Validation of Gene Expression Patterns for Oral Feeding Readiness: Transcriptional Analysis of Set of Genes in Neonatal Salivary Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Salivary Collection
2.2. RNA Extraction
2.3. RNA Quantification
2.4. Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. Statistical Analysis
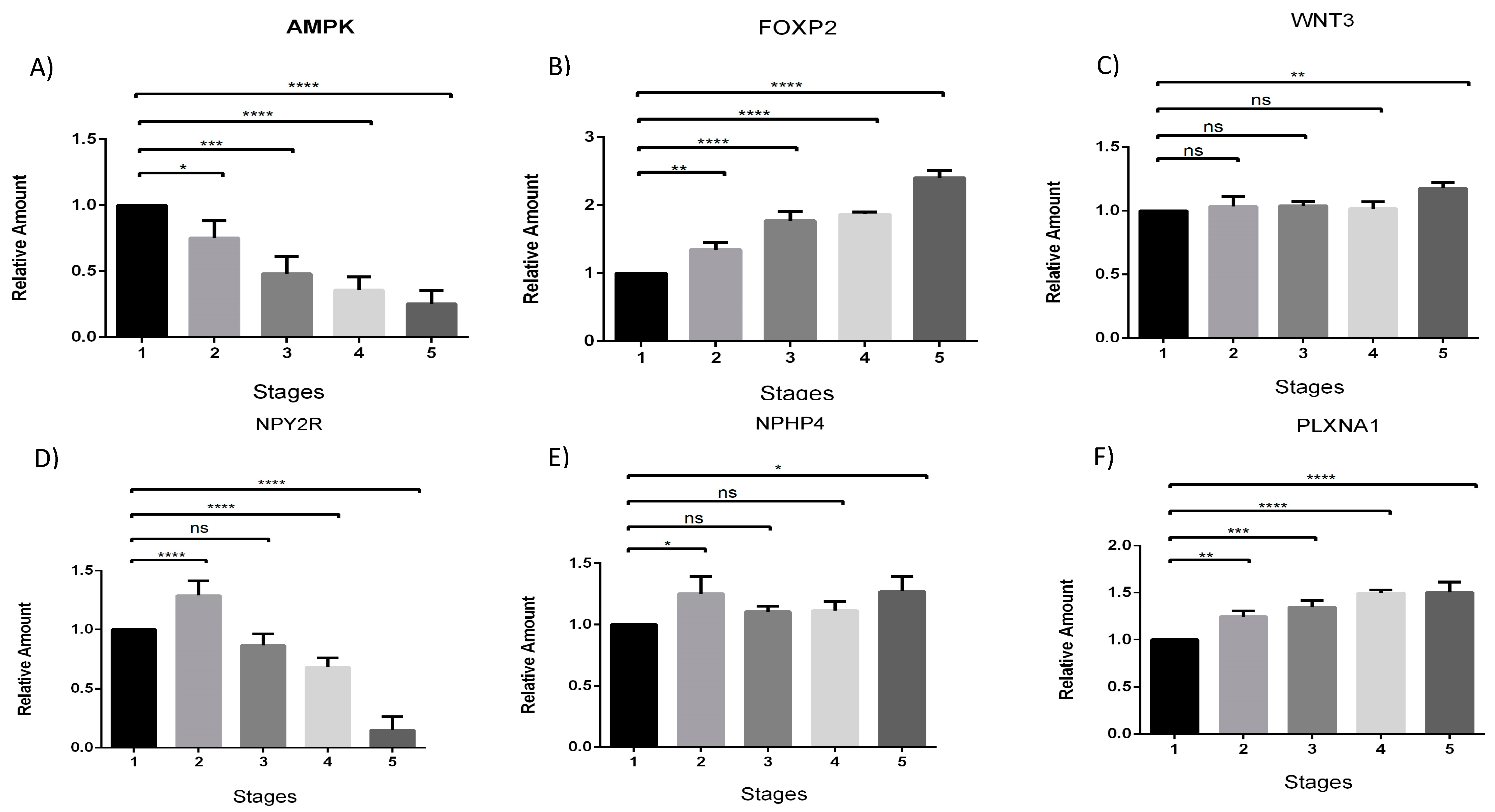
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18S | ribosomal 18S gene |
| AMPK | 5′ adenosine monophosphate-activated protein kinase gene |
| cDNA | complementary DNA |
| FOXP2 | Forkhead Box Protein P2 gene |
| GA | gestational age neonatal intensive care units (NICUs) |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase gene |
| mRNA | messenger RNA |
| NICUs | neonatal intensive care units |
| NPHP4 | Nephrocystin 4 gene |
| NPY2R | neuropeptide Y receptor 2 gene |
| PLXNA1 | Plexin A1 gene |
| qPCR | quantitative polymerase chain reaction |
| RNA-seq | RNA sequencing |
| RT-qPCR | reverse transcription quantitative PCR |
| WNT3 | proto-oncogene—WNT3 |
References
- Rodriguez Gonzalez, P.; Perez-Cabezas, V.; Chamorro-Moriana, G.; Ruiz Molinero, C.; Vazquez-Casares, A.M.; Gonzalez-Medina, G. Effectiveness of Oral Sensory-Motor Stimulation in Premature Infants in the Neonatal Intensive Care Unit (NICU) Systematic Review. Children 2021, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Griffith, T.T.; Bell, A.F.; Vincent, C.; White-Traut, R.; Medoff-Cooper, B.; Rankin, K. Oral Feeding Success: A Concept Analysis. Adv. Neonatal Care 2019, 19, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kamity, R.; Kapavarapu, P.K.; Chandel, A. Feeding Problems and Long-Term Outcomes in Preterm Infants—A Systematic Approach to Evaluation and Management. Children 2021, 8, 1158. [Google Scholar] [CrossRef] [PubMed]
- Lucena, S.L.; Rocha, A.D.; Costa, A.M.; Ramos, J.R.M.; Lopes, J.M.A.; Moreira, M.E.L. A non-invasive technique for evaluation of respiratory efforts in preterm infants during feeding. J. Neonatal Nurs. 2014, 20, 171–177. [Google Scholar] [CrossRef]
- Yamamoto, R.C.C.; Prade, L.S.; Bolzan, G.P.; Weimmann, A.R.M.; Soares, M.K. Prontidão para início da alimentação oral e função motora oral de recém-nascidos pré-termo. Rev. CEFAC 2017, 19, 503–509. [Google Scholar] [CrossRef]
- Rocha, A.D.; Lopes, J.M.; Ramos, J.R.; Gomes, S.C., Jr.; Lopes Lucena, S.; Medeiros, A.; Lopes Moreira, M.E. Development of a technique for evaluating temporal parameters of sucking in breastfeeding preterm newborns. Early Hum. Dev. 2011, 87, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Bolzan, G.P.; Berwig, L.C.; Prade, L.S.; Cuti, L.K.; Yamamoto, R.C.C.; Silva, A.M.T.; Weinmann, A.R.M. Avaliação para o início da alimentação oral de recém-nascidos