Examination of the Expression Profile of Resistance Genes in Yuanjiang Common Wild Rice (Oryza rufipogon)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gene Download
2.2.2. Pathogen Culture
2.2.3. Inoculation Induction Treatment
2.2.4. Fluorescence Quantitative PCR
2.2.5. Bioinformatics Analysis
3. Results
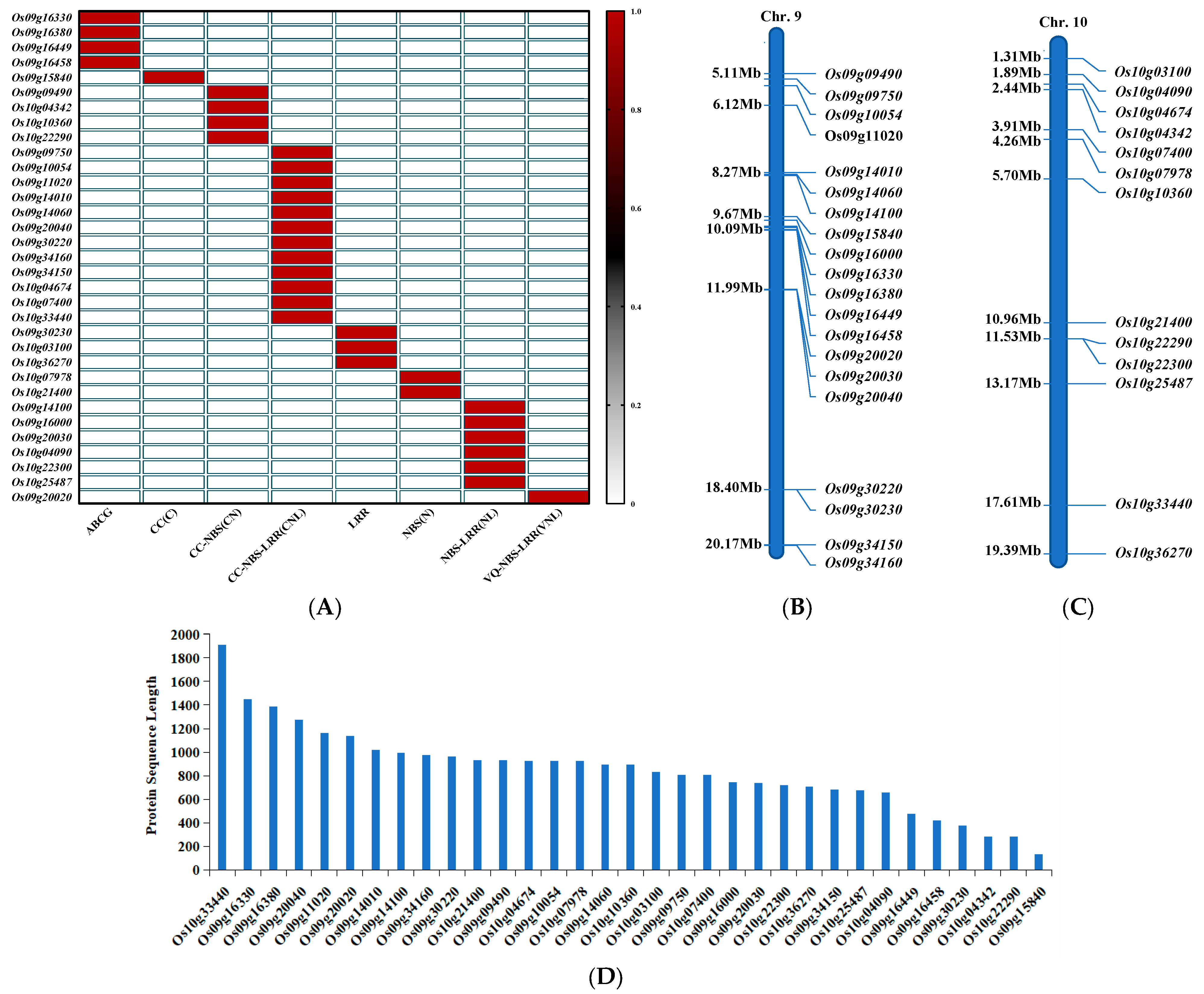
3.1. Basic Information of NLR and ABC Family Genes in Rice Chromosomes 9 and 10
3.2. The Expression of 33 Downloaded Genes Induced by Pathogenic Bacteria
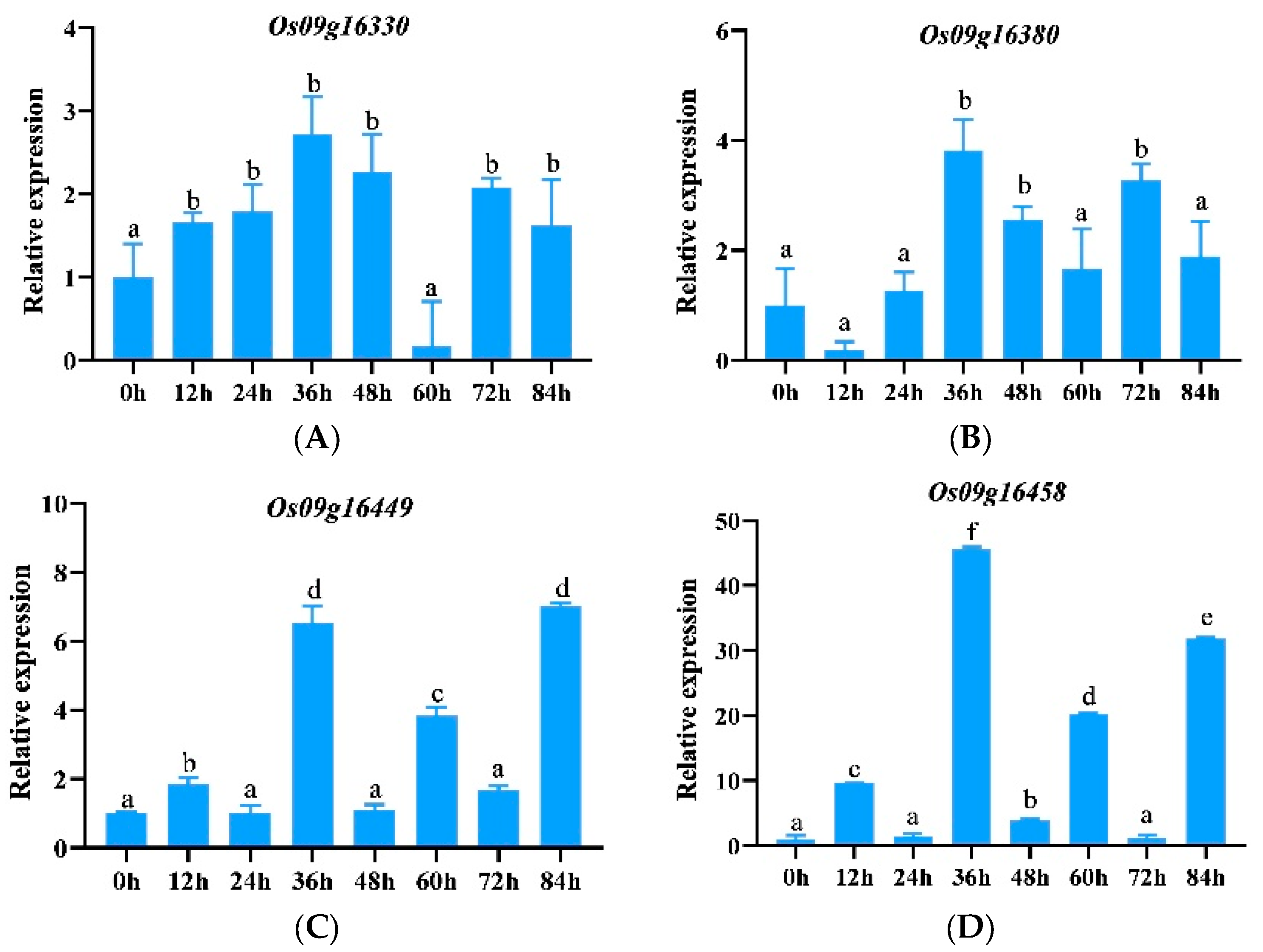
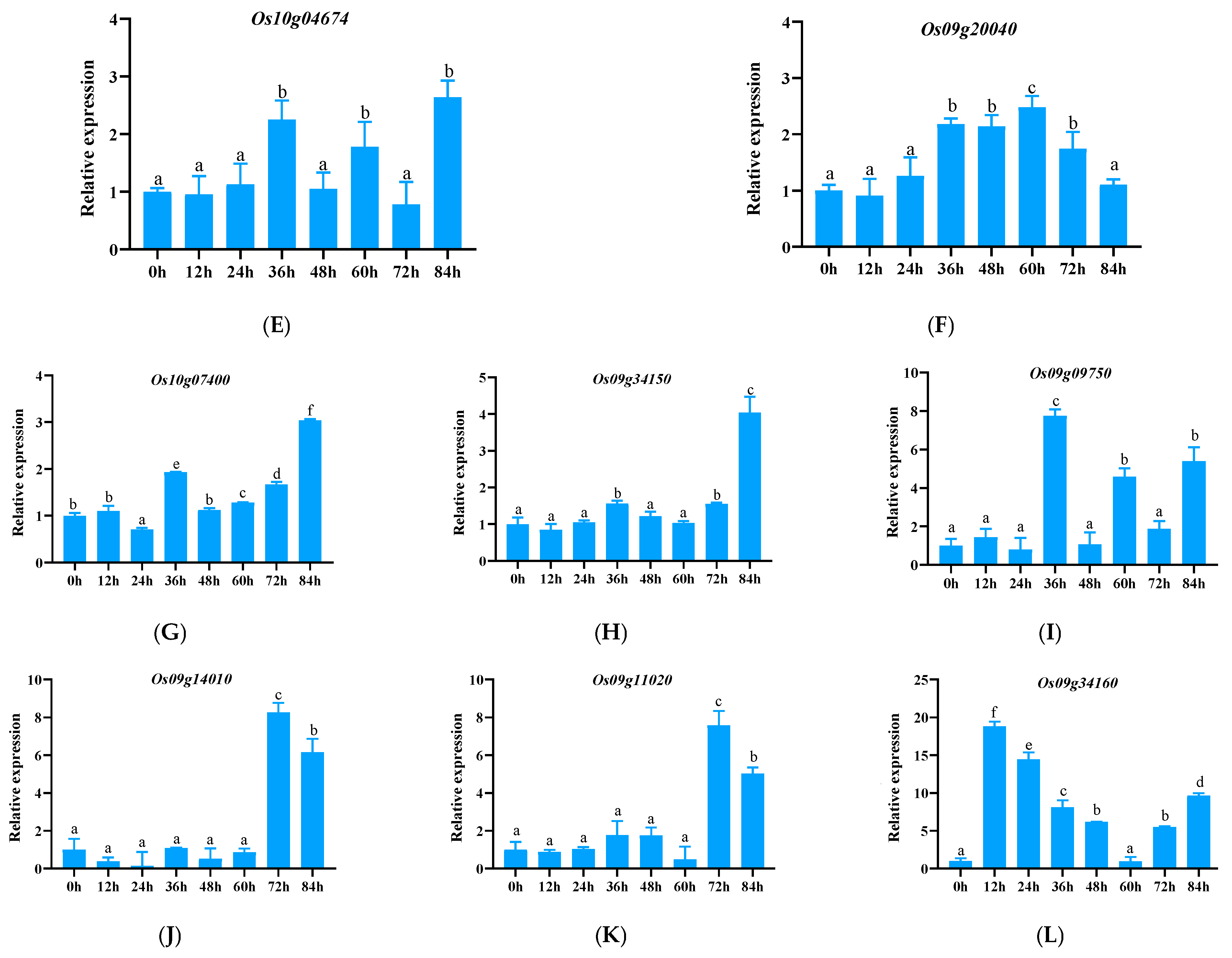
3.2.1. Expression Pattern Analysis of ABCG Subfamily Genes
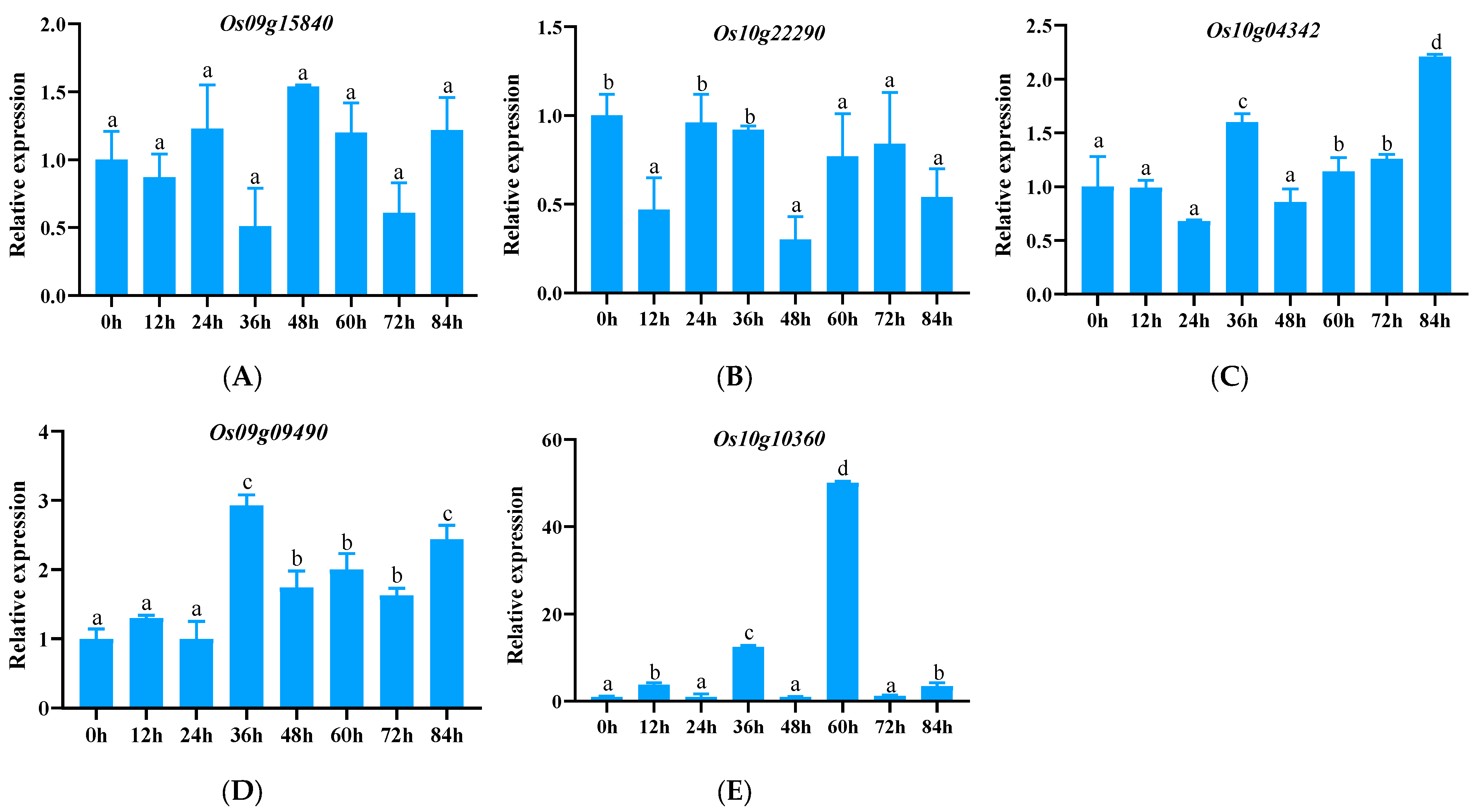
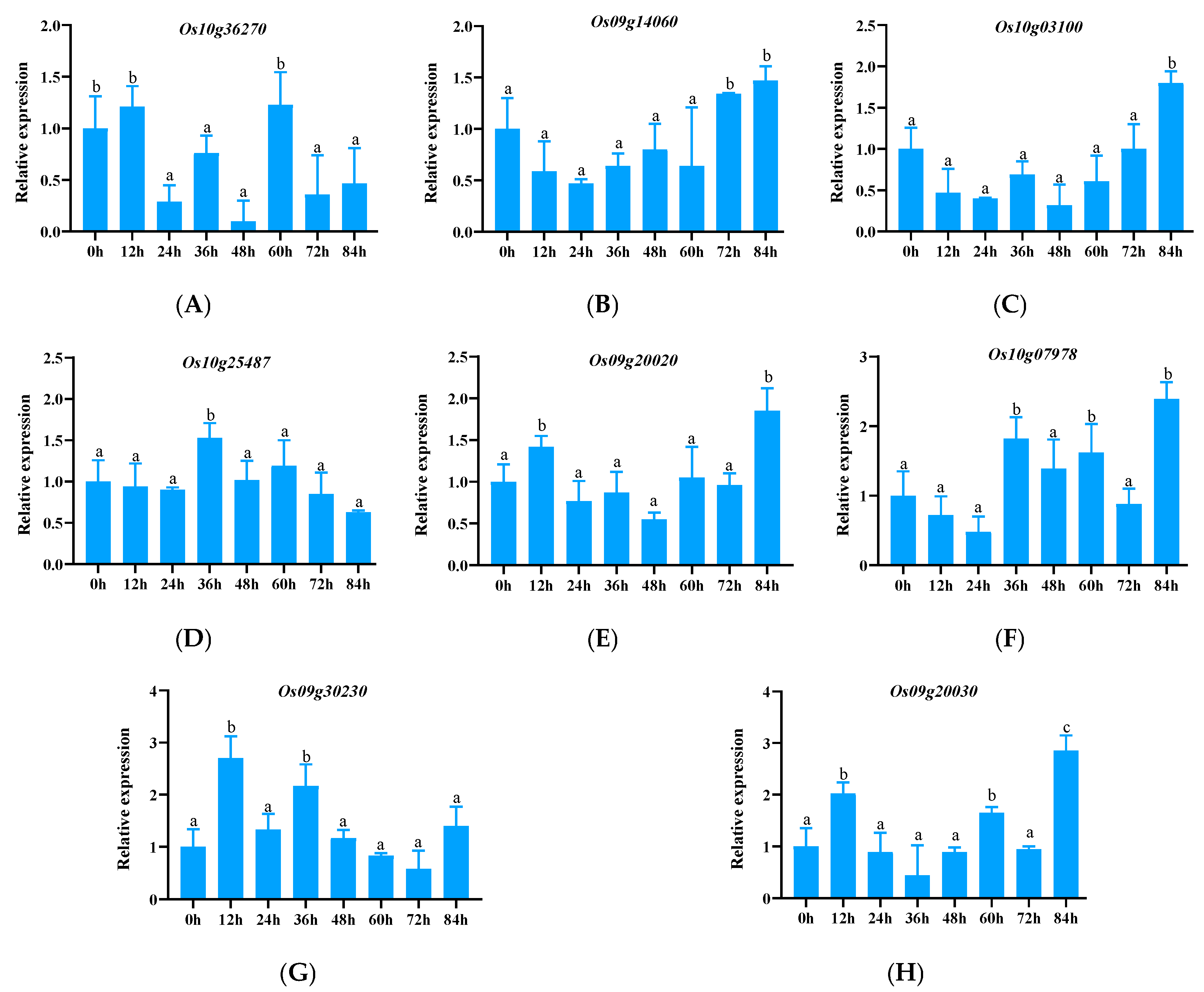
3.2.2. Analysis of Expression Patterns of NLR Family Genes of the CC (C) Type and CC-NBS (CN) Type in this Study
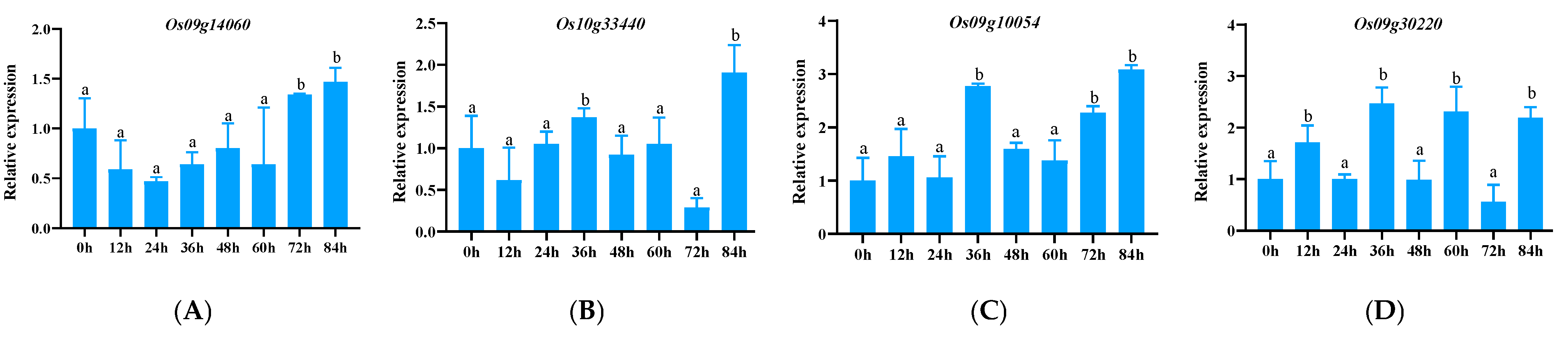
3.2.3. Expression Pattern Analysis of CC-NBS-LRR (CNL)-Type NLR Family Genes
3.2.4. Expression Pattern Analysis of Other NLR Family Genes
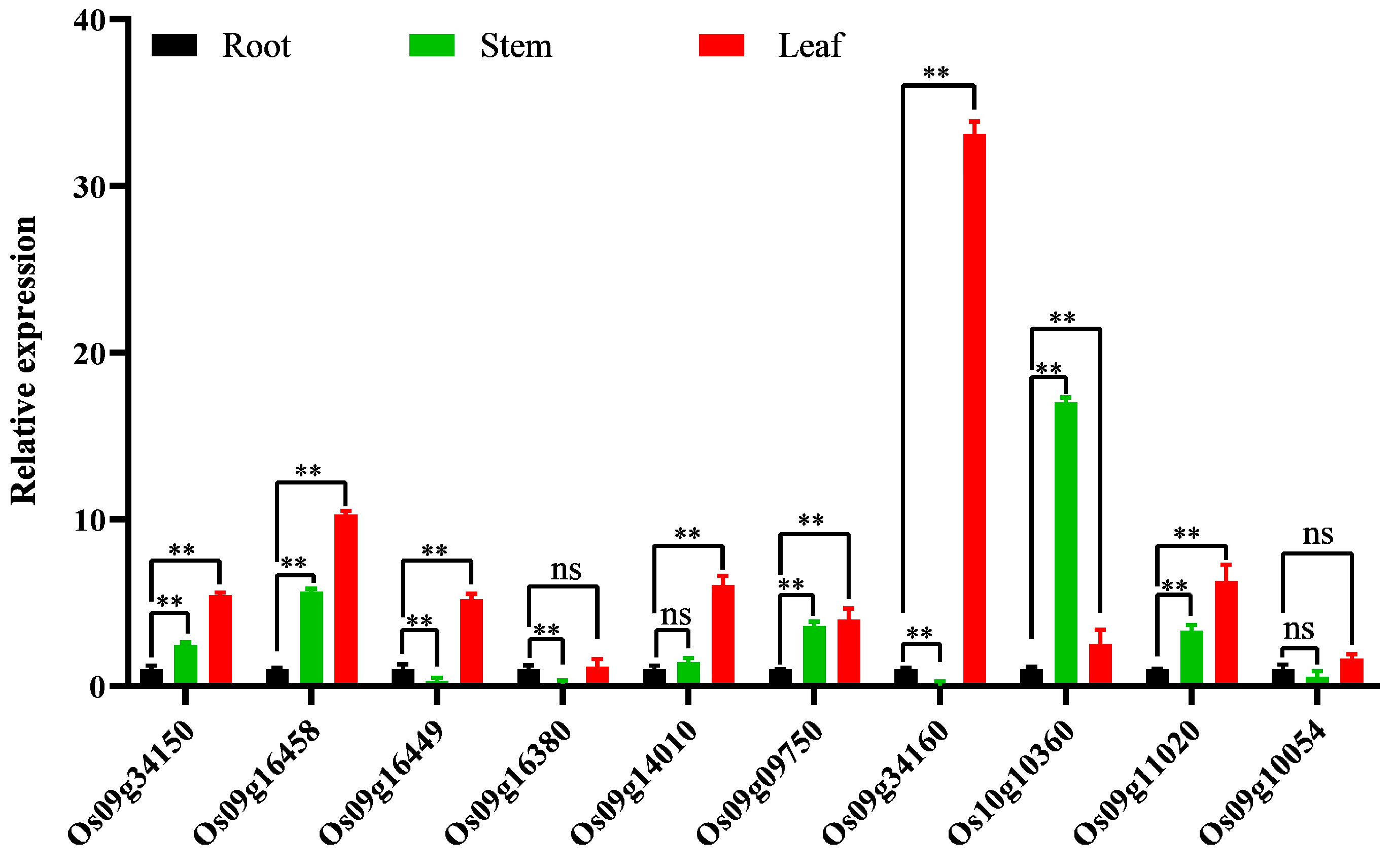
3.2.5. Tissue-Specific Expression Analysis of Significantly Up-Regulated Genes
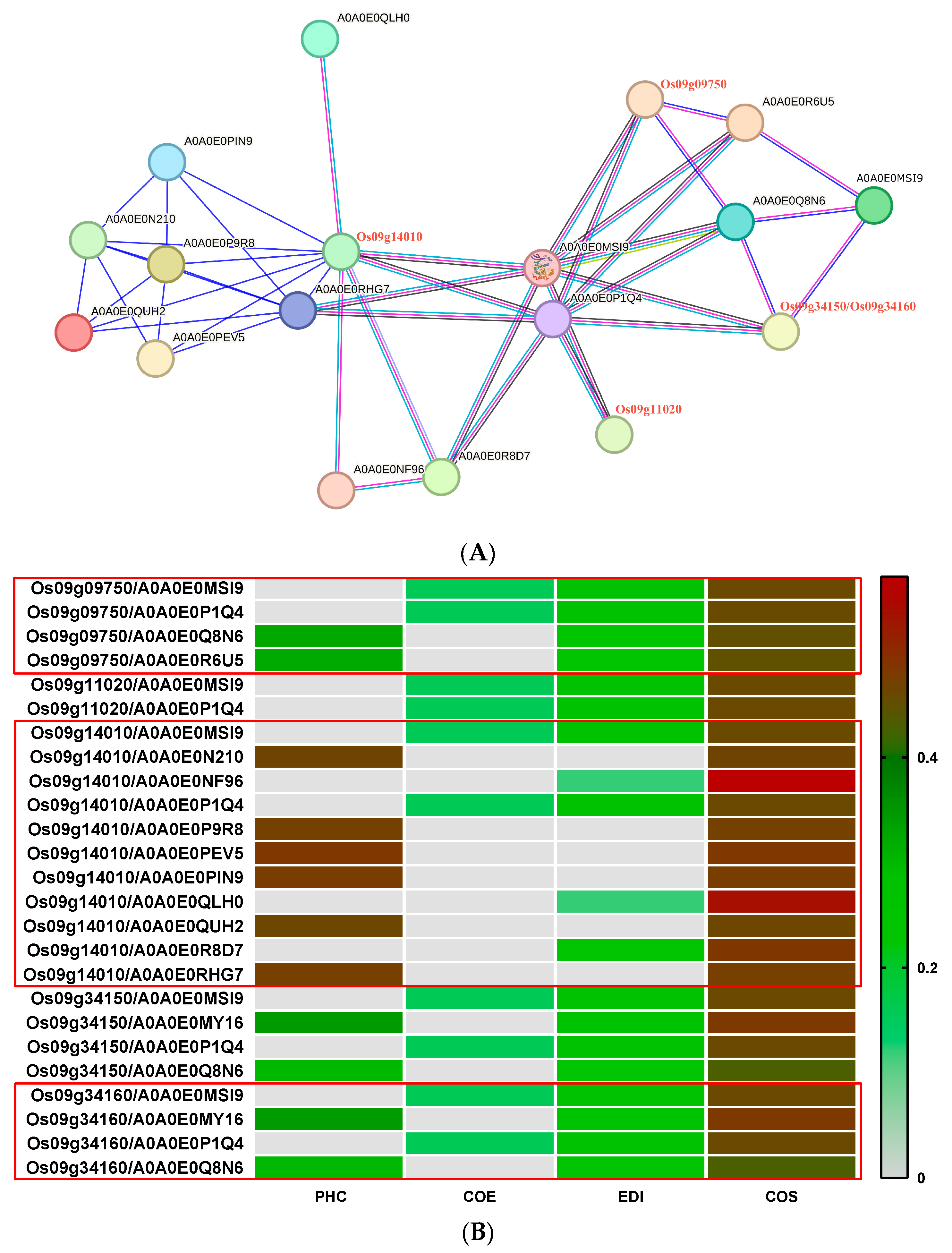
3.2.6. Prediction of ABCG-Type Regulatory Network of Highly Expressed Genes
3.2.7. Prediction of CC-NBS-LRR (CNL)-Type Regulatory Network of Highly Expressed Genes
4. Discussion
4.1. CC-NBS-LRR (CNL) Family Genes Play a Key Role in the Resistance of Yuanjiang Common Wild Rice to Bacterial Blight
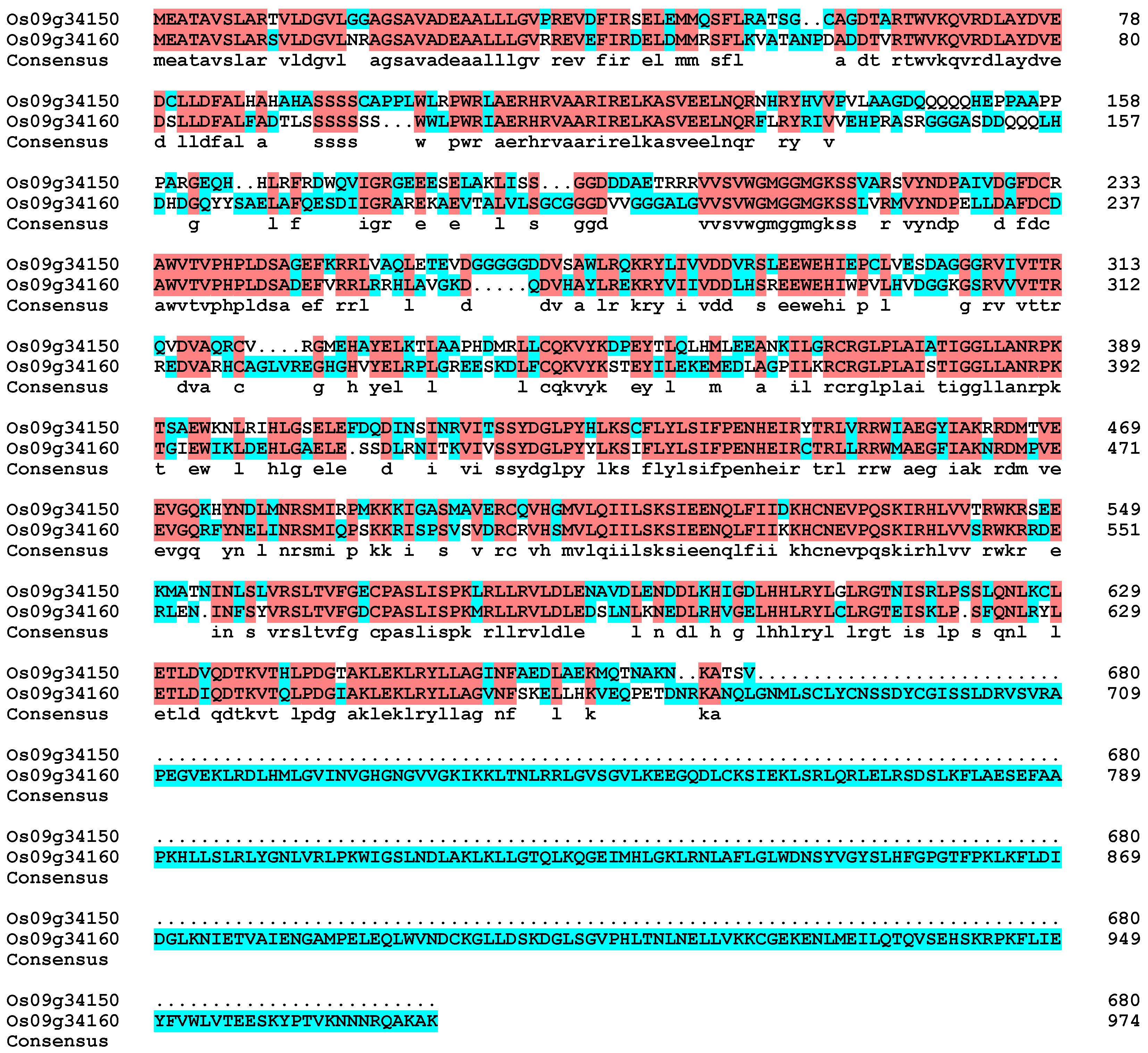
4.2. Five CC-NBS-LRR (CNL) Proteins, including Os09g34150, Mediate Complex Regulatory Pathways
4.3. ABCG-like Proteins Are Essential in the Disease Resistance Process of Yuanjiang Common Wild Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaku, H.; Noda, T.; Ochiai, H. Occurrence of bacterial leaf blight of rice in Kyushu Island in 1993, and collection and evaluation of the isolates of the causal bacterium. Insect Mol. Biol. 2009, 18, 183–194. [Google Scholar]
- Li, Z. Isolation, Cloning and Germplasm Creation of Rice White Leaf Blight Resistance Gene Xa48(t); Yunnan University: Kunming, China, 2022. [Google Scholar]
