Exploring the Influence of Fok1/Apa1 Polymorphic Variants on Adolescent Mental Health and Response to Vitamin D Supplementation in Embryonic Hippocampal Cell Lines
Abstract
:1. Introduction
2. Results
2.1. VDR Polymorphic Variant in Adolescents
2.2. Difference in Depressive and Anxious Symptoms in Relation to Fok 1 and Apa1 Polymorphic Variants
2.3. VDR Polymorphic Variant in Embryonic Hippocampal Cell Lines
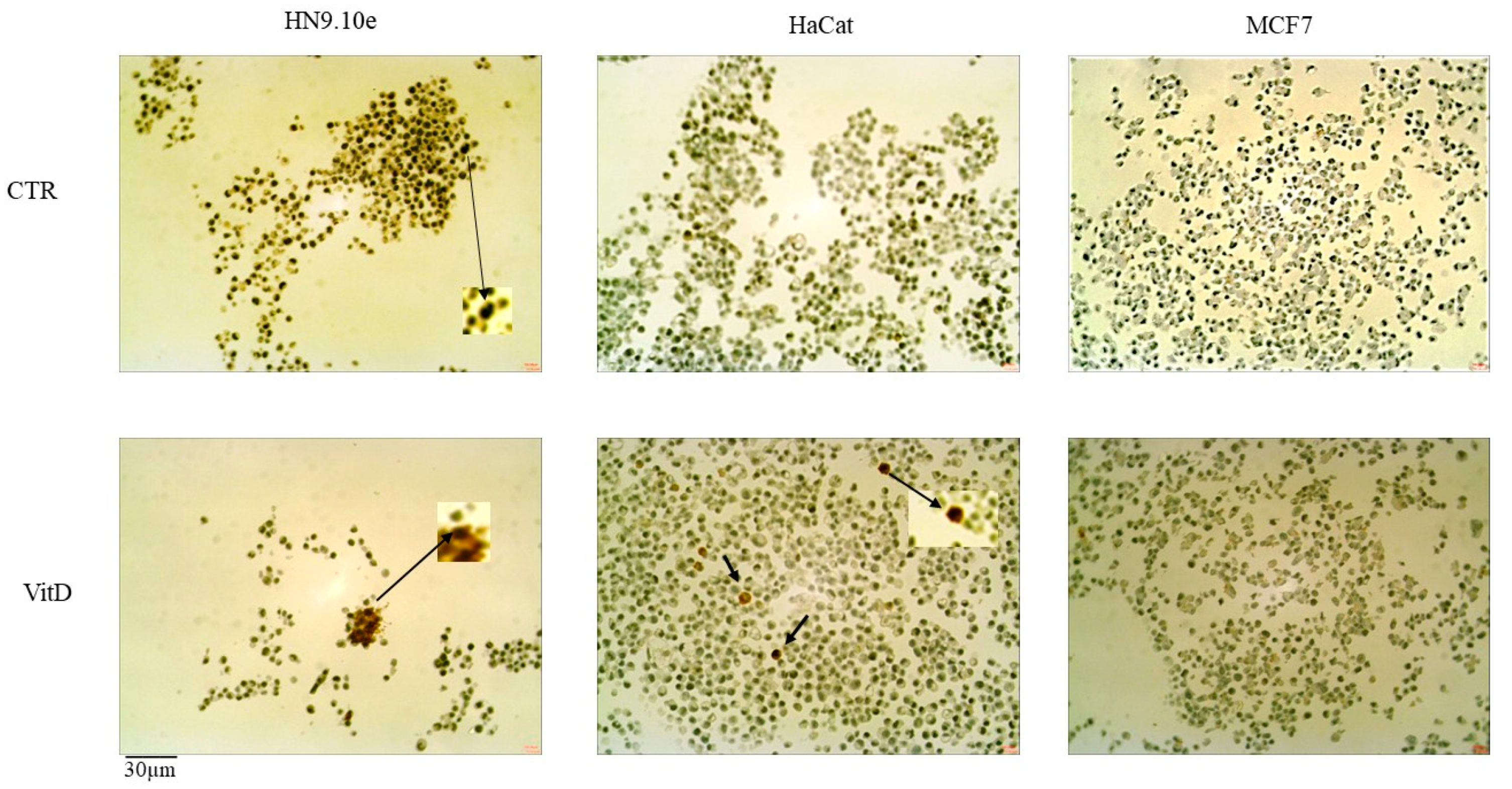
2.4. Effect of Vitamin D3 on Cell Lines with Different VDR Polymorphic Variants
3. Discussion
4. Materials and Methods
4.1. Experimental Plan
4.2. Study Population
4.3. Ethics Approval and Informed Consent
4.4. Assessment of Anxiety Symptoms
4.5. Assessment of Depression Symptoms
4.6. Collection of Saliva Samples
4.7. Genomic DNA Extraction
4.8. Genotyping of Fok1 and Apo1 SNPs
4.9. Cell Culture and Treatments
4.10. Quantitative Real-Time RT-PCR
4.11. Immunocytochemistry
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlberg, C.; Raczyk, M.; Zawrotna, N. Vitamin D: A master example of nutrigenomics. Redox Biol. 2023, 62, 102695. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Liberman, U.; Bikle, D.D. Disorders in the Action of Vitamin D. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. [Google Scholar]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Genomic signaling of vitamin D. Steroids 2023, 198, 109271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Hua, L.; Li, P.; Wu, J.; Shang, S.; Deng, F.; Luo, J.; Liao, M.; Wang, N.; et al. Vitamin D receptor (VDR) on the cell membrane of mouse macrophages participates in the formation of lipopolysaccharide tolerance: mVDR is related to the effect of artesunate to reverse LPS tolerance. Cell Commun Signal. 2023, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Sitrin, M.D.; Bissonnette, M.; Bolt, M.J.; Wali, R.; Khare, S.; Scaglione-Sewell, B.; Skarosi, S.; Brasitus, T.A. Rapid effects of 1,25(OH)2 vitamin D3 on signal transduction systems in colonic cells. Steroids 1999, 64, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Kanemoto, Y.; Sawada, T.; Nojiri, K.; Kurokawa, T.; Tsutsumi, R.; Nagasawa, K.; Kato, S. Differential gene regulation by a synthetic vitamin D receptor ligand and active vitamin D in human cells. PLoS One 2023, 18, e0295288. [Google Scholar] [CrossRef] [PubMed]
- Seuter, S.; Neme, A.; Carlberg, C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 2016, 44, 4090–4104. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Mailhot, G.; White, J.H. Vitamin D and Immunity in Infants and Children. Nutrients 2020, 12, 1233. [Google Scholar] [CrossRef]