pré-termo. CoDAS 2016, 28, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Smith, E.O. A novel approach to assess oral feeding skills of preterm infants. Neonatology 2011, 100, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, C.I.; Moraes, S.A.D.; Zamberlan-Amorim, N.E.; Castral, T.C.; Silva, A.D.A.; Scochi, C.G.S. Validação clínica do Instrumento de Avaliação da Prontidão do Prematuro para Início da Alimentação Oral. Rev. Lat.-Am. Enferm. 2013, 21, 140–145. [Google Scholar] [CrossRef]
- Neiva, F.C.; Leone, C.R.; Leone, C.; Siqueira, L.L.; Uema, K.A.; Evangelista, D.; Delgado, S.; Rocha, A.D.; Buhler, K.B. Non-nutritive sucking evaluation in preterm newborns and the start of oral feeding: A multicenter study. Clinics 2014, 69, 393–397. [Google Scholar] [CrossRef]
- Maron, J.L.; Hwang, J.S.; Pathak, S.; Ruthazer, R.; Russell, R.L.; Alterovitz, G. Computational Gene Expression Modeling Identifies Salivary Biomarker Analysis that Predict Oral Feeding Readiness in the Newborn. J. Pediatr. 2015, 166, 282–288.e5. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ilyin, S.E.; Belkowski, S.M.; Plata-Salamán, C.R. Biomarker discovery and validation: Technologies and integrative approaches. Trends Biotechnol. 2004, 22, 411–416. [Google Scholar] [CrossRef]
- Fábryová, H.; Celec, P. On the origin and diagnostic use of salivary RNA. Oral Dis. 2014, 20, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, H.; Wong, D.T. Salivary biomarkers for clinical applications. Mol. Diagn. Ther. 2009, 13, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.L.; Nguyet, N.M.; Quyen, N.T.H.; Ngoc, T.V.; Tram, T.V.; Gan, T.T.; Tung, N.T.; Dung, N.T.; Chau, N.V.V.; Wills, B.; et al. An evaluation of dried blood spots and oral swabs as alternative specimens for the diagnosis of dengue and screening for past dengue virus exposure. Am. J. Trop. Med. Hyg. 2012, 87, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, M.; Esmaeili, M. Evaluation of HBs-Ag and anti-HBc levels in serum and saliva of patients with hepatitis B. Med. J. Islam. Repub. Iran 2020, 34, 101. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Measles and rubella laboratory network: 2007 meeting on use of alternative sampling techniques for surveillance. Wkly. Epidemiol. Rec. 2008, 83, 229–232. [Google Scholar]
- Vyse, A.J.; Cohen, B.J.; Ramsay, M.E. A comparison of oral fluid collection devices for use in the surveillance of virus diseases in children. Public Health 2001, 115, 201–207. [Google Scholar] [CrossRef]