- Yang, Y.; Zhou, Y.H.; Sun, J.; Liang, W.F.; Chen, X.Y.; Wang, X.M.; Zhou, J.; Chen, Y.; Wang, J.M.; Wu, S.L.; et al. Research progress on cloning and function of Xa genes against rice bacterial blight. Front. Plant Sci. 2022, 13, 847199. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, K.; Huang, Y.; Shi, C.; Hu, W.S.; Zhang, Y.; Zhang, Q.J.; Xia, E.H.; Hutang, G.R.; Zhu, X.G.; et al. SMRT sequencing of the Oryza rufipogon genome reveals the genomic basis of rice adaptation. Commun Biol. 2020, 3, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.J.; Lu, C.K.; Wan, J.P.; Yang, J.; Liu, L.; Zhang, F.F.; Wu, Z.Q.; Zhang, X.; Chang, G.M.; Yu, D.Q.; et al. Genetic dissection of stem branch trait and envisioning of fixing heterosis by vegetative reproduction in Oryza rufipogon. Agronomy 2022, 12, 1503. [Google Scholar] [CrossRef]
- Sugiyama, S.; Sakuta, M.; Tsujimura, Y.; Yamaguchi, Y.; Htun, T.M.; Inoue, C.; Numaguchi, K.; Ishii, T.; Ishikawa, R. Detection of novel loci involved in non-seed-shattering behaviour of an indica rice cultivar, Oryza sativa IR36. Mol. Genet. Genom. 2023, 298, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.F.; Tan, L.B.; Fu, Y.C.; Liu, F.X.; Cai, H.W.; Xie, D.X.; Wu, F.; Wu, J.Z.; Matsumoto, T.; Sun, C.Q. Genetic control of inflorescence architecture during rice domestication. Nat. Commun. 2013, 4, 2200. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Wang, D.; Tan, L.B.; Fu, Y.C.; Liu, F.X.; Xiao, L.T.; Zhu, Z.F.; Fu, Q.; Sun, X.Y.; Gu, P.; et al. LABA1, a domestication gene associated with Long, barbed awns in wild rice. Plant Cell 2015, 27, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.B.; Li, X.R.; Liu, F.X.; Sun, X.Y.; Li, C.G.; Zhu, Z.F.; Fu, Y.C.; Cai, H.W.; Wang, X.K.; Xie, D.X.; et al. Control of a key transition from prostrate to erect growth in rice domestication. Nat. Genet. 2008, 40, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.X.; Zhang, D.Y.; Yin, F.Y.; Zhong, Q.F.; Wang, B.; Xiao, S.Q.; Ke, X.; Wang, L.X.; Zhang, Y.; Zhao, C.M.; et al. Identification and Fine-Mapping of a New Bacterial Blight Resistance Gene, Xa47(t), in G252, an introgression line of Yuanjiang Common Wild Rice (Oryza rufipogon). Plant Dis. 2021, 105, 4106–4112. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.D.; Zhong, Q.F.; Xiao, S.Q.; Wang, B.; Ke, X.; Zhang, Y.; Yin, F.Y.; Zhang, D.Y.; Jiang, C.; Liu, L.; et al. A new NLR disease resistance gene Xa47 confers durable and broad-spectrum resistance to bacterial blight in rice. Front. Plant Sci. 2022, 13, 1037901. [Google Scholar] [CrossRef] [PubMed]
