A Unique Comprehensive Model to Screen Newborns for Severe Combined Immunodeficiency—An Ontario Single-Centre Experience Spanning 2013–2023
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Newborn Screening
2.3. Measurement of TREC levels
2.4. SCID Diagnosis
3. Results
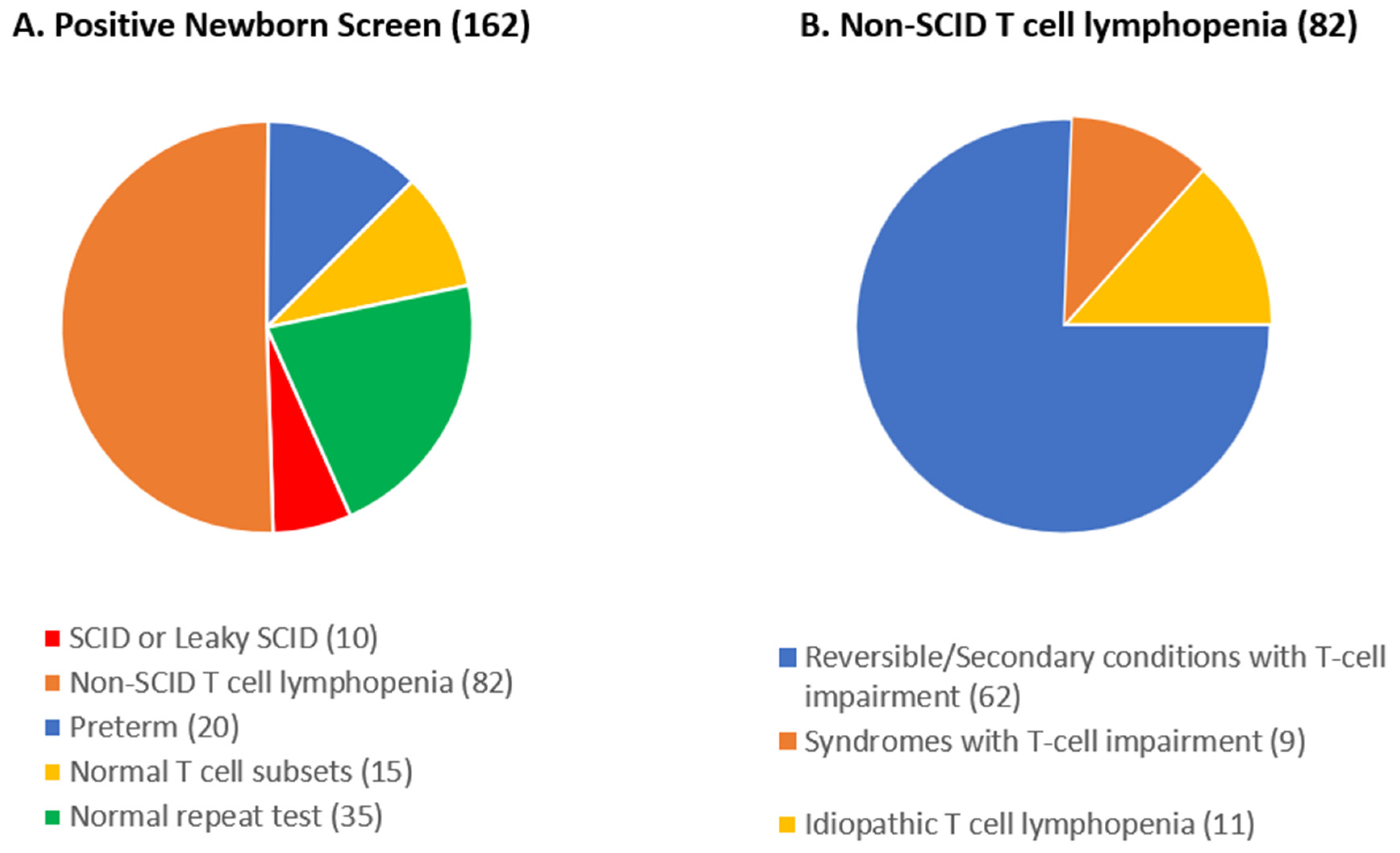
3.1. SCID NBS within Catchment Area
3.2. SCID Infants Detected by NBS in Ontario
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roifman, C.M. Primary T-cell Immunodeficiencies. In Clinical Immunology: Principles and Practice, 5th ed.; Rich, R., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef]
- Ovadia, A.; Roifman, C.M. Principles of Treatment of Primary Immunodeficiencies. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Elsevier: Waltham, MA, USA, 2016; pp. 513–521. [Google Scholar]
- Brown, L.; Xu-Bayford, J.; Allwood, Z.; Slatter, M.; Cant, A.; Davies, E.G.; Veys, P.; Gennery, A.R.; Gaspar, H.B. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: The case for newborn screening. Blood 2011, 117, 3243–3246. [Google Scholar] [CrossRef] [PubMed]
- Thakar, M.S.; Logan, B.R.; Puck, J.M.; Dunn, E.A.; Buckley, R.H.; Cowan, M.J.; O’Reilly, R.J.; Kapoor, N.; Satter, L.F.; Pai, S.-Y.; et al. Measuring the effect of newborn screening on survival after haematopoietic cell transplantation for severe combined immunodeficiency: A 36-year longitudinal study from the Primary Immune Deficiency Treatment Consortium. Lancet 2023, 402, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Puck, J.M. Development of population-based newborn screening for severe combined immunodeficiency. J. Allergy Clin. Immunol. 2005, 115, 391–398. [Google Scholar] [CrossRef]
- Kwan, A.; Puck, J.M. History and current status of newborn screening for severe combined immunodeficiency. Semin. Perinatol. 2015, 39, 194–205. [Google Scholar] [CrossRef]
- Puck, J.M. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia. Immunol. Rev. 2019, 287, 241–252. [Google Scholar] [CrossRef]
- Verbsky, J.; Thakar, M.; Routes, J. The Wisconsin approach to newborn screening for severe combined immunodeficiency. J. Allergy Clin. Immunol. 2012, 129, 622–627. [Google Scholar] [CrossRef]
- Kwan, A.; Hu, D.; Song, M.; Gomes, H.; Brown, D.R.; Bourque, T.; Gonzalez-Espinosa, D.; Lin, Z.; Cowan, M.J.; Puck, J.M. Successful newborn screening for SCID in the Navajo Nation. Clin. Immunol. 2015, 158, 29–34. [Google Scholar] [CrossRef]
- Cross, C. Ontario newborns now screened for SCID. Can. Med. Assoc. J. 2013, 185, E616. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.E.; Ovadia, A.; Schejter, Y.D. Managing Newborn Screening for SCID in a Referral Centre. LymphoSign J. 2017, 4, 77–79. [Google Scholar] [CrossRef]
