Abstract
Closely-related plant groups with distinct microbiomes, chemistries and ecological characteristics represent tractable models to explore mechanisms shaping species spread, competitive dynamics and community assembly at the interface of native and introduced ranges. We investigated phyllosphere microbial communities, volatile organic compound (VOC) compositions, and potential interactions among introduced S. trilobata, native S. calendulacea and their hybrid in South China. S. trilobata exhibited higher α diversity but significantly different community composition compared to the native and hybrid groups. However, S. calendulacea and the hybrid shared certain microbial taxa, suggesting potential gene flow or co-existence. The potent antimicrobial VOC profile of S. trilobata, including unique compounds like p-cymene (13.33%), likely contributes to its invasion success. The hybrid’s intermediate microbial and VOC profiles suggest possible consequences for species distribution, genetic exchange, and community assembly in heterogeneous environments. This hybrid deserves further study as both an opportunity for and threat to diversity maintenance. These differentiating yet connected plant groups provide insight into ecological and evolutionary dynamics shaping microbiome structure, species co-occurrence and competitive outcomes during biological exchange and habitat transformation. An interdisciplinary approach combining chemical and microbial ecology may reveal mechanisms underlying community stability and change, informing management of species spread in a globalized world.
1. Introduction
The phyllosphere, primarily consisting of the aerial parts of plant leaves, is a vast but often overlooked microbial habitat [1,2,3]. The global leaf surface area, estimated at 1,017,260,200 km2, is approximately twice that of the land surface. Assuming 106 to 107 bacteria per cm2 of leaf surface, the phyllosphere may host up to 1026 bacterial cells [1], with even higher numbers when including yeasts, filamentous fungi, and protists. The phyllosphere includes both epiphytic (leaf surface) and endophytic (leaf interior) microbiota. As epiphytic microbiota are more diverse and abundant [4,5], this study focuses on these surface-dwelling microbes.
In recent decades, our understanding of plant–microbe interactions Has mainly focused on rhizosphere microbes. These diverse communities, often called the plant’s second genome [6,7], are crucial for plant health, while some beneficial microbes like plant growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi (AMF) and nitrogen-fixing bacteria are well known, they represent only a small fraction of rhizosphere communities. Our understanding of rhizosphere microbe assembly and function remains limited [8]. The knowledge gap for phyllosphere microbe is even larger. However, studies on Arabidopsis, grapevine, and mustard plant Boechera stricta have revealed significant overlaps between key community members in the phyllosphere and rhizosphere [9,10,11]. This suggests that, phyllosphere microbes should be considered when investigating host-microbe interactions.
Phyllosphere microorganisms are crucial for plant and ecosystem health. They benefit host plants by improving nutrient uptake, increasing tolerance, protecting pathogens, regulating hormones, and degrading environmental toxins [12,13]. These plant–microbe interactions enhance ecosystem resilience and sustainable plant growth [14,15,16]. Certain phyllosphere bacteria, such as Microbacterium, Thermus, and Methylobacterium, produce growth regulators like IAA and fix nitrogen, improving plant growth and nutrition [17,18]. Atmospheric dinitrogen fixed by genera, like Beijerinckia and Azotobacter, can be absorbed by leaves or transported to roots, affecting plant growth [19]. In rice, interleaf microbes fix nitrogen and produce antimicrobial substances, indirectly promoting plant growth [20]. Phyllosphere microorganisms contribute to nutrient cycling and influence microbial communities in soil, water, and air, with implications for conservation and restoration efforts [21,22,23]. This emerging field has broad implications for plant and ecosystem health, sustainable agriculture and land use management.
Phyllosphere microorganism sources are complex, influenced by both biotic factors (host characteristics, insect activity, and microbial interactions) and abiotic factors (soil, climate, and temperature) [4,11,24,25,26]. Plant-emitted volatile organic compounds significantly shape phyllosphere microbial communities, serving as antimicrobial agents or carbon sources [27,28]. For instance, Methylobacterium and Candida boidinii can utilize methanol as a growth substrate [27,29]. Plant volatiles also contain fungicidal substances that help plants resist pest stress and impact microbial community structure. Understanding leaf VOCs can provide insights into phyllosphere community composition and plant–microbe interactions.
Global trade and cultural exchanges have led to the introduction of exotic species, some becoming invasive and damaging natural ecosystems. S. trilobata (L.) Pruski, a South American perennial herb introduced to China in the 1970s, is now one of the world’s 100 most noxious invasive species. In southern China, it has displaced the native S. calendulacea. Recent studies on S. trilobata have focused on its ecological impacts [30], phytochemical properties [31], and eco-physiological traits [32,33].