- Prigozhin, D.M.; Krasileva, K.V. Analysis of intraspecies diversity reveals a subset of highly variable plant immune receptors and predicts their binding sites. Plant Cell 2021, 33, 998–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Chen, M.; Sun, L.; Wang, Y.; Yin, J.M.; Liu, J.; Sun, X.Q.; Hang, Y.Y. Genome-Wide identification and evolutionary analysis of NBS-LRR genes from dioscorea rotundata. Front Genet. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Feng, Q.; Liu, P.Q.; He, X.; Zhao, J.H.; Xu, Y.J.; Zhang, L.L.; Huang, Y.; Zhao, J.; Fan, J.; et al. RPW8.1 enhances the ethylene-signaling pathway to feedback-attenuate its mediated cell death and disease resistance in Arabidopsis. New Phytol. 2020, 229, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, J.M.; Wang, H.P.; Zhong, X.P.; Li, H.L.; Yu, J.N.; Kang, J.G. Identification and expression profiling analysis of NBS–LRR genes involved in Fusarium oxysporum f.sp. conglutinans resistance in cabbage. 3 Biotech 2019, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Zuo, C.K.; Zhao, Y.Q.; Huang, S.X.; Wang, T.K.; Zhu, M.; Li, J.; Tao, X.R. Determination of key residues in tospoviral NSm required for Sw-5b recognition, their potential ability to overcome resistance, and the effective resistance provided by improved Sw-5b mutants. Mol. Plant Pathol. 2021, 23, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Nayak, S. Perspectives of genomic diversification and molecular recombination towards R-gene evolution in plants. Physiol. Mol. Biol. Plants 2013, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, Y.; Hu, Q.; Chen, J.J.; Li, K.P.; Chen, L.; Liu, H.; Wang, W.; Kuang, H.H. Dynamic nucleotide-binding site and leucine-rich repeat-encoding genes in the grass family. Plant Physiol. 2012, 159, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hayashi, N.; Wang, C.; Fukuoka, S.; Kawasaki, S.; Takatsuji, H.; Jiang, C.J. Rice blast resistance gene Pikahei-1(t), a member of a resistance gene cluster on chromosome 4, encodes a nucleotide-binding site and leucine-rich repeat protein. Mol. Breed. 2014, 34, 691–700. [Google Scholar] [CrossRef]
