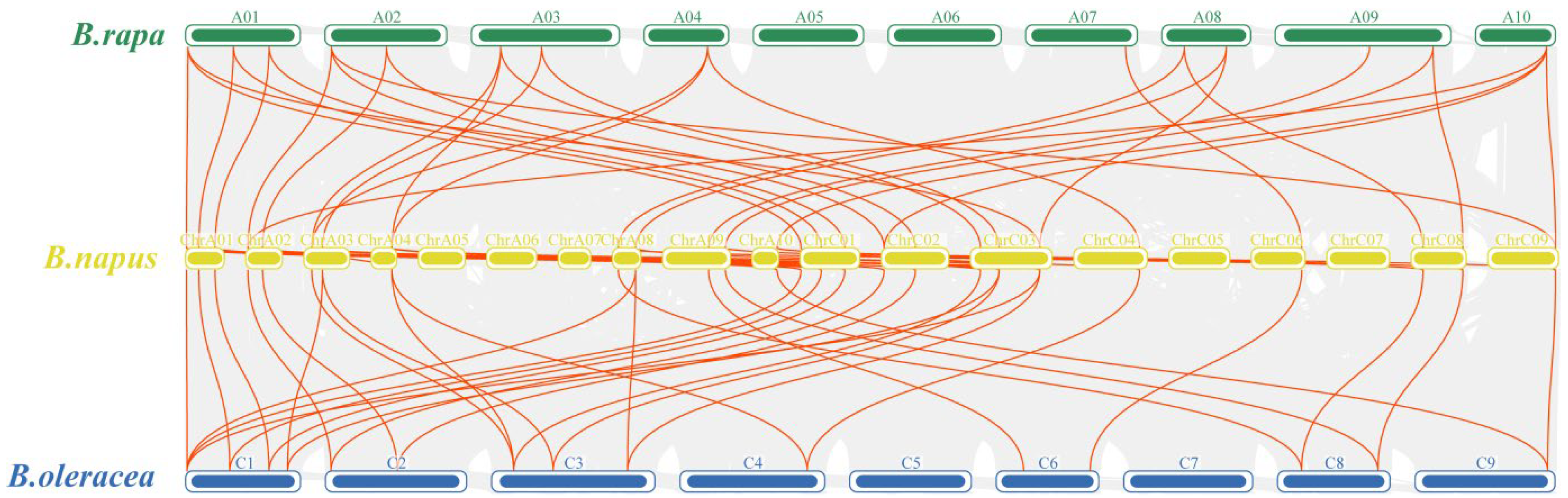
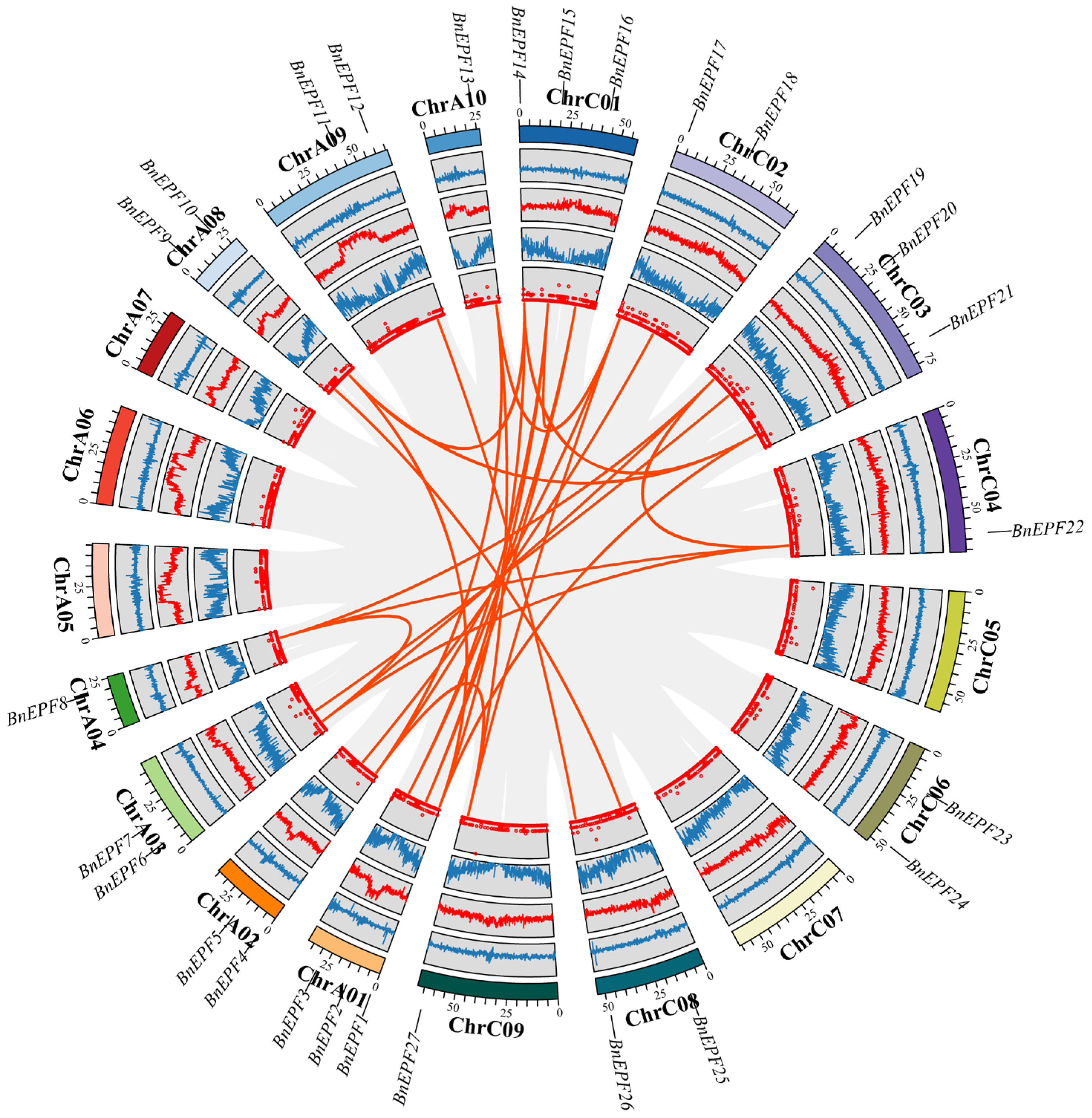
2.4. Collinearity Analysis of EPF Genes
2.5. Prediction of the Interaction between miRNA and BnEPFs
2.6. Analysis of Transcriptome-Wide Expression Patterns
2.7. Materials and Treatment
2.8. qRT-PCR Analysis of EPF Gene Expression Patterns
2.9. Statistical Procedures Were Applied for Data Analysis
3. Results
3.1. Identification and Characterization of EPFs in Rapeseed
3.2. Physical and Chemical Characteristics along with Subcellular Localization Prediction of BnEPF Proteins
3.3. Evolutionary Analysis of the EPF Family
3.4. Analysis of EPF Family Structure, Conserved Motifs, and Cis-Regulatory Elements in the Promoter
3.5. Collinearity Analysis of EPF Gene Family
3.6. Prediction of miRNA Targeting Relationships with BnEPFs
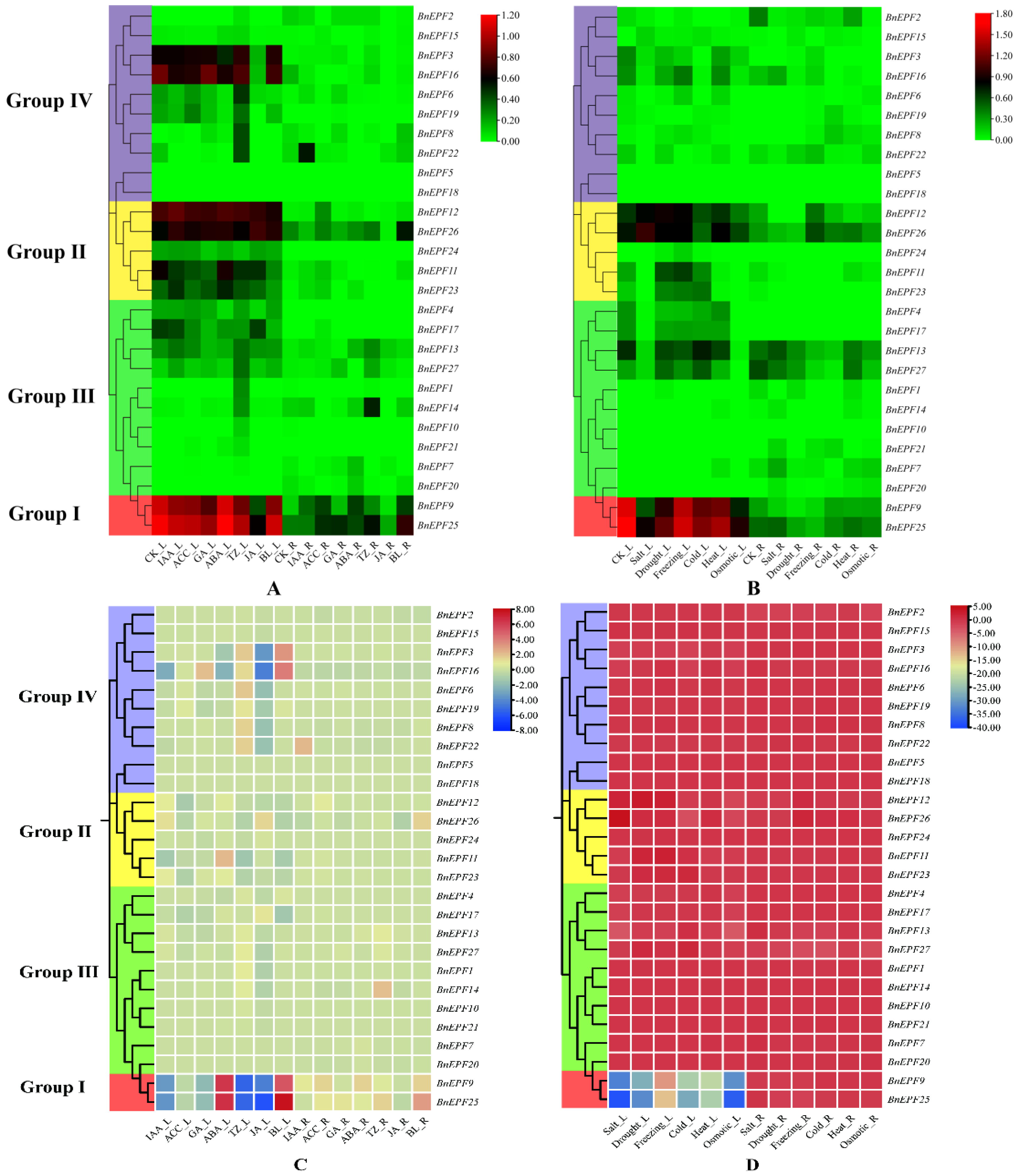
3.7. Examination of Transcriptome Expression Profiles of BnEPFs across Various Exogenous Hormone Treatments and Abiotic Stress Conditions
3.8. Analysis of BnEPF Expression under Salt Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harwood, J.L.; Woodfield, H.K.; Chen, G.; Weselake, R.J. Chapter 6—Modification of oil crops to produce fatty acids for industrial applications. In Fatty Acids; Ahmad, M.U., Ed.; AOCS Press: Champaign, IL, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 187–236. [Google Scholar]
- Zubair, M.; Maqbool, F.; Mehmood, I.; Muzammil, S.; Waseem, M.; Imran, M.; Nadeem, H.U.; Azeem, F.; Siddique, M.H. Chapter 4–Rapeseed oil. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, B.R., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–55. [Google Scholar]
- Sun, R. Economic/Academic Importance of Brassica rapa; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Neik, T.X.; Barbetti, M.J.; Batley, J. Current status and challenges in identifying disease resistance genes in Brassica napus. Front. Plant Sci. 2017, 8, 1788. [Google Scholar] [CrossRef]
- Hasanuzzaman; Zhou, M.; Shabala, S. How Does Stomatal Density and Residual Transpiration Contribute to Osmotic Stress Tolerance? Plants 2023, 12, 494. [Google Scholar] [CrossRef]