While many studies have investigated the invasion mechanism of S. trilobata, focusing on chemosensory effects and stress response [34,35], limited research exists on its phyllosphere microbial communities and growth-promoting VOCs. Endophytic bacteria and leaf-emitted VOCs have been shown to facilitate S. trilobata’s growth and competitive advantages [36,37,38], suggesting that both microbial community structure and secondary metabolites may contribute to its successful invasion. To investigate the invasion mechanism of S. trilobata, we studied the invasive plant, its native counterpart S. calendulacea, and their hybrids. We used Illumina NovaSeq high-throughput sequencing of the 16S rRNA V3-V4 fragment to analyze phyllosphere microbiome formation, succession, and development. Additionally, we used headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography mass spectrometry (GC-MS) to identify the composition and proportion of volatile compounds in Sphagneticola species. Our research aims to explore the relationship between phyllosphere microbial composition and volatile compounds, providing insights into the invasion mechanisms of S. trilobata.
2. Materials and Methods
2.1. Sample Preparation
In March 2022, we collected S. calendulacea, S. trilobata, and their F1 hybrids from the field in Beidou Town, Taishan City, Guangdong Province (21.879192° N, 112.410689° E). For each species/taxon, we randomly selected 15 uniform growing samples. For each sample, we collected two intact, healthy leaves at the same height using sterilized scissors and stored them in sterile 50mL conical tubes. All leaf samples were stored at −80 °C until assay analysis.
2.2. DNA Extraction
We extract phyllosphere microbiome DNA following methods from [39,40]. Briefly, we weighed 10 g of the leaf samples, cut them into pieces, and immersed them in sterile TE buffer (10 mmol/L Tris-HCl, 1 mmol/L EDTA, Ph 8.0) at a 1:20 ratio (leaf weight/buffer volume). The material was sealed with a sterilized membrane and shaken at 200 r/min for 30 min (HY-4, Ruihua Changzhou, China) at room temperature to separate microbial cells from the leaf surface. The mixture was then sonicated at 40 kHz for 15 min (JP-010T, Jiemeng, Shenzhen, China). Microorganisms were collected onto a 0.22 µm filter membrane using vacuum filtration in a sterile environment. Total DNA was extracted from filters using a FastDNA spin kit (Qbiogene, Irvine, CA, USA) per manufacturer’s instructions. Purified DNA was dissolved in 100 µL of ddH2O and stored at −20 °C.
2.3. PCR Amplification, Sequence Processing and Analysis
Sample DNA was diluted to 1 ng/µL with sterile water. The V3-V4 region of 16S rRNA was amplified using bacterial specific primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR reaction mixture contained 15 µL 2× Phusion Master Mix, 3 µL each of forward and reverse primers (2 µmol/L), 7 µL genomic DNA (1 ng/µL), and 2 µL ddH2O. PCR reaction conditions were the following: 98 °C for 1 min; 30 cycles of 98 °C for 10 s, 50 °C for 30 s, and 72 °C for 30 s; and final extension at 72 °C for 5 min. Equal amounts of PCR productions were mixed, homogenized, and purified. Paired-end sequencing of 16S rRNA hypervariable regions was performed using the Illumina NovaSeq platform (Illumina, San Diego, CA, USA).
Raw sequencing data were analyzed using the QIIME2.0 DADA2 and USEARCH pipelines. After assembly, filtering, and quality control, amplicon sequence variants (ASVs) were defined at 100% similarity. Bacterial sequences were classified using the SILVA database (Release 138, http://www.arb-silva.de accessed on 12 May 2022). ASVs and basic analysis results were obtained for each sample, with composition structure statistics and diversity analysis conducted at various taxonomic levels.
2.4. Sample Prepration and Treatment for Volatile Compounds Detection
Harvested materials were immediately weighed, frozen in sterile 50 mL conical tubes, and stored at −80 °C. One gram of sample powder was transferred to a 20 mL headspace vial (Agilent, Palo Alto, CA, USA) containing NaCl-saturated solution to inhibit enzyme reactions. Vials were sealed with crimp-top caps using TFE-silicone headspace septa (Agilent Technologies, Santa Clara, CA, USA). For the solid-phase microextraction (SPME) assay [41], each vial was heated to 60 °C for 5 min; then, a 120 µm DVB/CWR/PDMS fiber (Agilent Technologies, Santa Clara, CA, USA) was exposed to the sample’s headspace for 15 min at 100 °C.
2.5. GC-MS Conditions
After sampling, we desorbed VOCs from the fiber coating in the GC injection port (Model 8890; Agilent, Santa Clara, CA, USA) at 250 °C for 5 min in splitless mode. VOCs were identified and quantified using an Agilent Model 8890 GC and a 7000D mass spectrometer (Agilent, Santa Clara, CA, USA), equipped with a 30 m × 0.25 mm × 0.25 μm DB-5 MS capillary column (5% phenyl-polymethylsiloxane). Helium served as the carrier gas at 1.2 mL/min. The injector and detector temperatures were 250 °C and 280 °C, respectively. Oven temperature was programmed from 40 °C (3.5 min), increasing to 100 °C at 10 °C/min, to 180 °C at 7 °C/min, to 280 °C at 25 °C/min, and held for 5 min. Mass spectra were recorded in electron impact (EI) ionization mode at 70 eV. Quadrupole mass detector, ion source, and transfer line temperatures were set at 150 °C, 230 °C, and 280 °C, respectively. We used selected ion monitoring (SIM) mode for analyte identification and quantification.