- Ashikawa, I.; Hayashi, N.; Yamane, H.; Kanamori, H.; Wu, J.Z.; Matsumoto, T.; Ono, K.; Yano, M. Two adjacent nucleotide-binding site–leucine-rich repeat class genes arer equired to confer pikm-specific rice blast resistance. Genetics 2008, 180, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lu, G.D.; Zeng, L.R.; Wang, G.L. Two broad-spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome 6. Mol. Genet. Genom. 2002, 267, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Song, M.Y.; Seo, Y.S.; Kim, H.K.; Ko, S.; Cao, P.J.; Suh, J.P.; Yi, G.; Roh, J.H.; Lee, S.; et al. Rice Pi5-Mediated resistance to magnaporthe Oryzae requires the presence of two coiled-coil–nucleotide-binding–leucine-rich repeat genes. Genetics 2009, 181, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, Y.; Kanzaki, H.; Abe, A.; Yoshida, K.; Muluneh, T.; Saitoh, H.; Fujibe, T.; Matsumura, H.; Shenton, M.; Galam, D.C.; et al. A multifaceted genomics approach allows the isolation of the rice Pia-blast resistance gene consisting of two adjacent NBS-LRR protein genes. Plant J. 2011, 66, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.H.; Ji, Z.Y.; Liu, B.; He, C.; Liu, H.; Liu, S.Z.; Yang, B.; Chen, G.Y. Xa1 allelic R genes activate rice blight resistance suppressed by interfering TAL effectors. Plant Commun. 2020, 1, 100087. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, Y.; Wu, D.; Rao, W.W.; Guo, J.P.; Ma, Y.H.; Wang, Z.Z.; Shangguan, X.X.; Wang, H.Y.; Xu, C.X.; et al. The coiled-coil and nucleotide binding domains of BROWN PLANTHOPPER RESISTANCE14 function in signaling and resistance against planthopper in Rice. Plant Cell 2017, 29, 3157–3185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, J.; Wang, Z.Z.; Jing, S.L.; Wang, Y.; Ouyang, Y.D.; Cai, B.N.; Xin, X.Y.; Liu, X.; Zhang, C.X.; et al. Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc. Nalt. Acad. Sci. USA 2016, 113, 12850–12855. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ji, L.X.; Zhu, B.H.; Yuan, X.J.; Jiang, D.M.; Xie, G.S. OsCML16 interacts with a novel CC-NBS-LRR protein OsPi304 in the Ca2+/Mg2+ dependent and independent manner in rice. Biochem. Bioph. Res. Commun. 