Liver Transcriptomic Profiles of Ruminant Species Fed Spent Hemp Biomass Containing Cannabinoids
Abstract
:1. Introduction
2. Materials and Methods
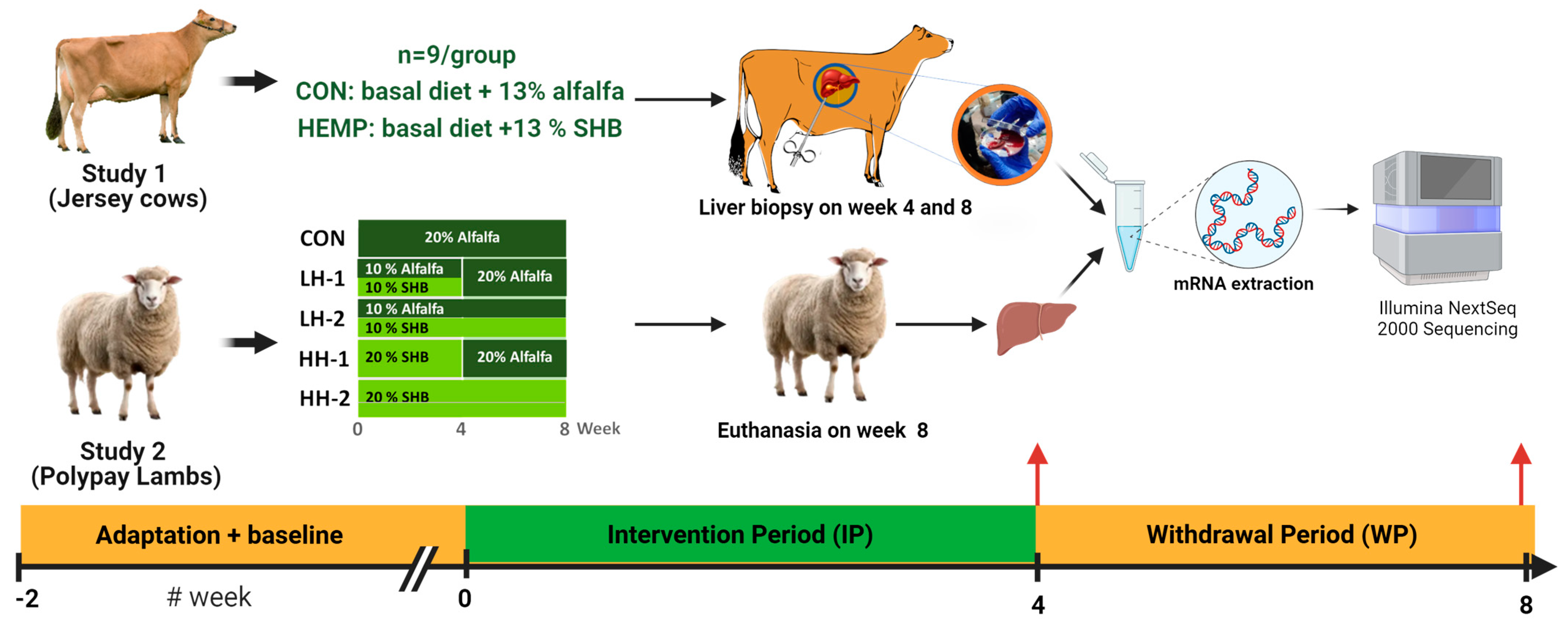
2.1. In Vivo Study and Experimental Designs
2.2. Sample Collections