2.6. Bioinformatics and Statistical Analysis
Analyses were performed in R 3.4.3. We filtered out ASVs classified as chloroplasts and mitochondria. α-diversity was estimated using Chao1, Shannon’s and Simpson’s indices on absolute abundance matrices. ANOVA was used to analyze significant differences for those indices among three Sphagneticola species, with Tukey’s honest significant difference test for post-hoc comparisons. Relationships among bacterial community structures of the three Sphagneticola species were assessed using principal coordinate analysis (NMDS) based on Bray–Curtis distances and UPGMA clustering tree structures.
3. Results
3.1. Varied Species Richness and Diversity of Phyllosphere Microbes among Two Sphagneticola Species and Their F1 Hybrids
High-throughput sequencing of S. trilobata, S. calendulacea and their F1 hybrid yielded 3,418,736 raw sequences, with 3,413,498 high-quality sequences retained after filtering. Rarefaction curves indicate sufficient sequencing depth (Figure S1). α-diversity analyses revealed the following: ➀ Chao1 index: F1 hybrids (1082.00 ± 78.89) showed significantly higher species richness than both parents. No significant difference was observed between S. calendulacea (774.76 ± 122.40) and S. trilobata (763.33 ± 114.79) (Figure 1A). ②. Shannon index: S. trilobata (4.14 ± 0.30) exhibited significantly higher diversity than S. calendulacea (3.60 ± 0.13) (Figure 1B). The F1 hybrid (4.01 ± 0.18) did not differ significantly from either parent (Figure 1B). ③. Simpson index: No significant differences were found among S. calendulacea (0.90 ± 0.04), S. trilobata (0.95 ± 0.02), and the F1 hybrid (0.93 ± 0.01) (Figure 1C).
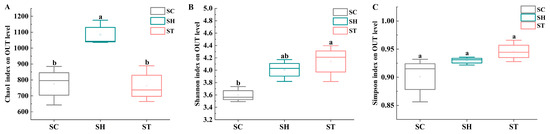
Figure 1.
α-diversity indices for the 16S rRNA gene sequences. Box plots of the Chao1 (A), Shannon (B), and Simpson (C) indices in phyllosphere samples from both S. calendulacea (SC), S. trilobata (ST) and their F1 hybrids (SH). Whiskers represent the minimum and maximum values. The bar represents the median. Bonferroni correction-adjusted p-value was calculated from Dunn’s test of multiple comparisons using rank sums. The different letters represent significant differences between groups (p < 0.05).
3.2. Distinct Phyllosphere Microbial Community Structure at Family and Genus Levels among Three Sphagneticola Groups
Taxonomic annotation revealed significant differences in community structure between the host plant groups at both the family and genus levels. Taxa with < 0.01 relative abundance were grouped as “others”. At the family level, 10 bacterial families dominated the phyllosphere communities, comprising > 50% of relative abundance (Figure 2A). The family Beijerinckiaceae was most abundant on S. trilobata (32.0%) and the hybrid (23.7%), but lower on S. calendulacea (22.4%). In contrast, the family Oxalobacteraceae was most prevalent on S. calendulacea (23.8%) but rarer on S. trilobata (4.8%) and the hybrid (5.9%). At the genus level (Figure 2B), Methylobacterium-Methylorubrum dominated on S. trilobata (31.7%) and the hybrid (22.9%), but were less abundant on S. calendulacea (21.9%). Duganella was most prominent on S. calendulacea (22.5%) but rarer on S. trilobata (4.3%) and the hybrid (5.3%). While the hybrid’s microbial community was intermediate between its parents at the family level, it more closely resembled S. trilobata at the genus level.
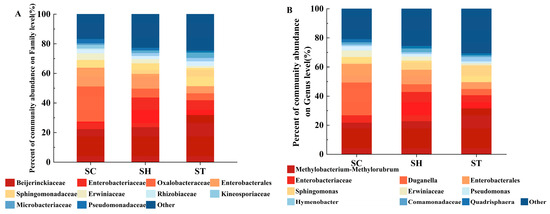
Figure 2.
Average relative abundances of bacterial at the family level (A) and the genus level (B) in the different species. SC, S. calendulacea; SH, the hybrids; ST, S. trilobata.
3.3. Distinct Phyllosphere Microbial Community Assemblies among Three Sphagneticola Species Groups
β-diversity analyses compared overall bacterial community compositions between host plant groups. Non-metric multidimensional scaling (NMDS) and UPGMA clustering both revealed significantly distinct phyllosphere community structures among S. trilobata, S. calendulacea and their hybrid. NMDS analysis separated samples into three mutually exclusive groups (Figure 3A). S. trilobata clustered alone in Group 1, while S. calendulacea and the hybrid formed separate clusters in Groups 2 and 3, respectively. UPGMA clustering showed a slightly different pattern. S. trilobata remained in a distinct cluster, but S. calendulacea and hybrid communities intermingled, suggesting higher similarity and shared bacterial membership.