2018, 504, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.U.; Song, W.Y.; Hong, D.; Ko, D.; Yamaoka, Y.; Jang, S.; Yim, S.; Lee, E.; Khare, D.; Kim, K.Y.; et al. Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant 2016, 9, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Laurent, C.; Geisler, M. Correction: Learning from each other: ABC transporter regulation by protein phosphorylation in plant and mammalian systems. Biochem. Soc. Trans. 2015, 43, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.M.; George, A.M. The ABC transporter structure and mechanism: Perspectives on recent research. Cell. Mol. Life Sci. 2004, 61, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Garcia, O.; Bouige, P.; Forestier, C.L.; Dassa, E. Inventory and comparative analysis of rice and arabidopsis ATP-Binding Cassette (ABC) Systems. J. Mol. Biol. 2004, 343, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Zhang, S.N.; Guo, H.P.; Wang, S.K.; Xu, L.; Li, C.Y.; Qian, Q.; Chen, F.; Geisler, M.; Qi, Y.H.; et al. OsABCB14 functions in auxin transport and iron homeostasis in rice (Oryza sativa L.). Plant J. 2014, 79, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Wu, Z.; Liu, Y.; Shen, Z.D.; Xu, F.; Zhang, M.Y.; Ye, H. Mitochondrial ABC Transporter ATM3 Is Essential for Cytosolic Iron-Sulfur Cluster Assembly. Plant Physiol. 2017, 173, 2096–2109. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, L.; Zhang, X.D.; Yang, Z.M. A pleiotropic drug resistance family protein gene is required for rice growth, seed development and zinc homeostasis. Rice Sci. 2023, 30, 127–137. [Google Scholar] [CrossRef]
- Tagashira, Y.; Shimizu, T.; Miyamoto, M.; Nishida, S.; Yoshida, K. Overexpression of a gene involved in phytic acid biosynthesis substantially increases phytic acid and total phosphorus in rice seeds. Plants 2015, 4, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yamaji, N.; Chen, Z.C.; Feng, J. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2011, 69, 857–867. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shi, Y.Z.; Zhang, X.B.; Xu, X.Q.; Wang, H.M.; Li, L.J.; Zhang, Z.H.; Shang, H.H.; Wang, Z.H.; Wu, J.L. The OsABCI7 transporter interacts with OsHCF222 to stabilize the thylakoid membrane in Rice. Plant Physiol. 2020, 184, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.L.; Li, X.; Wang, P.; Cai, C.; Zhang, B.; Sun, C.D.; Zhang, W.S.; Xu, C.J.; Ferguson, I.; Chen, K.S. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with di erent lignin accumulation. Planta 2008, 227, 1243–1254. [Google Scholar] [CrossRef]