- Bollen, S.E.; Bass, J.J.; Fujita, S.; Wilkinson, D.; Hewison, M.; Atherton, P.J. The Vitamin D/Vitamin D receptor (VDR) axis in muscle atrophy and sarcopenia. Cell. Signal. 2022, 96, 110355. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Baldi, E.; Bucherelli, C.; Ferri, I.; Sidoni, A.; Codini, M.; Conte, C.; Beccari, T.; Traina, G. Vitamin D receptor expression and acid sphingomyelinase activity in prefrontal region of a learning animal model. Arch. Ital. Biol. 2019, 157, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Arcuri, C.; Cataldi, S.; Mecca, C.; Codini, M.; Ceccarini, M.R.; Patria, F.F.; Beccari, T.; Albi, E. Niemann-Pick Type A Disease: Behavior of Neutral Sphingomyelinase and Vitamin D Receptor. Int. J. Mol. Sci. 2019, 20, 2365. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Arcuri, C.; Hunot, S.; Mecca, C.; Codini, M.; Laurenti, M.E.; Ferri, I.; Loreti, E.; Garcia-Gil, M.; Traina, G.; et al. Effect of Vitamin D in HN9.10e Embryonic Hippocampal Cells and in Hippocampus from MPTP-Induced Parkinson’s Disease Mouse Model. Front. Cell. Neurosci. 2018, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Markov, G.V.; Laudet, V. Origin and evolution of the ligand-binding ability of nuclear receptors. Mol. Cell. Endocrinol. 2011, 334, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Sahli, H.; Souissi, C.; Sellami, S.; Ben Ammar El Gaaied, A. polymorphic variant in VDR gene in Tunisian postmenopausal women are associated with osteopenia phenotype. Climacteric: The journal of the International Menopause. Society 2015, 18, 624–630. [Google Scholar]
- Hallak, J.A.; Tibrewal, S.; Mohindra, N.; Gao, X.; Jain, S. Single Nucleotide polymorphic variant in the BDNF, VDR, and DNASE 1 Genes in Dry Eye Disease Patients: A Case-Control Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5990–5996. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.M.; Esmaieli-Bandboni, A.; Veisi Malekshahi, Z.; Shahbaz Sardood, M.; Hashemi, M.; Majidzadeh, K.; Kadkhodazadeh, M.; Esmaili, R.; Negahdari, B. Vitamin D receptor gene polymorphic variant and risk of breast cancer in Iranian women. Ann. Med. Surg. 2021, 73, 103150. [Google Scholar] [CrossRef]
- Jiang, P.; Zhu, W.Y.; He, X.; Tang, M.M.; Dang, R.L.; Li, H.D.; Xue, Y.; Zhang, L.H.; Wu, Y.Q.; Cao, L.J. Association between Vitamin D Receptor Gene polymorphic variant with Childhood Temporal Lobe Epilepsy. Int. J. Environ. Res. Public Health 2015, 12, 13913–13922. [Google Scholar] [CrossRef]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, L.; Chen, B.; Wang, X. Vitamin D receptor rs2228570 polymorphism and Parkinson’s disease risk in a Chinese population. Neurosci. Lett. 2020, 717, 134722. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, X. The Correlation Between Vitamin D Receptor (VDR) Gene polymorphic variant and Autism: A Meta-analysis. J. Mol. Neurosci. 2020, 70, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Guerini, F.R.; Bolognesi, E.; Chiappedi, M.; Mensi, M.M.; Fumagalli, O.; Rogantini, C.; Zanzottera, M.; Ghezzo, A.; Zanette, M.; Agliardi, C.; et al. Vitamin D Receptor polymorphic variant Associated with Autism Spectrum Disorder. Autism. Res. 2020, 13, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Handoko, H.Y.; Nancarrow, D.J.; Mowry, B.J.; McGrath, J.J. Polymorphisms in the vitamin D receptor and their associations with risk of schizophrenia and selected anthropometric measures. Am. J. Hum. Biol. 2006, 18, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.J.; Refsum, H.; Warden, D.R.; Medway, C.; Wilcock, G.K.; Smith, A.D. The vitamin D receptor gene is associated with Alzheimer’s disease. Neurosci. Lett. 2011, 504, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Kuningas, M.; Mooijaart, S.P.; Jolles, J.; Slagboom, P.E.; Westendorp, R.G.; van Heemst, D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol. Aging 2009, 30, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Lye, M.S.; Tey, Y.Y.; Tor, Y.S.; Shahabudin, A.F.; Ibrahim, N.; Ling, K.H.; Stanslas, J.; Loh, S.P.; Rosli, R.; Lokman, K.A.; et al. Predictors of recurrence of major depressive disorder. PLoS ONE 2020, 15, e0230363. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.M.; Rawlins, J.N.; McHugh, S.B.; Deacon, R.M.; Yee, B.K.; Bast, T.; Zhang, W.N.; Pothuizen, H.H.; Feldon, J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Idunkova, A.; Lacinova, L.; Dubiel-Hoppanova, L. Stress, depression, and hippocampus: From biochemistry to electrophysiology. Gen. Physiol. Biophys. 2023, 42, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Lye, M.S.; Tor, Y.S.; Tey, Y.Y.; Shahabudin, A.; Loh, S.P.; Ibrahim, N.; Stanslas, J.; Rosli, R.; Ling, K.H. BsmI-ApaI-TaqI TAC (BAt) Haplotype of Vitamin D Receptor Gene Is Associated with Increased Risk of Major Depressive Disorder. J. Mol. Neurosci. 2021, 71, 981–990. [Google Scholar] [CrossRef]
- Bozdogan, S.T.; Kutuk, M.O.; Tufan, E.; Altıntaş, Z.; Temel, G.O.; Toros, F. No Association between polymorphic variant of Vitamin D and Oxytocin Receptor Genes and Autistic Spectrum Disorder in a Sample of Turkish Children. Clin. Psychopharmacol. Neurosci. 2018, 16, 415–421. [Google Scholar] [CrossRef]
- Can, M.Ş.; Baykan, H.; Baykan, Ö.; Erensoy, N.; Karlıdere, T. Vitamin D Levels and Vitamin D Receptor Gene Polymorphism in Major Depression. Psychiatr. Danub. 2017, 29, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Usategui-Martín, R.; De Luis-Román, D.A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.L. Vitamin D Receptor (VDR) Gene polymorphic variant Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef]