- Roifman, C.M.; Vong, L. Management of newborn screening for severe combined immunodeficiency at a quaternary referral centre—An updated algorithm. LymphoSign J. 2023, 10, 36–41. [Google Scholar] [CrossRef]
- Abstracts from the Immunodeficiency Canada—8th SCID Symposium, 22 October 2020. LymphoSign J. 2020, 7, 122–151. [CrossRef]
- Abstracts of the Immunodeficiency Canada 10th PID Symposium, 19 October 2023, Ottawa. LymphoSign J. 2023, 10, 48–65. [CrossRef]
- Suresh, S.; Dadi, H.; Reid, B.; Vong, L.; Bulman, D.E.; Roifman, C.M. Time-dependent decline of T-cell receptor excision circle levels in ZAP-70 deficiency. J. Allergy Clin. Immunol. Pract. 2019, 8, 806–808.e2. [Google Scholar] [CrossRef] [PubMed]
- Douek, D.C.; McFarland, R.D.; Keiser, P.H.; Gage, E.A.; Massey, J.M.; Haynes, B.F.; Polis, M.A.; Haase, A.T.; Feinberg, M.B.; Sullivan, J.L.; et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998, 396, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Shearer, W.T.; Dunn, E.; Notarangelo, L.D.; Dvorak, C.C.; Puck, J.M.; Logan, B.R.; Griffith, L.M.; Kohn, D.B.; O’Reilly, R.J.; Fleisher, T.A.; et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: The Primary Immune Deficiency Treatment Consortium experience. J. Allergy Clin. Immunol. 2014, 133, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Blom, M.; Zetterström, R.H.; Stray-Pedersen, A.; Gilmour, K.; Gennery, A.R.; Puck, J.M.; van der Burg, M. Recommendations for uniform definitions used in newborn screening for severe combined immunodeficiency. J. Allergy Clin. Immunol. 2022, 149, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, J.; Junker, A.; Thibodeau, M.L.; Grenier, D.; Turvey, S.E.; Yacoub, W.; Embree, J.; Haddad, E.; Langley, J.M.; Ramsingh, R.M.; et al. Severe Combined Immunodeficiency (SCID) in Canadian Children: A National Surveillance Study. J. Clin. Immunol. 2013, 33, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.; Abraham, R.S.; Currier, R.; Brower, A.; Andruszewski, K.; Abbott, J.K.; Baker, M.; Ballow, M.; Bartoshesky, L.E.; Bonagura, V.R.; et al. Newborn Screening for Severe Combined Immunodeficiency in 11 Screening Programs in the United States. JAMA 2014, 312, 729–738. [Google Scholar] [CrossRef]
- Biggs, C.M.; Haddad, E.; Issekutz, T.B.; Roifman, C.M.; Turvey, S.E. Newborn screening for severe combined immunodeficiency: A primer for clinicians. Can. Med. Assoc. J. 2017, 189, E1551–E1557. [Google Scholar] [CrossRef]