- Kenneth, J.L.; Thomas, D.S. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Qian, L.H.; Wang, Y.; Chen, M.; Liu, J.; Lu, R.; Zou, X.; Sun, X.Q.; Zhang, Y.M. Genome-wide identification and evolutionary analysis of NBS-LRR genes from secale cereal. Front. Genet. 2021, 12, 771814. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.M.; Hamel, L.P.; Moffett, P. Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol. Plant Microbe Interact. 2011, 24, 918–931. [Google Scholar] [CrossRef]
- Schreiber, K.J.; Bentham, A.R.; Williams, S.J.; Kobe, B.; Staskawicz, B.J. Multiple domain associations within the arabidopsis immune receptor RPP1 regulate the activation of programmed cell death. PLoS Pathog. 2016, 12, e1005769. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.W.; Zhai, K.R.; Xie, Z.; Yang, D.Y.; Zhu, X.D.; Liu, J.Z.; Wang, X.; Qin, P.; Yang, Y.Z.; Zhang, G.M.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science. 2017, 355, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, B.H.; Guo, Q.; Shi, L.; He, M.; Qin, Z.Y.; Li, L.H.; He, P.; Wang, Z.C.; Hu, D.Y.; et al. A Time-Course Proteomic Analysis of Rice of Rice Triggered by Plant Activator BTH. J. Plant Growth Regul. 2015, 34, 392–409. [Google Scholar] [CrossRef]
- Bonardi, V.; Cherkis, K.; Nishimura, M.T.; Dangl, J.L. A new eye on NLR proteins: Focused on clarity or diffused by complexity? Curr. Opin. Immunol. 2012, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Guan, S.H.; Sun, Y.; Deng, Z.P.; Tang, W.Q.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, W.F. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Panstruga, R.; Parker, J.E.; Schulze, L.P. SnapShot: Plant immune response pathways. Cell 2009, 136, 978.e1–978.e3. [Google Scholar] [CrossRef]
- Gillet, J.P.; Boonen, M.; Jadot, M.; Gottesman, M.M. The dual role of ABC transporters in drug metabolism and resistance to chemotherapy. Liver Biol. Pathobiol. 2020, 76, 1007–1014. [Google Scholar] [CrossRef]
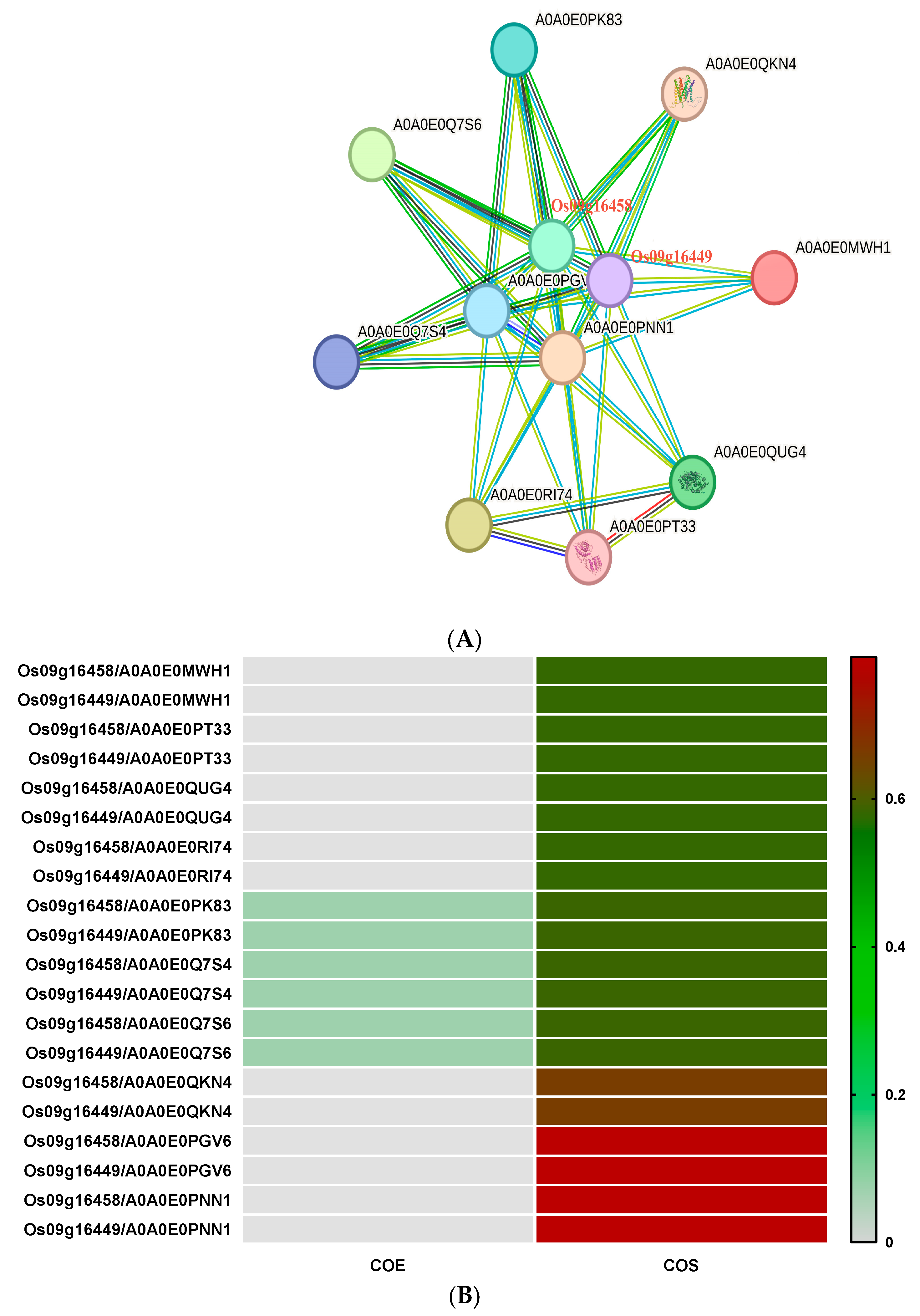
| Interacting Proteins | Annotation | WoLF PSORT Prediction | CELLO v.2.5 Prediction |