- Gandhi, V.; O’Brien, M.H.; Yadav, S. High-Quality and High-Yield RNA Extraction Method from Whole Human Saliva. Biomark. Insights 2020, 15, 1177271920929705. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Mrkusich, E.M. The state of RT-quantitative PCR: Firsthand observations of implementation of minimum information for the publication of quantitative real-time PCR experiments (MIQE). J. Mol. Microbiol. Biotechnol. 2014, 24, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, E.; Maki, M.; Maron, J. Salivary FOXP2 expression and oral feeding success in premature infants. Cold Spring Harb. Mol. Case Stud. 2016, 2, a000554. [Google Scholar] [CrossRef] [PubMed]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Ferreira, I.; Darleans Dos Santos Cunha, W.; Henrique Ferreira Gomes, L.; Azevedo Cintra, H.; Lopes Cabral Guimarães Fonseca, L.; Ferreira Bastos, E.; Clinton Llerena, J., Jr.; Farias Meira de Vasconcelos, Z.; da Cunha Guida, L. A rapid and accurate methylation-sensitive high-resolution melting analysis assay for the diagnosis of Prader Willi and Angelman patients. Mol. Genet. Genomic Med. 2019, 7, e637. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.R.; Costa, R.A.; Gomes, L.H.F.; dos Santos Cunha, W.D.; Tyszler, L.S.; Freitas, S.; Llerena Junior, J.C.; de Vasconcelos, Z.F.M.; Nicholls, R.D.; Guida, L.D.C. A newborn screening pilot study using methylation-sensitive high resolution melting on dried blood spots to detect Prader-Willi and Angelman syndromes. Sci. Rep. 2020, 10, 13026. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, L.L.C.G.; Rocha, D.N.; Cintra, H.A.; de Araújo, L.L.; Dos Santos, G.L.M.; de Faria, L.L.; Salú, M.D.S.; Leite, S.H.D.S.; Rocha, A.D.; Lopes, M.D.C.B.; et al. Establishing a Standardized DNA Extraction Method Using NaCl from Oral Mucosa Cells for Its Application in Imprinting Diseases Such as Prader-Willi and Angelman Syndromes: A Preliminary Investigation. Genes 2024, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wells, J.; Dykes, F. Gene expression profiling as a tool for assessing feeding readiness in premature and newborn infants. J. Neonatal Nurs. 2023, 29, 123–130. [Google Scholar]
- Jones, L.; Patel, S.; Johnson, R. The role of gene expression biomarkers in determining feeding readiness in premature infants: A systematic review. J. Perinatol. 2024, 44, 210–218. [Google Scholar]
- Duran, I.; Betancourt, L.H. Challenges and opportunities in omics approaches: A review of current methodologies and future directions. Front. Genet. 2023, 14, 685512. [Google Scholar]
- Maheshwari, A.; Jilling, T.; Saha, S. Cellular and molecular processes in the development of the feeding premature infant: Implications for nutrition and advancement of enteral feeding. Pediatr. Res. 2017, 81, 222–230. [Google Scholar] [CrossRef]