2.3. RNA Extraction, Library Preparation, and Sequencing
2.4. Quality Control, Processing, and Alignment of Reads
2.5. Differential Gene Expression Analysis
2.6. Bioinformatic Analyses
3. Results
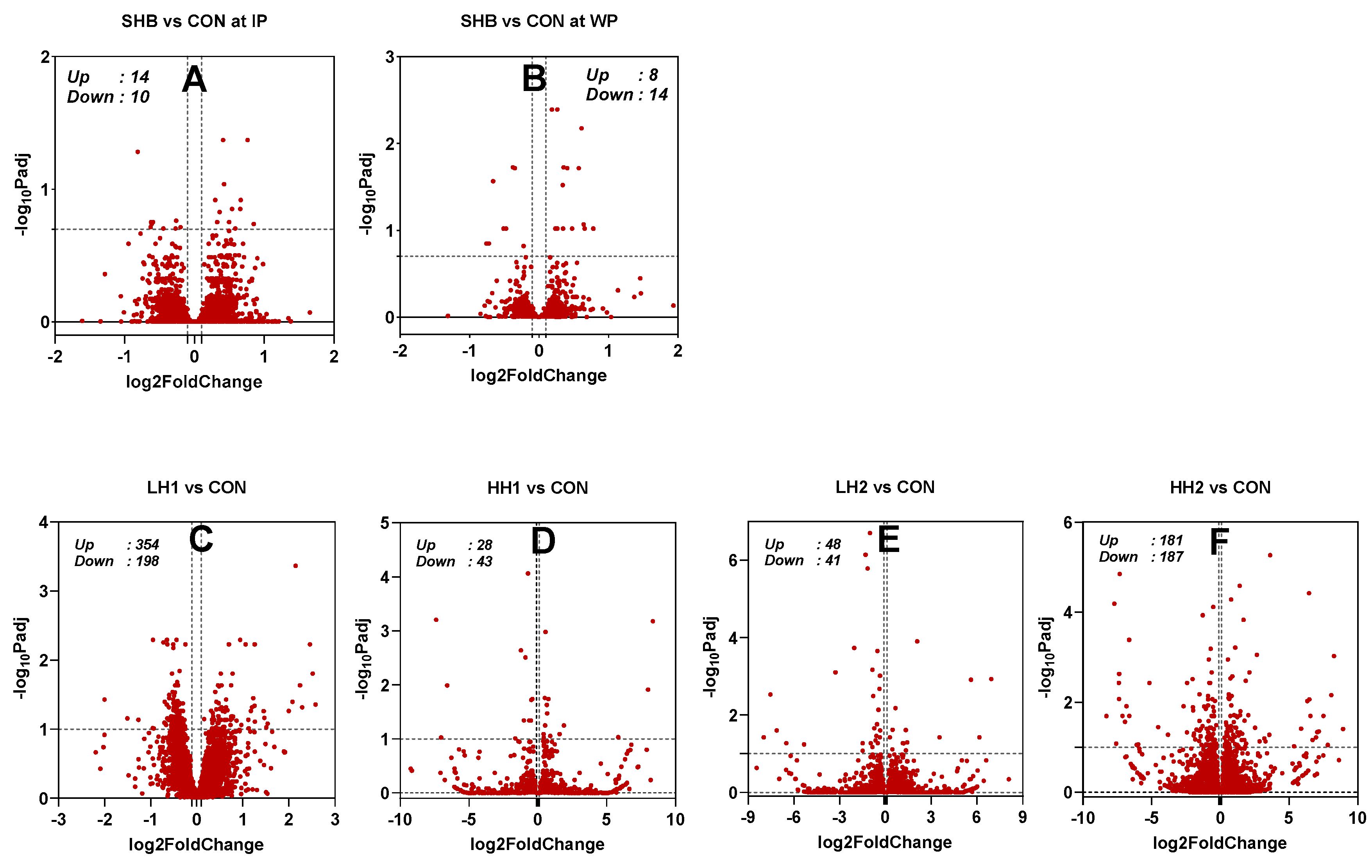
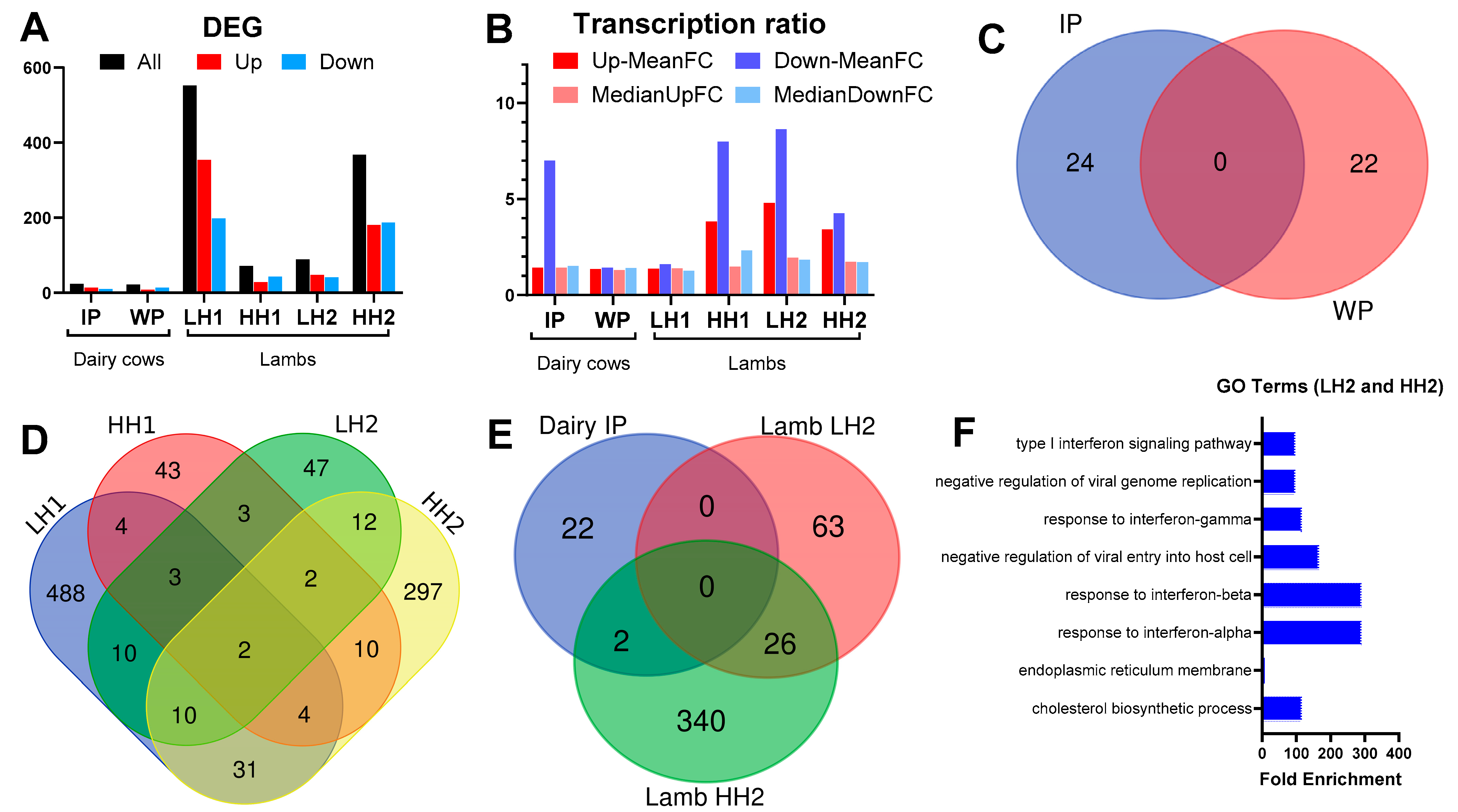
3.1. Differential Gene Expression Analysis