|---|---|---|---|
| Os09g16458 | ABC transporter domain-containing protein (ABCG subfamily genes protein) | Cytoplasmic | Cytoplasmic |
| Os09g16449 | ABC transporter domain-containing protein (ABCG subfamily genes protein) | Plasma membrane | Plasma membrane |
| A0A0E0PK83 | Abi domain-containing protein (Uncharacterized protein) | Nuclear, chloroplast | Plasma membrane |
| A0A0E0Q7S4 | Uncharacterized protein (Uncharacterized protein) | Nuclear | Nuclear |
| A0A0E0Q7S6 | Abi domain-containing protein (Uncharacterized protein) | Chloroplast, cytoplasmic | Plasma membrane |
| A0A0E0MWH1 | DHquinase_I domain-containing protein (Uncharacterized protein) | Chloroplast, cytoplasmic | Plasma membrane, chloroplast |
| A0A0E0PGV6 | HECT domain-containing protein (E3 ubiquitin-protein ligases) | Nuclear | Nuclear |
| A0A0E0PNN1 | HECT domain-containing protein (E3 ubiquitin-protein ligases) | Cytoplasmic, nuclear | Nuclear, plasma membrane |
| A0A0E0PT33 | Belongs to the EPSP synthase family, herbicide glyphosate-resistant protein | Chloroplast | Chloroplast |
| A0A0E0QKN4 | COX15-CtaA domain-containing protein (Uncharacterized protein) | Chloroplast, plasma membrane | Plasma membrane |
| A0A0E0QUG4 | DHQ synthase domain-containing protein | Chloroplast | Chloroplast |
| A0A0E0RI74 | DHquinase_I domain-containing protein (Uncharacterized protein) | Chloroplast, mitochondrion | Mitochondrion |
| Preferred Name | Annotation | WoLF PSORT Prediction | CELLO v.2.5 Prediction |
|---|---|---|---|
| Os09g14010 | Belongs to the disease resistance CC-NBS-LRR family | Chloroplast, nuclear | Nuclear |
| Os09g09750 | Belongs to the disease resistance CC-NBS-LRR family | Chloroplast, nuclear | Cytoplasmic, nuclear |
| Os09g11020 | Belongs to the disease resistance CC-NBS-LRR family | Cytoplasmic, chloroplast | Nuclear, cytoplasmic |
| Os09g34150 | Belongs to the disease resistance CC-NBS-LRR family | Cytoplasmic | Cytoplasmic |
| Os09g34160 | Belongs to the disease resistance CC-NBS-LRR family | Nuclear, cytoplasmic | Cytoplasmic, nuclear |
| A0A0E0MSI9 | Belongs to the protein kinase superfamily | Cytoplasmic | Mitochondria, cytoplasmic |
| A0A0E0N210 | F-box domain-containing protein | Chloroplast, nuclear, cytoplasmic | Plasma membrane, cytoplasmic |