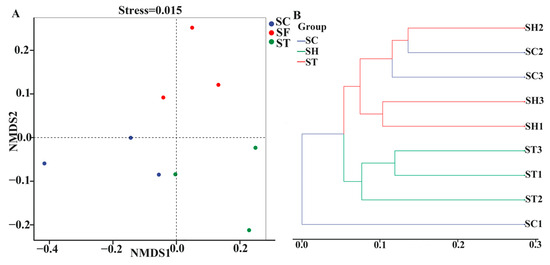
Figure 3.
Principal coordinate analysis (NMDS) (A) and UPGMA clustering analysis (B) of the bacterial communities in different species based on Bray–Curtis distances. SC: S. calendulacea; SH: the hybrids; ST: S. trilobata.
One S. calendulacea sample (SC1) formed an outgroup branch, indicating the most dissimilar community. This outlier may result from localized environmental variability or stochastic microbial association. The remaining S. calendulacea and hybrid communities grouped together with interspersed branches, reflecting an intermediate state between complete differentiation and homogeneity.
3.4. Distinct Profile of Volatile Organic Compounds among Three Sphagneticola Species
S. calendulacea contained the most diverse VOCs profile, with 72 total compounds detected, eighteen comprising > 1% relative abundance (Table 1). The hybrid and S. trilobata contained 60 and 73 VOCs, respectively, with 18 and 13 at > 1% relative abundance (Table 1). While all groups were dominated by terpenes and aromatics, S. calendulacea also had more esters and alcohols, suggesting niche differentiation in their VOC profiles.

Table 1.
Composition of volatile compounds in S. calendulacea, S. trilobata and their hybrids.
The native S. calendulacea exhibited a distinct VOC profile, including styrene, p-xylene, cis-muurola-4(14),5-diene, and benzyl benzoate, with β-cubebene as the most abundant compound (14.39%; Table 1). The hybrid was characterized by copaene, β-cis-ocimene, 1,4-cadinadiene, α-muurolene, and α-cadinene, with (E)-germacrene D predominant (24.59%; Table 1). While sharing some VOCs with S. calendulacea, such as β-cubebene, the hybrid’s profile was intermediate yet distinct, with unique compounds and different relative quantities. S. trilobata displayed the greatest differentiation, containing several unique VOCs including p-cymene, (1S)-(—)-α-pinene, β-ocimene, cis-muurola-3,5-diene, and phenylethyl alcohol. Its major VOC was α-phellandrene (20.37%; Table 1). The presence of multiple unique VOCs and differences in the relative abundances of shared compounds demonstrates that S. trilobata’s volatile signature was the most divergent among the three groups.
Fold change analysis quantified VOC differences among S. calendulacea, S. trilobata and their hybrid. S. calendulacea upregulated primarily hydrocarbons and aldehydes compared to the hybrid, with 2-thujene showing the greatest increase (3.41-fold; Figure 4A). Conversely, (E)-germacrene D was downregulated 5.64-fold in S. calendulacea relative to the hybrid. Comparing the hybrid and S. trilobata revealed distinct differences, with phenylethyl alcohol and D-limonene upregulated 4.16-fold in S. trilobata, while terpenes and aromatics were downregulated (Figure 4B). S. trilobata had fewer upregulated VOCs compared to S. calendulacea but showed an increase in phenylethyl alcohol (3.42-fold), D-limonene (2.35-fold) and humulene (1.36-fold), while β-cubebene decreased 2.82-fold (Figure 4C). β-cubebene’s abundance in S. calendulacea indicates its volatile signature was most differentiated from S. trilobata.
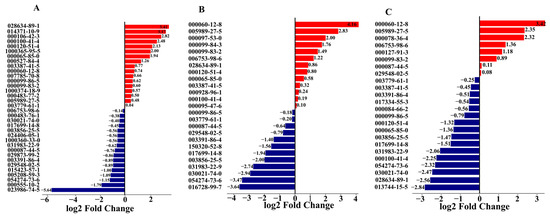
Figure 4.
Comparison of differences between SC and SH (A), ST and SH (B), SC and ST (C) volatile compounds based on fold change. SC: S. calendulacea; SH: the hybrids; ST: S. trilobata. Red and blue bar indicates upregulation and downregulation respectively.
However, in the absence of data on the ecological roles and impacts of these specific VOCs, further work is required to determine whether the differentiation in volatile signatures observed here provides a fitness advantage. Analyzing VOC profiles from additional populations of native, introduced and hybrid Sphagneticola, especially in areas of introduction and hybridization, would provide a broader understanding of how volatile evolution shapes diverse ecological interactions within and outside a plant’s native range.
4. Discussion
4.1. Divergent Diversity and Assembly of Phyllosphere Microbial Communities among Sphagneticola Species and Their Hybrids
α-diversity indices (Chao1, Shannon, and Simpson) revealed higher species richness and diversity in S. trilobata’s phyllosphere microbial communities compared to S. calendulacea. The F1 hybrid exhibited comparable diversity and evenness to both parent groups, suggesting inheritance of a diverse, well-distributed assortment of microbial species, which may influence ecological interactions and adaptation [42].