- Peng, P.; Li, R.; Chen, Z.-H.; Wang, Y. Stomata at the crossroad of molecular interaction between biotic and abiotic stress responses in plants. Front. Plant Sci. 2022, 13, 1031891. [Google Scholar] [CrossRef]
- Harrison, E.L.; Cubas, L.A.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop. J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Katsir, L.; Davies, K.A.; Bergmann, D.C.; Laux, T. Peptide Signaling in Plant Development. Curr. Biol. 2011, 21, R356–R364. [Google Scholar] [CrossRef]
- Hara, K.; Yokoo, T.; Kajita, R.; Onishi, T.; Yahata, S.; Peterson, K.M.; Torii, K.U.; Kakimoto, T. Epidermal Cell Density is Autoregulated via a Secretory Peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis Leaves. Plant Cell Physiol. 2009, 50, 1019–1031. [Google Scholar] [CrossRef]
- Lee, J.S.; Hnilova, M.; Maes, M.; Lin, Y.-C.L.; Putarjunan, A.; Han, S.-K.; Avila, J.; Torii, K.U. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 2015, 522, 439–443. [Google Scholar] [CrossRef]
- Takata, N.; Yokota, K.; Ohki, S.; Mori, M.; Taniguchi, T.; Kurita, M. Evolutionary Relationship and Structural Characterization of the EPF/EPFL Gene Family. PLoS ONE 2013, 8, e65183. [Google Scholar] [CrossRef]
- Caine, R.S.; Chater, C.C.; Kamisugi, Y.; Cuming, A.C.; Beerling, D.J.; Gray, J.E.; Fleming, A.J. An ancestral stomatal patterning module revealed in the non-vascular land plant Physcomitrella patens. Development 2016, 143, 3306–3314. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef]
- Lu, J.; He, J.; Zhou, X.; Zhong, J.; Li, J.; Liang, Y.-K. Homologous genes of epidermal patterning factor regulate stomatal development in rice. J. Plant Physiol. 2019, 234–235, 18–27. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2018, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Hunt, L.; Afsharinafar, M.; Al Meselmani, M.; Mitchell, A.; Howells, R.; Wallington, E.; Fleming, A.J.; E Gray, J. Reduced stomatal density in bread wheat leads to increased water-use efficiency. J. Exp. Bot. 2019, 70, 4737–4748. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, T.; Zhang, C.; Li, J.; Wang, Z.; Li, H.; Liu, X.; Yin, L.; Wang, S.; Zhang, S.; et al. Overexpression of the potato StEPF2 gene confers enhanced drought tolerance in Arabidopsis. Plant Biotechnol. Rep. 2020, 14, 479–490. [Google Scholar] [CrossRef]