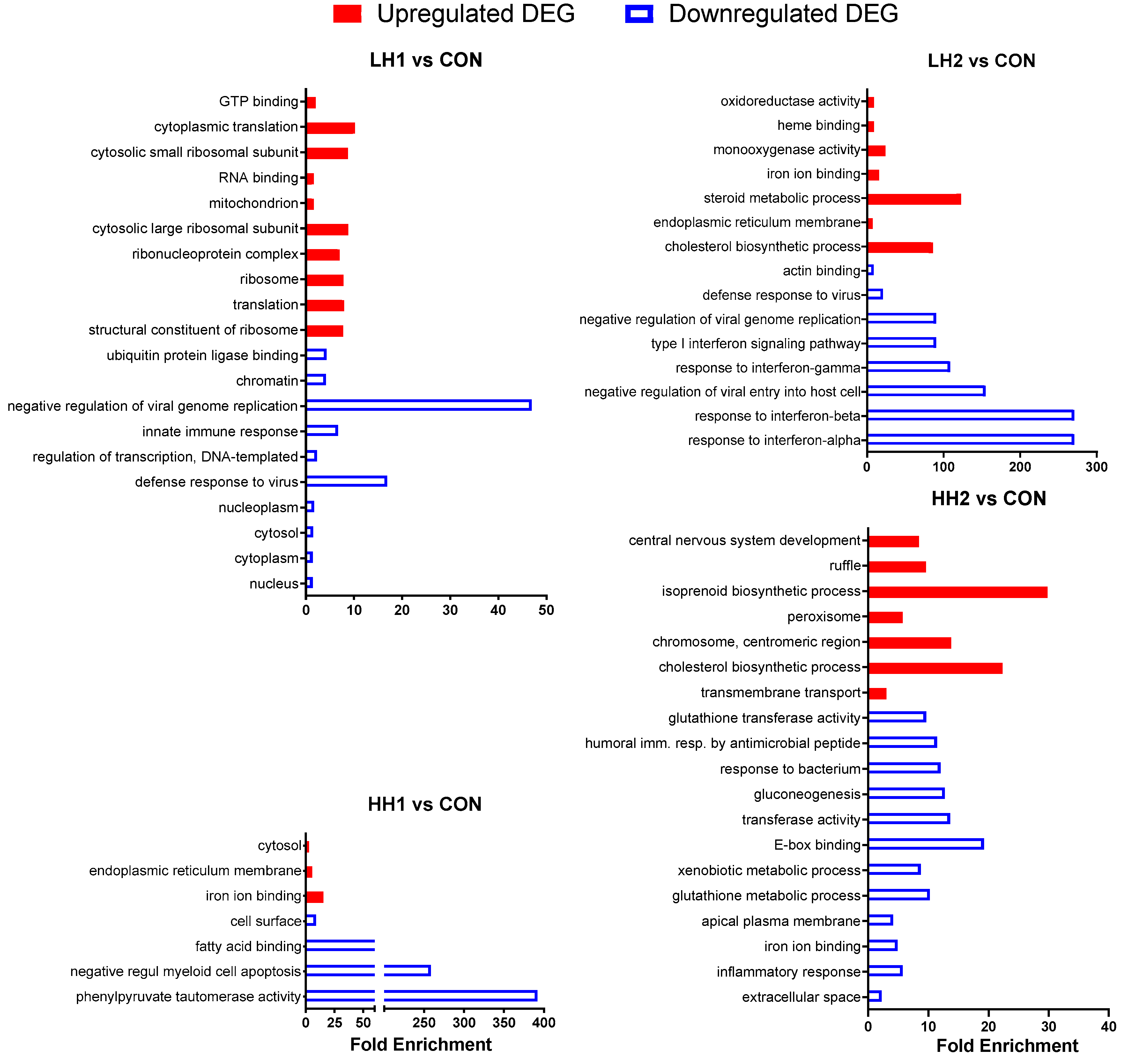
3.2. Functional Analysis of Differentially Expressed Genes
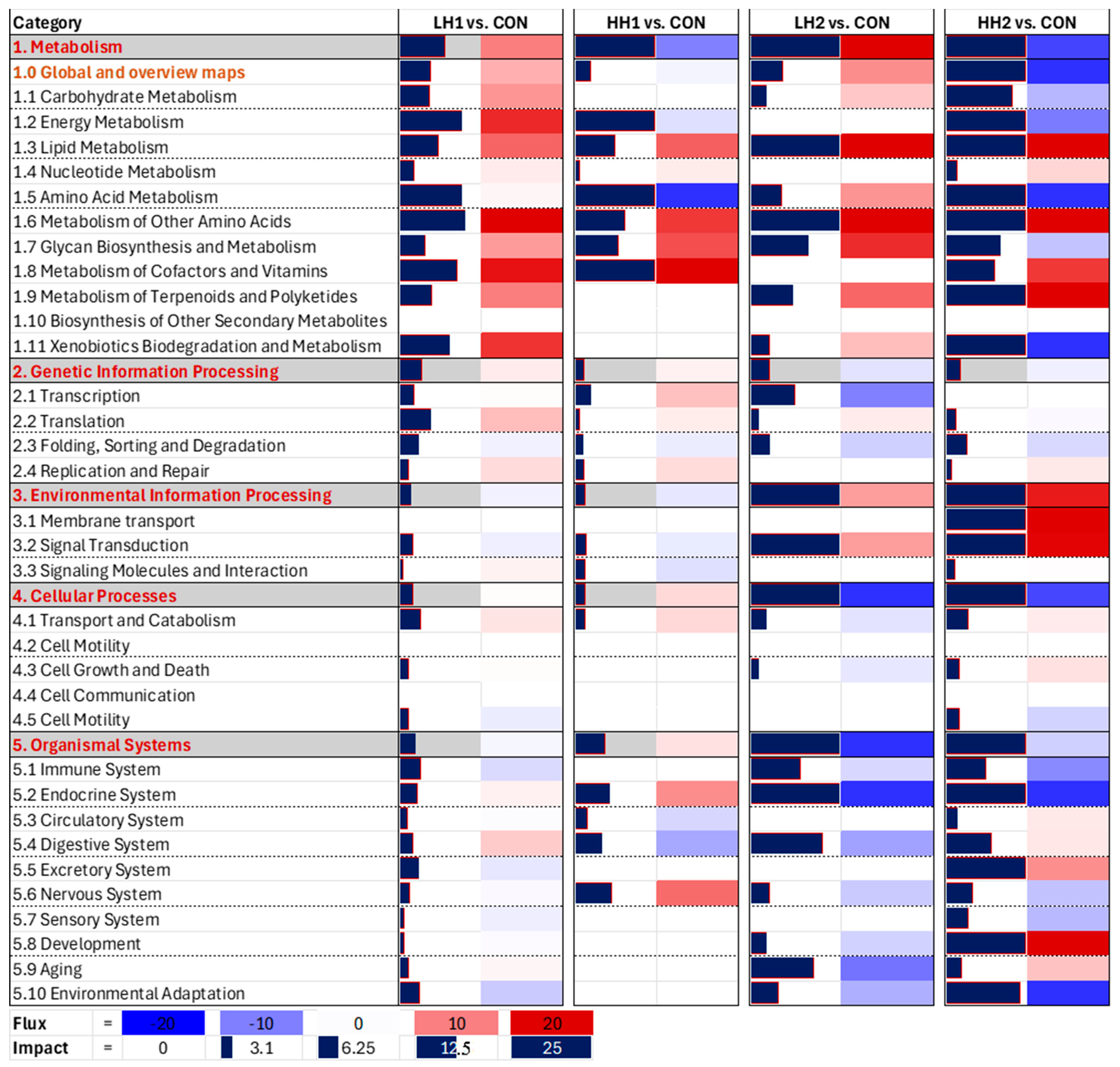
3.3. KEGG Pathways Analysis
4. Discussion
4.1. Most Cannabinoid Receptors Are Virtually Not Expressed in Liver
4.2. Feeding SHB Has a Minor Effect on the Liver Transcriptome
4.3. Feeding SHB Affects Genes Related to Amino Acids and Steroid Synthesis
4.4. Spent Hemp Biomass Does Not Induce Inflammation
4.5. Data Do Not Support an Effect of SHB on PPAR Signaling Pathway
4.6. Activation of Cytochrome P450 Pathway by SHB
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raymunt, M. Hemp Cultivation in Europe: Key Market Details and Opportunities. 2020, 1–26. Available online: https://hempindustrydaily.com/wp-content/uploads/2020/07/hemp-in-europe-2020-FINAL.pdf (accessed on 20 January 2024).