β-diversity analyses (NMDS and UPGMA clustering) showed distinct bacterial community structures among S. trilobata, S. calendulacea, and the hybrid. While S. trilobata consistently formed a separate group, S. calendulacea and the hybrid displayed some shared membership, indicating heterogeneity or intermediate character in their assembly. This supports the influence of plant genotype, environmental heterogeneity, and microbial dispersal on plant–microbe interactions [2,4,11,24,26]. Further research is needed to understand the drivers of both differentiation and occasional convergence in these phyllosphere communities [43].
These findings have potential ecological and evolutionary implications. S. trilobata’s higher microbial diversity may provide a competitive advantage over native species, offering a broader range of functional traits and potential benefits [44]. The shared membership between S. calendulacea and the hybrid suggests certain microbial taxa are well suited to colonize both native and hybrid hosts, possibly due to adaptability to various environmental conditions or host genotypes [45].
Understanding the complex interactions between plants and their associated microbial communities is crucial for predicting the ecological and evolutionary consequences of these relationships. For example, the higher species richness and diversity in S. trilobata may contribute to its success as an invasive species. A diverse microbiome can enhance plant resilience to environmental stressors and promote nutrient acquisition [11,44]. Furthermore, the shared microbial membership between S. calendulacea and the hybrid may facilitate gene flow between native and introduced species, potentially leading to the emergence of novel genotypes and phenotypes [42].
4.2. Chemosensitive Compounds and Invasive Success: The Role of Volatile Composition in S. trilobata’s Dominance Over Native Plant Species
Exotic species generate chemosensitive compounds that hinder the growth of indigenous plants through the volatilization of stems and leaves and the secretion of roots [37,38]. Infusions from various parts of S. trilobata negatively affect common crops like rice and tomato [46,47]. The volatile composition of Sphagneticola species differs, and examining their phyllosphere VOCs can shed light on the potent chemosensory effects of S. trilobata. Terpenoids, prevalent in the phyllosphere volatiles of the three Sphagneticola species, possess excellent fungicidal properties and are commonly used in fungicides [48].
S. trilobata has fewer VOCs with a relative content above 1% compared to native species and hybrids, but it has a higher relative content of compounds with fungicidal effects, such as α-Phellandrene and Caryophyllene. The unique compound p-cymene, found in S. trilobata, has a relative content of 13.33%, second only to α-Phellandrene and D-Limonene among all volatiles. p-Cymene exhibits various pharmacological properties, including antibacterial [49], antiparasitic [50], and antiviral activities [51]. Fold-change analysis reveals that the most downregulated volatile in S. trilobata compared to S. calendulacea and their hybrids is phenylethyl alcohol. This compound, which has an aromatic odor, is known to inhibit a range of pathogenic bacteria [52].
This evidence suggests that S. trilobata contains more bacteriostatic volatile substances than S. calendulacea and the hybrids. Studies have shown that the growth performance of S. trilobata supplemented with its own extract is superior to that of S. calendulacea in any cultivation environment, indicating that the VOCs of invasive plants can inhibit other plants without causing autotoxicity [53,54]. These antibacterial and anti-pest VOCs can help protect S. trilobata from most pests and diseases during its invasion, providing it with a competitive advantage over native plants. The stronger chemosensitization of S. trilobata compared to S. calendulacea and their hybrids further contributes to its ability to outcompete and suppress the growth of surrounding native plants.
In conclusion, the unique volatile composition of S. trilobata, including compounds like p-cymene, phenylethyl alcohol, α-Phellandrene, and Caryophyllene, may play a critical role in its invasive success. These chemosensitive substances not only inhibit the growth of native plants but also provide protection against pests and diseases, allowing S. trilobata to thrive in various environments and outcompete other plant species.
4.3. Volatile-Mediated Plant–Microbe Interactions among the Sphagneticola Species
Plant leaves emit VOCs that can influence the composition of their phyllosphere microbial communities. Compounds like terpenoids, phenylpropanoids and phencylides have antimicrobial properties and can inhibit colonization [55,56]. S. trilobata produces high amounts of terpenoids with insecticidal and antimicrobial activity, which may contribute to its lower microbial diversity relative to the other Sphagneticola groups.
The VOCs from S. trilobata could interfere with native plants and microbes, enabling the formation of single-species communities. Antifungal VOCs may also allow S. trilobata to avoid native pathogens, facilitating invasion success. However, microbial communities can reciprocally shape the production of plant VOCs. For example, Bacteroides, Bacillales and Methylobacterium-Methylorubrum, found on raspberries, produce VOCs that increase pest/disease resistance [57,58].
In this study, Methylobacterium-Methylorubrum was most abundant across all Sphagneticola groups, especially the hybrid. This suggests the hybrid, not just S. trilobata, may possess strong chemical effects that reduce suitable habitat for native species. Plant–microbe interactions and their shared chemical mediators warrant further study as determinants of community structure and potential drivers of species invasion.
The reciprocal relationships between plants, microbes and their volatile signals are complex but ecologically significant. While terpenoid-rich S. trilobata may chemically inhibit competitors to dominate communities, associated microbes like M. methylorubrum could simultaneously enhance its defenses and stress tolerance in new ranges. However, whether the high abundance of M. methylorubrum provides benefits, and what microbial-derived VOCs are involved, requires experimental confirmation. The hybrid’s combined volatile chemistry and microbial profile may represent an intermediate or novel state that similarly influences community assembly and species distributions.