| A0A0E0R8D7 | AAA domain-containing protein belongs to the disease resistance NB-LRR family | Cytoplasmic | Cytoplasmic |
| A0A0E0RHG7 | No functional domain belongs to the uncharacterized protein | Cytoplasmic | Cytoplasmic |
| A0A0E0P1Q4 | Belongs to the protein kinase superfamily | Cytoplasmic | Cytoplasmic |
| A0A0E0Q8N6 | Rx_N domain-containing protein, belongs to the uncharacterized protein | Cytoplasmic | Cytoplasmic |
| A0A0E0R6U5 | Rx_N domain-containing protein, belongs to the uncharacterized protein | Cytoplasmic | Mitochondrial, nuclear |
| A0A0E0PEV5 | DUF247 domain-containing protein belongs to the uncharacterized protein | Cytoplasmic, nuclear | Nuclear, cytoplasmic |
| A0A0E0QLH0 | RING-type domain-containing protein | Nuclear | Nuclear |
| A0A0E0MY16 | PWWP domain-containing protein | Nuclear | Nuclear |
| A0A0E0QUH2 | DUF247 domain-containing protein belongs to the uncharacterized protein | Cytoplasmic, chloroplast, Nuclear | Plasma membrane, nuclear |
| A0A0E0PIN9 | DUF247 domain-containing protein belongs to the uncharacterized protein | Plasma membrane | PlasmaMembrane |
| A0A0E0P9R8 | F-box domain-containing protein | Chloroplast, mitochondria | Plasma membrane, nuclear |
| A0A0E0NF96 | AvrRpt-cleavage domain-containing protein can be found in the RPM1 disease resistance | Vacuole, extracellular | Nuclear, extracellular |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, W.; Chen, L.; Wang, B.; Liu, L.; Yin, F.; Zhong, Q.; Li, J.; Zhang, D.; Xiao, S.; Zhang, Y.; et al. Examination of the Expression Profile of Resistance Genes in Yuanjiang Common Wild Rice (Oryza rufipogon). Genes 2024, 15, 924. https://doi.org/10.3390/genes15070924
Kan W, Chen L, Wang B, Liu L, Yin F, Zhong Q, Li J, Zhang D, Xiao S, Zhang Y, et al. Examination of the Expression Profile of Resistance Genes in Yuanjiang Common Wild Rice (Oryza rufipogon). Genes. 2024; 15(7):924. https://doi.org/10.3390/genes15070924
Chicago/Turabian StyleKan, Wang, Ling Chen, Bo Wang, Li Liu, Fuyou Yin, Qiaofang Zhong, Jinlu Li, Dunyu Zhang, Suqin Xiao, Yun Zhang, and et al. 2024. "Examination of the Expression Profile of Resistance Genes in Yuanjiang Common Wild Rice (Oryza rufipogon)" Genes 15, no. 7: 924. https://doi.org/10.3390/genes15070924