- Liu, S.; Jia, F.; Jiao, Z.; Wang, J.; Xia, X.; Yin, W. Ectopic expression of secretory peptide PdEPF3 in Arabidopsis confers drought tolerance with reduced stomatal density. Acta Soc. Bot. Pol. 2019, 88, 1–12. [Google Scholar] [CrossRef]
- Uchida, N.; Lee, J.S.; Horst, R.J.; Lai, H.-H.; Kajita, R.; Kakimoto, T.; Tasaka, M.; Torii, K.U. Regulation of inflorescence architecture by intertissue layer ligand–receptor communication between endodermis and phloem. Proc. Natl. Acad. Sci. USA 2012, 109, 6337–6342. [Google Scholar] [CrossRef] [PubMed]
- Abrash, E.B.; Davies, K.A.; Bergmann, D.C. Generation of Signaling Specificity in Arabidopsis by Spatially Restricted Buffering of Ligand–Receptor Interactions. Plant Cell 2011, 23, 2864–2879. [Google Scholar] [CrossRef]
- Chen, H.; Wang, T.; He, X.; Cai, X.; Lin, R.; Liang, J.; Wu, J.; King, G.; Wang, X. BRAD V3.0: An upgraded Brassicaceae database. Nucleic Acids Res. 2021, 50, D1432–D1441. [Google Scholar] [CrossRef]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M.; et al. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2011, 40, D1202–D1210. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; López, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-S.; Chen, Y.-C.; Lu, C.-H.; Hwang, J.-K. Prediction of protein subcellular localization. Proteins 2006, 64, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z. KaKs_Calculator 3.0: Calculating Selective Pressure on Coding and Non-Coding Sequences. Genom. Proteom. Bioinform. 2022, 20, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, S.; Wei, L.; Huang, Y.; Liu, D.; Jia, Y.; Luo, C.; Lin, Y.; Liang, C.; Hu, Y.; et al. BnIR: A multi-omics database with various tools for Brassica napus research and breeding. Mol. Plant 2023, 16, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Livak, K.J.; Schmittgen, T.J.M. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hua, L.; Zhu, Z.; Tan, L.; Zhao, X.; Zhang, W.; Liu, F.; Fu, Y.; Cai, H.; Sun, X.; et al. GAD1 Encodes a Secreted Peptide That Regulates Grain Number, Grain Length, and Awn Development in Rice Domestication. Plant Cell 2016, 28, 2453–2463. [Google Scholar] [CrossRef]
- Jiao, Z.; Han, S.; Li, Z.; Huang, M.; Niu, M.-X.; Yu, X.; Liu, C.; Wang, H.-L.; Yin, W.; Xia, X. PdEPFL6 reduces stomatal density to improve drought tolerance in poplar. Ind. Crops Prod. 2022, 182, 114873. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.P.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Liang, Y.; Chen, S.; Yuan, Y.; Chen, Y.; Hu, H. Bna.EPF2 Enhances Drought Tolerance by Regulating Stomatal Development and Stomatal Size in Brassica napus. Int. J. Mol. Sci. 2023, 24, 8007. [Google Scholar] [CrossRef]
- Richardson, L.G.L.; Torii, K.U. Take a deep breath: Peptide signalling in stomatal patterning and differentiation. J. Exp. Bot. 2013, 64, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Gabaldón, T.; Koonin, E.V. Functional and evolutionary implications of gene orthology. Nature reviews. Genetics 2013, 14, 360–366. [Google Scholar] [PubMed]
- Shen, X.X.; Salichos, L.; Rokas, A. A genome-scale investigation of how sequence, function, and treebased gene properties influence phylogenetic inference. Genome Biol. Evol. 2016, 8, 2565–2580. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Wang, J.; Shi, Y.; Wang, Z.; Zhang, J.; Du, Q.; Liu, B.; Jia, X.; Niu, J.; Gu, C.; et al. Genome-Wide Identification and Analysis of the EPF Gene Family in Sorghum bicolor (L.) Moench. Plants 2023, 12, 3912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Rajaofera, M.J.N.; Zhu, L.; Liu, W.; Zheng, F.; Miao, W. WY7 is a newly identified promoter from the rubber powdery mildew pathogen that regulates exogenous gene expression in both monocots and dicots. PLoS ONE 2020, 15, e233911. [Google Scholar] [CrossRef]