- Heng, Y.J.; Pennell, C.E.; Chua, H.N.; Perkins, J.; Lye, S.J. Whole Blood Gene Expression Profile Associated with Spontaneous Preterm Birth in Women with Threatened Preterm Labor. PLoS ONE 2014, 9, e96901. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.V.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F. Early nutrition and its effect on growth, body composition, and later obesity. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 180–185. [Google Scholar] [CrossRef]
- Moran, T.H.; Bi, S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Roseberry, A.G.; Liu, H.; Jackson, A.C.; Cai, X.; Friedman, J.M. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron 2004, 41, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Herzog, H.; Hort, Y.J.; Ball, H.J.; Hayes, G.; Shine, J.; Selbie, L.A. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc. Natl. Acad. Sci. USA 1992, 89, 5794–5798. [Google Scholar] [CrossRef] [PubMed]
- Primeaux, S.; Wilson, S.; Cusick, M.C.; York, D.A.; Wilson, M.A. Effects of Altered Amygdalar Neuropeptide Y Expression on Anxiety-Related Behaviors. Neuropsychopharmacology 2005, 30, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of maternal obesity on the insulin-like growth factor system, insulin resistance and growth in the ovine fetus. Rev. Chil. Nutr. 2015, 42, 192–197. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK: Positive and negative regulation, and its role in whole-body energy homeostasis. Curr. Opin. Cell Biol. 2015, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Lin, S.C. AMPK Promotes Autophagy by Facilitating Mitochondrial Fission. Cell Metab. 2016, 23, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Shriberg, L.D.; Ballard, K.J.; Tomblin, J.B.; Duffy, J.R.; Odell, K.H.; Williams, C.A. Speech and prosody characteristics of adults with Prader-Willi syndrome. J. Commun. Disord. 2010, 43, 127–138. [Google Scholar] [CrossRef]
- Vargha-Khadem, F.; Gadian, D.G.; Copp, A.; Mishkin, M. FOXP2 and the neuroanatomy of speech and language. Nat. Rev. Neurosci. 2005, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Schreiweis, C.; Bornschein, U.; Burguière, E.; Kerimoglu, C.; Schreiter, S.; Dannemann, M.; Goyal, S.; Rea, E.; French, C.A.; Puliyadi, R.; et al. Humanized Foxp2 accelerates learning by enhancing transitions from declarative to procedural performance. Proc. Natl. Acad. Sci. USA 2014, 111, 14253–14258. [Google Scholar] [CrossRef] [PubMed]
- Estruch, S.B.; Graham, S.A.; Chinnappa, S.M.; Deriziotis, P.; Fisher, S.E. Functional characterization of rare FOXP2 variants in neurodevelopmental disorder. J. Neurodev. Disord. 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Konopka, G.; Roberts, T.F. Insights into the Neural and Genetic Basis of Vocal Communication. Cell 2016, 164, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; de Paiva Alves, E.; Veenstra, G.J.; Hoppler, S. Tissue- and stage-specific Wnt target gene expression is controlled subsequent to β-catenin recruitment to cis-regulatory modules. Development 2016, 143, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Reya, T.; Clevers, H. Wnt signalling in stem cells and cancer. Nature 2005, 434, 843–850. [Google Scholar] [CrossRef]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef]