Overall, volatile-mediated plant–microbe interactions deserve greater consideration as mechanisms not only promoting but also potentially limiting or regulating the spread of invasive species outside their native ranges. S. trilobata’s chemical effects on and associations with other organisms likely depend on attributes of both its new and ancestral environments, as well as its resident microbiota. The hybrid’s unique characteristics may also derive from a combination of introduced and native influences that shape its biological and ecological relationships in a distinct manner. Further work could explore how terpenoid profiles, microbial communities and their interactive VOCs differ quantitatively and qualitatively between these groups across native and introduced populations. Manipulative experiments are also needed to determine the causal roles of plant and microbial volatiles in community dynamics, species coexistence and habitat generation or loss. By integrating chemical and biological approaches, deeper insights may be gained into the complex factors underlying plant invasion, competitive exclusion of natives, and conservation of diversity even between closely-related species.
5. Conclusions
In this study, we examined the phyllosphere microbial community structure and VOCs in three Sphagneticola species: the introduced S. trilobata, the native S. calendulacea and their hybrid. Despite their close relationships, these species exhibit distinct microbial communities, volatile chemistries and ecological characteristics that together may determine their spread and competitive ability.
Specifically, firstly, S. trilobata harbors higher microbial diversity and produces more potent antimicrobial volatiles, likely facilitating its invasion success and dominance in new environments. However, the presence of certain shared microbes among the three groups indicates a shared resilience in coping with similar environment stresses. Secondly, the hybrid’s intermediate traits warrant further study as potential drivers of habitat generation, genetic exchange, or modulation of chemical effects on community structure. Thirdly, volatile-mediated plant–microbe interactions influence invasion, competitive exclusion, and coexistence. These interactions depend on attributes of both ancestral and new ranges as well as resident organisms. Finally, an interdisciplinary approach combining chemical and microbial ecology is needed to understand plant–microbe dynamics at fine scales, which may have significant consequences for species distributions and ecosystem properties. Findings could reveal opportunities for novel control strategies or harnessing positive interactions for diversity conservation in a changing world.
This system provides a tractable model to advance understanding of mechanisms that generate and maintain biodiversity—or lead to its loss. By examining closely-related native, introduced, and hybrid groups across ranges, we can gain new insights into the complex relationships shaping community assembly at the invasion front. These findings could inform novel control strategies or methods to harness positive interactions for diversity conservation in changing environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes15070955/s1, Figure S1: Sample rarefaction curves.
Author Contributions
Conceptualization, H.Z., S.L. and W.W.; methodology, H.Z., Y.L. and W.W.; formal analysis, H.Z. and S.L.; investigation, P.C. and Y.L.; data curation, H.Z., S.L. and P.C.; writing—original draft preparation, H.Z., S.L.; writing—review and editing, Y.L. and W.W.; visualization, S.L. and S.Z.; supervision, W.G. and W.W.; project administration, W.G. and W.W.; funding acquisition, W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Natural Science Foundation of China (NSFC) (Grant no. 31670384) to Wei Wu and Guangdong Province University Innovative Team Project: Innovation and Development Application of Ornamental Plant Germplasm with Lingnan Characteristics (2023KCXTD017) to Wei Guo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequence data supporting these findings were submitted to the NCBI Sequence Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/ (accessed on 28 June 2023)), under BioProject accession number PRJNA988409.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peñuelas, J.; Terradas, J. The foliar microbiome. Trends Plant Sci. 2014, 19, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E.; Brandl, M.T. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial Hub Taxa Link Host and Abiotic Factors to Plant Microbiome Variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Nomura, K.; Wang, X.; Sohrabi, R.; Xu, J.; Yao, L.; Paasch, B.C.; Ma, L.; Kremer, J.; Cheng, Y.; et al. A plant genetic network for preventing dysbiosis in the phyllosphere. Nature 2020, 580, 653–657. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Cordovez, V.; Dini-Andreote, F.; Carrión, V.J.; Raaijmakers, J.M. Ecology and Evolution of Plant Microbiomes. Annu. Rev. Microbiol. 2019, 73, 69–88. [Google Scholar] [CrossRef]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Remus-Emsermann M. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The soil microbiome influences grapevine-associated microbiota. MBio 2015, 6, e02527-14. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; Del Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Xiong, C.; Wei, Z.; Chen, Q.L.; Ma, B.; Zhou, S.Y.D.; Tan, J.; Zhang, L.M.; Cui, H.L.; Duan, G.L. Impacts of global change on the phyllosphere microbiome. New Phytol. 2022, 234, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Crombie, A.T.; Larke-Mejia, N.L.; Emery, H.; Dawson, R.; Pratscher, J.; Murphy, G.P.; McGenity, T.J.; Murrell, J.C. Poplar phyllosphere harbors disparate isoprene-degrading bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 13081–13086. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Prasanna, R. Prospecting the characteristics and significance of the phyllosphere microbiome. Ann. Microbiol. 2018, 68, 229–245. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Alex, T.H.H.; Ngoh, S.T.; Prithiviraj, B.; Ji, L. Leaf-residing Methylobacterium species fix nitrogen and promote biomass and seed production in Jatropha curcas. Biotechnol. Biofuels 2015, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Abadi, V.A.J.M.; Sepehri, M.; Rahmani, H.A.; Zarei, M.; Ronaghi, A.; Taghavi, S.M.; Shamshiripour, M. Role of dominant phyllosphere bacteria with plant growth–promoting characteristics on growth and nutrition of maize (Zea mays L.). J. Soil. Sci. Plant Nutr. 2020, 20, 2348–2363. [Google Scholar] [CrossRef]
- Ruinen, J. Occurrence of Beijerinckia species in the ‘phyllosphere’. Nature 1956, 177, 220–221. [Google Scholar] [CrossRef]
- Mwajita, M.R.; Murage, H.; Tani, A.; Kahangi, E.M. Evaluation of rhizosphere, rhizoplane and phyllosphere bacteria and fungi isolated from rice in Kenya for plant growth promoters. SpringerPlus 2013, 2, 606. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Bortfeld-Miller, M.; Ackermann, M.; Vorholt, J.A. A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet. 2014, 10, e1004283. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; Jones, E.; Kline, J.; Northcutt, D.; Stenson, J.; Womack, A.M.; Bohannan, B.J.; Brown, G.Z.; Green, J.L. Architectural design influences the diversity and structure of the built environment microbiome. ISME J. 2012, 6, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.W.; Bodenhausen, N.; Beilsmith, K.; Meng, D.; Muegge, B.D.; Subramanian, S.; Vetter, M.M.; Vilhjálmsson, B.J.; Nordborg, M.; Gordon, J.I.; et al. Genome-wide association study of Arabidopsis thaliana leaf microbial community. Nat. Commun. 2014, 5, 5320. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.T.; Whiteman, N.K. Insect herbivory reshapes a native leaf microbiome. Nat. Ecol. Evol. 2020, 4, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Wall, C.B.; Costantini, M.S.; Rollins, R.L.; Atkins, M.L.; Cabrera, F.P.; Cetraro, N.D.; Feliciano, C.K.; Greene, A.L.; Kitamura, P.K.; et al. Plant part and a steep environmental gradient predict plant microbial composition in a tropical watershed. ISME J. 2021, 15, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Sy, A.; Timmers, A.C.; Knief, C.; Vorholt, J.A. Methylotrophic metabolism is advantageous for Methylobacterium extorquens during colonization of Medicago truncatula under competitive conditions. Appl. Environ. Microbiol. 2005, 71, 7245–7252. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, S.; Krumbein, A.; Schreiner, M. Composition of the phyllospheric microbial populations on vegetable plants with different glucosinolate and carotenoid compositions. Microb. Ecol. 2008, 56, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Yurimoto, H.; Oku, M.; Sakai, Y. Yeast methylotrophy and autophagy in a methanol-oscillating environment on growing Arabidopsis thaliana leaves. PLoS ONE 2011, 6, e25257. [Google Scholar] [CrossRef]
- Xu, G.S.; Huang, Z.Q.; Lin, G.H.; Li, Z.X. Wedelia trilobata invasion alters soil nematode communities and soil ecosystem functions in a subtropical forest ecosystem. Pedosphere 2018, 28, 641–652. [Google Scholar]
- Azizan, K.A.; Ghani, N.H.A.; Nawawi, M.F. GC-MS based metabolomics and multivariate statistical analysis of Wedelia trilobata extracts for the identification of potential phytochemical properties. Plant Omics 2015, 8, 537–543. [Google Scholar]
- Zhang, Q.; Huang, J.; Ke, W.; Cai, M.; Chen, G.; Peng, C. Responses of Sphagneticola trilobata, Sphagneticola calendulacea and their hybrid to drought stress. Int. J. Mol. Sci. 2021, 22, 11288. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zeng, L.; Cai, M.; Chauvat, M.; Forey, E.; Tariq, A.; Graciano, C.; Zhang, Z.; Gu, Y.; Zeng, F.; et al. An invasive and native plant differ in their effects on the soil food-web and plant-soil phosphorus cycle. Geoderma 2022, 410, 115672. [Google Scholar] [CrossRef]