- Gleba, J.J.; Kłopotowska, D.; Banach, J.; Turlej, E.; Mielko, K.A.; Gębura, K.; Bogunia-Kubik, K.; Kutner, A.; Wietrzyk, J. Polymorphism of VDR Gene and the Sensitivity of Human Leukemia and Lymphoma Cells to Active Forms of Vitamin D. Cancers 2022, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Maj, E.; Trynda, J.; Maj, B.; Gębura, K.; Bogunia-Kubik, K.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Differential response of lung cancer cell lines to vitamin D derivatives depending on EGFR, KRAS, p53 mutation status and VDR polymorphism. J. Steroid Biochem. Mol. Biol. 2019, 193, 105431. [Google Scholar] [CrossRef]
- Cataldi, S.; Ceccarini, M.R.; Patria, F.; Beccari, T.; Mandarano, M.; Ferri, I.; Lazzarini, A.; Curcio, F.; Albi, E. The Effect of Vitamin D3 and Silver Nanoparticles on HaCaT Cell Viability. Int. J. Mol. Sci. 2022, 23, 1410. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, M.R.; Fittipaldi, S.; Ciccacci, C.; Granese, E.; Centofanti, F.; Dalla Ragione, L.; Bertelli, M.; Beccari, T.; Botta, A. Association Between DRD2 and DRD4 polymorphic variant and Eating Disorders in an Italian Population. Front. Nutr. 2022, 9, 838177. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, F.; Domenis, R.; Dalla, E.; Cataldi, S.; Conte, C.; Mandarano, M.; Sidoni, A.; Cifù, A.; Beccari, T.; Mirarchi, A.; et al. Ceramide releases exosomes with a specific miRNA signature for cell differentiation. Sci. Rep. 2023, 13, 10993. [Google Scholar] [CrossRef]
- Codini, M.; Fiorani, F.; Mandarano, M.; Cataldi, S.; Arcuri, C.; Mirarchi, A.; Ceccarini, M.R.; Beccari, T.; Kobayashi, T.; Tomishige, N.; et al. Sphingomyelin Metabolism Modifies Luminal A Breast Cancer Cell Line under a High Dose of Vitamin C. Int. J. Mol. Sci. 2023, 24, 17263. [Google Scholar] [CrossRef]

| Fok1 genotype | ||||||||
| AA | GG | AG | Total | |||||
| n | % | n | % | n | % | n | % | |
| Total population | 79 | 42.9 | 18 | 9.8 | 87 | 47.3 | 184 | 100 |
| Males | 30 | 38.0 | 5 | 27.8 | 33 | 37.9 | 68 | 37 |
| Females | 49 | 62.0 | 13 | 72.2 | 53 | 62.1 | 116 | 63 |
| Apa1 genotype | ||||||||
| CC | AA | CA | Total | |||||
| n | % | n | % | n | % | n | % | |
| Total population | 57 | 31 | 46 | 25 | 81 | 44 | 184 | 100 |
| Males | 24 | 42.1 | 17 | 37.0 | 27 | 33.3 | 68 | 37 |
| Females | 33 | 57.9 | 29 | 63.0 | 54 | 66.7 | 116 | 63 |
| Fok1 genotypes | |||
| AA | GG | AG | |
| % | % | % | |
| Italian parents | 40 | 9 | 51 |
| Foreign parents | 53 | 9 | 38 |
| Foreign mother | 34 | 7 | 59 |
| Foreign father | 64 | 9 | 27 |