- Dvorak, C.C.; Haddad, E.; Heimall, J.; Dunn, E.; Buckley, R.H.; Kohn, D.B.; Cowan, M.J.; Pai, S.-Y.; Griffith, L.M.; Cuvelier, G.D.; et al. The diagnosis of severe combined immunodeficiency (SCID): The Primary Immune Deficiency Treatment Consortium (PIDTC) 2022 Definitions. J. Allergy Clin. Immunol. 2023, 151, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, A.A.; Pagliarulo, F.; Faes, L.; Vavassori, S.; Güngör, T.; Bachmann, L.M.; Schmid, J.P. Causes of low neonatal T-cell receptor excision circles: A systematic review. J. Allergy Clin. Immunol. Pract. 2017, 5, 1457–1460.e22. [Google Scholar] [CrossRef] [PubMed]
- Currier, R.; Puck, J.M. SCID newborn screening: What we’ve learned. J. Allergy Clin. Immunol. 2021, 147, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, C.; Nennstiel, U.; Hönig, M.; Albert, M.H.; Ghosh, S.; Schuetz, C.; Brockow, I.; Hörster, F.; Niehues, T.; Ehl, S.; et al. Prospective Newborn Screening for SCID in Germany: A First Analysis by the Pediatric Immunology Working Group (API). J. Clin. Immunol. 2023, 43, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Heather, N.; de Hora, M.; Brothers, S.; Grainger, P.; Knoll, D.; Webster, D. Introducing Newborn Screening for Severe Combined Immunodeficiency—The New Zealand Experience. Int. J. Neonatal Screen. 2022, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Davis, J.; Pai, S.-Y.; Bonilla, F.A.; Puck, J.M.; Apkon, M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol. Genet. Metab. 2011, 104, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Modell, V.; Knaus, M.; Modell, F. An analysis and decision tool to measure cost benefit of newborn screening for severe combined immunodeficiency (SCID) and related T-cell lymphopenia. Immunol. Res. 2014, 60, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Thompson, J.D.; Kobrynski, L.; Ojodu, J.; Zarbalian, G.; Grosse, S.D. Cost-Effectiveness/Cost-Benefit Analysis of Newborn Screening for Severe Combined Immune Deficiency in Washington State. J. Pediatr. 2016, 172, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.C.; Mahlaoui, N.; Mignot, C.; Le Bihan, C.; Rabetrano, H.; Hoang, L.; Neven, B.; Moshous, D.; Cavazzana, M.; Blanche, S.; et al. Systematic neonatal screening for severe combined immunodeficiency and severe T-cell lymphopenia: Analysis of cost-effectiveness based on French real field data. J. Allergy Clin. Immunol. 2015, 135, 1589–1593. [Google Scholar] [CrossRef]
- Bessey, A.; Chilcott, J.; Leaviss, J.; de la Cruz, C.; Wong, R. A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK. Int. J. Neonatal Screen. 2019, 5, 28. [Google Scholar] [CrossRef]