- Chen, X.L.; Song, R.T.; Yu, M.Y.; Sui, J.M.; Wang, J.S.; Qiao, L.X. Cloning and functional analysis of the chitinase gene promoter in peanut. Genet. Mol. Res. 2015, 14, 12710–12722. [Google Scholar] [CrossRef]
- Bessho-Uehara, K.; Wang, D.R.; Furuta, T.; Minami, A.; Nagai, K.; Gamuyao, R.; Asano, K.; Angeles-Shim, R.B.; Shimizu, Y.; Ayano, M.; et al. Loss of function at RAE2, a previously unidentified EPFL, is required for awnlessness in cultivated Asian rice. Proc. Natl. Acad. Sci. USA 2016, 113, 8969–8974. [Google Scholar] [CrossRef]




| Gene Name | Forward | Reverse |
|---|---|---|
| BnEPF3 | GCTCCCACCGTCGTTTCA | TGACCCACCTGCCTCCTT |
| BnEPF9 | TGATTGGGTCGACGGCAC | TGCCTTCAACGGGGACTTG |
| BnEPF12 | GCATTTTCCGACGCACCC | CACAAGACGGCATGGGGA |
| BnEPF13 | CAAGTCCCCACTCTGCCG | GGGAGGAAGGAGGCCGTA |
| BnEPF16 | TGCTCCCACCGTCGTTTC | GAGGCGGTTGCGGAGTAG |
| BnEPF17 | CCGAGCTGCCACAACAGA | GGGAGGAAGGAGGCCGTA |
| BnEPF25 | TGATTGGGTCGACGGCAC | TGCCTTCAACGGGGACTTG |
| BnEPF26 | GTCTAGGCTGCCGGACTG | GCGATGCACACACGAAGC |
| BnEPF27 | CTACACCACCGAGCTGCC | GGGAGGAAGGAGGCCGTA |
| Actin | TGGGTTTGCTGGTGACGAT | TGCCTAGGACGACCAACAATACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, W.; Chen, J.; Wan, H.; Zhao, H.; Liu, X.; Dai, X.; Zeng, C.; Xu, D. Comprehensive Identification and Expression Profiling of Epidermal Pattern Factor (EPF) Gene Family in Oilseed Rape (Brassica napus L.) under Salt Stress. Genes 2024, 15, 912. https://doi.org/10.3390/genes15070912
Wang S, Wang W, Chen J, Wan H, Zhao H, Liu X, Dai X, Zeng C, Xu D. Comprehensive Identification and Expression Profiling of Epidermal Pattern Factor (EPF) Gene Family in Oilseed Rape (Brassica napus L.) under Salt Stress. Genes. 2024; 15(7):912. https://doi.org/10.3390/genes15070912
Chicago/Turabian StyleWang, Shanshan, Wei Wang, Jingdong Chen, Heping Wan, Huixia Zhao, Xiaoyun Liu, Xigang Dai, Changli Zeng, and Danyun Xu. 2024. "Comprehensive Identification and Expression Profiling of Epidermal Pattern Factor (EPF) Gene Family in Oilseed Rape (Brassica napus L.) under Salt Stress" Genes 15, no. 7: 912. https://doi.org/10.3390/genes15070912