| Apa1 genotypes | |||
| CC | AA | CA | |
| % | % | % | |
| Italian parents | 31 | 24 | 45 |
| Foreign parents | 24 | 29 | 47 |
| Foreign mother | 28 | 28 | 44 |
| Foreign father | 18 | 32 | 50 |
| Age | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Depression | CTR | Anxiety | CTR | Depression | CTR | Anxiety | CTR | |
| total | 25.0 * | 75.0 | 30.9 * | 69.1 | 67.2 *§ | 32.8 | 67.2 *§ | 32.8 |
| 14 | 11.8 * | 88.2 | 35.3 * | 64.7 | 70.6 *§ | 29.4 | 60.8 *§ | 39.2 |
| 15 | 7.1 * | 92.9 | 7.1 * | 92.9 | 68.2 *§ | 31.8 | 68.2 *§ | 31.8 |
| 16 | 38.5 * | 61.5 | 30.8 * | 69.2 | 57.9 *§ | 42.1 | 71.1 *§ | 28.9 |
| 17 | 36.4 * | 63.6 | 54.5 * | 45.5 | 100 *§ | 0 | 100 *§ | 0 |
| Score Depression | Score Anxiety | ||||
|---|---|---|---|---|---|
| Anxiety | 62.10 (9.57) | p < 0.001 | Depression | 62.42 (8.52) | p < 0.001 |
| CTR | 47.27 (8.53) | CTR | 48.36 (7.57) | ||
| a | |||||||
| Model | Symptoms | Fok1 | Apa1 | ||||
| Genotype | F(2) | p | Genotype | F(2) | p | ||
| Allelic model | Depression | AA GG AG | 1.000 | 0.370 | CC AA AC | 0.703 | 0.496 |
| Anxiety | 0.029 | 0.971 | 2.300 | 0.103 | |||
| b | |||||||
| Model | Symptoms | Fok1 | Apa1 | ||||
| Genotype | t | p | Genotype | t | p | ||
| Dominant model | Depression | AA + AG versus GG | −0.407 | 0.684 | CC + CA versus AA | 0.790 | 0.430 |
| Anxiety | 0.162 | 0.872 | 1.897 | 0.059 | |||
| Recessive model | Depression | GG + AG versus AA | −182 | 0.239 | AA + CA versus CC | 0.510 | 0.610 |
| Anxiety | −0.218 | 0.828 | 0.187 | 0.852 | |||
| Cell Line | Fok1 | Apa1 |
|---|---|---|
| HN9.10e | AA | CC |
| HaCat | AG | AC |
| MCF7 | GG | AA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gizzi, G.; Fiorani, F.; Cataldi, S.; Mandarano, M.; Delvecchio, E.; Mazzeschi, C.; Albi, E. Exploring the Influence of Fok1/Apa1 Polymorphic Variants on Adolescent Mental Health and Response to Vitamin D Supplementation in Embryonic Hippocampal Cell Lines. Genes 2024, 15, 913. https://doi.org/10.3390/genes15070913
Gizzi G, Fiorani F, Cataldi S, Mandarano M, Delvecchio E, Mazzeschi C, Albi E. Exploring the Influence of Fok1/Apa1 Polymorphic Variants on Adolescent Mental Health and Response to Vitamin D Supplementation in Embryonic Hippocampal Cell Lines. Genes. 2024; 15(7):913. https://doi.org/10.3390/genes15070913
Chicago/Turabian StyleGizzi, Giulia, Federico Fiorani, Samuela Cataldi, Martina Mandarano, Elisa Delvecchio, Claudia Mazzeschi, and Elisabetta Albi. 2024. "Exploring the Influence of Fok1/Apa1 Polymorphic Variants on Adolescent Mental Health and Response to Vitamin D Supplementation in Embryonic Hippocampal Cell Lines" Genes 15, no. 7: 913. https://doi.org/10.3390/genes15070913