- Van der Ploeg, C.P.; Blom, M.; Bredius, R.G.; van der Burg, M.; Schielen, P.C.; Verkerk, P.H.; Van den Akker-van Marle, M.E. Cost-effectiveness of newborn screening for severe combined immunodeficiency. Eur. J. Pediatr. 2019, 178, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.T.F.; Keller, E.; Wiley, V.; Wong, M.; Farrar, M.A.; Chambers, G.M. Economic Evaluation of Newborn Screening for Severe Combined Immunodeficiency. Int. J. Neonatal Screen. 2022, 8, 44. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex | Initial TREC Level | PIDTC Diagnosis | Gene Affected |
|---|---|---|---|---|
| 1 | Male | 0 | SCID | IL2RG |
| 2 | Male | 0 | Leaky SCID | Unknown |
| 3 | Male | 45 | Leaky SCID | Unknown |
| 4 | Male | 1 | Leaky SCID | Unknown |
| 5 | Female | 0 | Leaky SCID | NHEJ1 |
| 6 | Male | 0 | SCID | DCLRE1C |
| 7 | Male | 0 | Leaky SCID | TRAC |
| 8 | Male | 0 | SCID | Unknown |
| 9 | Male | 8 | SCID | ADA |
| 10 | Male | 0 | SCID | RAC2 |
| 11 | Male | 0 | Leaky SCID | ADA |
| 12 | Male | 31 | Leaky SCID | ADA |
| 13 | Male | 0 | SCID * | ADA |
| 14 | Female | 25 | SCID | ADA |
| 15 | Male | 0 | SCID ** | IL2RG |
| 16 | Male | 0 | SCID | ADA |
| 17 | Male | 5 | Leaky SCID | RMRP |
| 18 | Male | 0 | SCID | CD3D |
| 19 | Male | 0 | SCID *** | IL2RG |
| 20 | Female | 0 | Leaky SCID | Unknown |
| 21 | Female | 0 | SCID | DCLRE1C |
| 22 | Male | 0 | SCID | CD3D |
| 23 | Male | 0 | SCID | CD3D |
| 24 | Male | 0 | Leaky SCID | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Ghamdi, A.; Pachul, J.W.; Al Shaqaq, A.; Fraser, M.; Watts-Dickens, A.; Yang, N.; Vong, L.; Kim, V.H.D.; Siu, V.M.; Pham-Huy, A.; et al. A Unique Comprehensive Model to Screen Newborns for Severe Combined Immunodeficiency—An Ontario Single-Centre Experience Spanning 2013–2023. Genes 2024, 15, 920. https://doi.org/10.3390/genes15070920
Al Ghamdi A, Pachul JW, Al Shaqaq A, Fraser M, Watts-Dickens A, Yang N, Vong L, Kim VHD, Siu VM, Pham-Huy A, et al. A Unique Comprehensive Model to Screen Newborns for Severe Combined Immunodeficiency—An Ontario Single-Centre Experience Spanning 2013–2023. Genes. 2024; 15(7):920. https://doi.org/10.3390/genes15070920
Chicago/Turabian StyleAl Ghamdi, Abdulrahman, Jessica Willett Pachul, Azhar Al Shaqaq, Meghan Fraser, Abby Watts-Dickens, Nicole Yang, Linda Vong, Vy H. D. Kim, Victoria Mok Siu, Anne Pham-Huy, and et al. 2024. "A Unique Comprehensive Model to Screen Newborns for Severe Combined Immunodeficiency—An Ontario Single-Centre Experience Spanning 2013–2023" Genes 15, no. 7: 920. https://doi.org/10.3390/genes15070920
APA StyleAl Ghamdi, A., Pachul, J. W., Al Shaqaq, A., Fraser, M., Watts-Dickens, A., Yang, N., Vong, L., Kim, V. H. D., Siu, V. M., Pham-Huy, A., Brager, R., Reid, B., & Roifman, C. M. (2024). A Unique Comprehensive Model to Screen Newborns for Severe Combined Immunodeficiency—An Ontario Single-Centre Experience Spanning 2013–2023. Genes, 15(7), 920. https://doi.org/10.3390/genes15070920