- Arts, H.H.; Knoers, N.V. Current insights into renal ciliopathies: What can genetics teach us? Pediatr. Nephrol. 2013, 28, 863–874. [Google Scholar] [CrossRef]
- Hildebrandt, F.; Benzing, T.; Katsanis, N. Ciliopathies. N. Engl. J. Med. 2011, 364, 1533–1543. [Google Scholar] [CrossRef]
- Tory, K.; Rousset-Rouvière, C.; Gubler, M.C.; Morinière, V.; Pawtowski, A.; Becker, C.; Guyot, C.; Gié, S.; Frishberg, Y.; Nivet, H.; et al. Mutations of NPHP2 and NPHP3 in infantile nephronophthisis. Kidney Int. 2009, 75, 839–847. [Google Scholar] [CrossRef]
- Otto, E.A.; Hurd, T.W.; Airik, R.; Chaki, M.; Zhou, W.; Stoetzel, C.; Patil, S.B.; Levy, S.; Ghosh, A.K.; Murga-Zamalloa, C.A.; et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat. Genet. 2010, 42, 840–850. [Google Scholar] [CrossRef]
- Wolf, M.T.; Hildebrandt, F. Nephronophthisis. Pediatr. Nephrol. 2011, 26, 181–194. [Google Scholar] [CrossRef]
- Tran, T.S.; Kolodkin, A.L.; Bharadwaj, R. Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 2007, 23, 263–292. [Google Scholar] [CrossRef]
- Boyer, N.P.; Gupton, S.L. Revisiting Netrin-1: One Who Guides (Axons). Front. Cell. Neurosci. 2018, 12, 221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.H.F.; Marques, A.B.; Dias, I.C.d.M.; Gabeira, S.C.d.O.; Barcelos, T.R.; Guimarães, M.d.O.; Ferreira, I.R.; Guida, L.C.; Lucena, S.L.; Rocha, A.D. Validation of Gene Expression Patterns for Oral Feeding Readiness: Transcriptional Analysis of Set of Genes in Neonatal Salivary Samples. Genes 2024, 15, 936. https://doi.org/10.3390/genes15070936
Gomes LHF, Marques AB, Dias ICdM, Gabeira SCdO, Barcelos TR, Guimarães MdO, Ferreira IR, Guida LC, Lucena SL, Rocha AD. Validation of Gene Expression Patterns for Oral Feeding Readiness: Transcriptional Analysis of Set of Genes in Neonatal Salivary Samples. Genes. 2024; 15(7):936. https://doi.org/10.3390/genes15070936
Chicago/Turabian StyleGomes, Leonardo Henrique Ferreira, Andressa Brito Marques, Isabel Cristina de Meireles Dias, Sanny Cerqueira de O. Gabeira, Tamara Rosa Barcelos, Mariana de Oliveira Guimarães, Igor Ribeiro Ferreira, Letícia Cunha Guida, Sabrina Lopes Lucena, and Adriana Duarte Rocha. 2024. "Validation of Gene Expression Patterns for Oral Feeding Readiness: Transcriptional Analysis of Set of Genes in Neonatal Salivary Samples" Genes 15, no. 7: 936. https://doi.org/10.3390/genes15070936