- Song, L.; Chow, W.S.; Sun, L.; Li, C.; Peng, C. Acclimation of photosystem II to high temperature in two Wedelia species from different geographical origins: Implications for biological invasions upon global warming. J. Exp. Bot. 2010, 61, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Hossen, K.; Das, K.R.; Okada, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and active substances from Wedelia chinensis (Osbeck). Foods 2010, 9, 1591. [Google Scholar] [CrossRef] [PubMed]
- Santonja, M.; Bousquet-Mélou, A.; Greff, S.; Ormeño, E.; Fernandez, C. Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol. 2019, 9, 8201–8213. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, X.; Li, S.; He, H.; Yan, X.; Chen, Y.; Dong, J.; Zhang, Z.; Li, S. Eudesmanolides from Wedelia trilobata (L.) Hitchc. as potential inducers of plant systemic acquired resistance. J. Agric. Food Chem. 2013, 61, 3884–3890. [Google Scholar] [CrossRef]
- Qi, S.S.; Liu, Y.J.; Dai, Z.C.; Wan, L.Y.; Du, D.L.; Ju, R.T.; Wan, J.S.; Bonser, S.P. Allelopathy confers an invasive Wedelia higher resistance to generalist herbivore and pathogen enemies over its native congener. Oecologia 2020, 192, 415–423. [Google Scholar] [CrossRef]
- Kembel, S.W.; Mueller, R.C. Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities. Botany 2014, 92, 303–311. [Google Scholar] [CrossRef]
- Ren, G.; Zhu, C.; Alam, M.S.; Tokida, T.; Sakai, H.; Nakamura, H.; Usui, Y.; Zhu, J.; Hasegawa, T.; Jia, Z. Response of soil, leaf endosphere and phyllosphere bacterial communities to elevated CO2 and soil temperature in a rice paddy. Plant Soil. 2015, 392, 27–44. [Google Scholar] [CrossRef]
- Alpendurada, M.F. Solid-phase microextraction: A promising technique for sample preparation in environmental analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [CrossRef]
- Arnold, A.E.; Lutzoni, F. Diversity and Host Range of Foliar Fungal Endophytes: Are Tropical Leaves Biodiversity Hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.R.; Copeland, J.; Wang, P.W.; Guttman, D.S.; Kotanen, P.M.; Johnson, M.T.J. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl. Acad. Sci. USA 2018, 115, E1157–E1165. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Luo, S.; Zeng, R.; Mo, M.; Li, H.; Lin, C. Allelopathic potential of Wedelia trilobata L.: Effects on germination, growth and physiological parameters of rice. In Proceedings of the 4th World Congress on Allelopathy, Wagga, Australia, 21–26 August 2005. [Google Scholar]
- Sun, J.; Wu, Y.; Wu, P.; Du, D. Allelopathic effect of alcohol extracts from different tissues of Wedelia trilobata L. Guangdong Agric. Sci. 2013, 40, 74–78. [Google Scholar]
- Kummer, R.; Estevão-Silva, C.F.; Bastos, R.L.; Grespan, R.; de Souza Silva-Comar, F.M.; Spironello, R.A.; Silva, E.L.; Bersani-Amado, C.A.; Kenji, R.; Cuman, N. Original Research Effect of p-Cymene on chemotaxis, phagocytosis and leukocyte behaviors. In Vitro 2008, 10, 100. [Google Scholar]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Chun, H.S. p-Cymene and its derivatives exhibit antiaflatoxigenic activities against Aspergillus flavus through multiple modes of action. Appl. Biol. Chem. 2018, 61, 489–497. [Google Scholar] [CrossRef]
- Shang, X.; Wang, Y.; Zhou, X.; Guo, X.; Dong, S.; Wang, D.; Zhang, J.; Pan, H.; Zhang, Y.; Miao, X. Acaricidal activity of oregano oil and its major component, carvacrol, thymol and p-cymene against Psoroptes cuniculi in vitro and in vivo. Vet. Parasitol. 2016, 226, 93–96. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.A.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell. Mol. Biol. 2017, 63, 42–47. [Google Scholar] [CrossRef]
- Mo, E.; Sung, C. Effect of Pichia anomala SKM-T and Galactomyces geotrichum SJM-59 dipping on storage property and sensory quality of strawberry. Food Sci. Biotechnol. 2005, 14, 487–492. [Google Scholar]
- Ullah, M.S.; Sun, J.; Rutherford, S.; Ullah, I.; Javed, Q.; Rasool, G.; Ajmal, M.; Du, D. Evaluation of the allelopathic effects of leachate from an invasive species (Wedelia triobata) on its own growth and performance and those of a native congener (W. chinensis). Biol. Invasions 2021, 23, 3135–3149. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; van Kleunen, M. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Ashour, H.M. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol. Ther. 2008, 7, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.G.; Bao, L.; Luciani, A.; Panighi, J.; Desjobert, J.M.; Costa, J.; Casanova, J.; Bolla, J.M.; Berti, L. (E)-Methylisoeugenol and elemicin: Antibacterial components of Daucus carota L. essential oil against Campylobacter jejuni. J. Agric. Food Chem. 2007, 55, 7332–7336. [Google Scholar] [CrossRef]
- Daranas, N.; Roselló, G.; Cabrefiga, J.; Donati, I.; Francés, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological control of bacterial plant diseases with Lactobacillus plantarum strains selected for their broad-spectrum activity. Ann. Appl. Biol. 2019, 174, 92–105. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Spinelli, F.; Pastore, C.; Farneti, B.; Savioli, S.; Rodriguez-Estrada, M.T.; Donati, I. Contribution of fruit microbiome to raspberry volatile organic compounds emission. Postharvest Biol. Technol. 2022, 183, 111742. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).