- Canada, G. Regulation of Hemp Products for Use in Livestock Feeds. 2024, 1–7. Available online: https://inspection.canada.ca/en/animal-health/livestock-feeds/regulatory-guidance/rg-1/chapter-3#c10 (accessed on 20 January 2024).
- Mark, T.; Shepherd, J.; Olson, D.; Snell, W.; Proper, S.; Thornsbury, S. Economic Viability of Industrial Hemp in the United States: A Review of State Pilot Programs United States Department of Agriculture; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2020.
- Parker, N.B.; Bionaz, M.; Ford, H.R.; Irawan, A.; Trevisi, E.; Ates, S. Assessment of Spent Hemp Biomass as a Potential Ingredient in Ruminant Diet: Nutritional Quality and Effect on Performance, Meat and Carcass Quality, and Hematological Parameters in Finishing Lambs. J. Anim. Sci. 2022, 100, skac263. [Google Scholar] [CrossRef] [PubMed]
- Irawan, A.; Puerto-Hernandez, G.M.; Ford, H.R.; Busato, S.; Ates, S.; Cruickshank, J.; Ranches, J.; Estill, C.T.; Trevisi, E.; Bionaz, M. Feeding Spent Hemp Biomass to Lactating Dairy Cows: Effects on Performance, Milk Components and Quality, Blood Parameters, and Nitrogen Metabolism. J. Dairy Sci. 2024, 107, 258–277. [Google Scholar] [CrossRef] [PubMed]
- Mosher, R.A.; Coetzee, J.F.; Cull, C.A.; Gehring, R.; Kukanich, B. Pharmacokinetics of Oral Meloxicam in Ruminant and Preruminant Calves. J. Vet. Pharmacol. Ther. 2012, 35, 373–381. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; da Silva, A.B.F. Cannabidiol as an Antioxidant; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128008270. [Google Scholar]
- Chen, S.; Kim, J.K. The Role of Cannabidiol in Liver Disease: A Systemic Review. Int. J. Mol. Sci. 2024, 25, 2370. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, F.; Zhou, H.; Ma, N.; Wu, S.; Huang, H.; Jin, G.; Wu, S.; Hong, W.; Zhuang, W.; Xia, H.; et al. A Novel Mechanism of Cannabidiol in Suppressing Hepatocellular Carcinoma by Inducing GSDME Dependent Pyroptosis. Front. Cell Dev. Biol. 2021, 9, 697832. [Google Scholar] [CrossRef] [PubMed]
- Palomares, B.; Ruiz-Pino, F.; Garrido-Rodriguez, M.; Eugenia Prados, M.; Sánchez-Garrido, M.A.; Velasco, I.; Vazquez, M.J.; Nadal, X.; Ferreiro-Vera, C.; Morrugares, R.; et al. Tetrahydrocannabinolic Acid A (THCA-A) Reduces Adiposity and Prevents Metabolic Disease Caused by Diet-Induced Obesity. Biochem. Pharmacol. 2020, 171, 113693. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.S.; Ragos, R.; Gurm, H.; Lapierre, M.; May, L.L.; Raha, S. Delta-9-Tetrahydrocannabinol Disrupts Mitochondrial Function and Attenuates Syncytialization in Human Placental BeWo Cells. Physiol. Rep. 2020, 8, e14476. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How Does Cannabidiol (CBD) Influence the Acute Effects of Delta-9-Tetrahydrocannabinol (THC) in Humans? A Systematic Review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Irawan, A.; Muchiri, R.N.; Parker, N.B.; van Breemen, R.B.; Ates, S.; Bionaz, M. Cannabinoid Residuals in Tissues of Lambs Fed Spent Hemp Biomass and Consumer’s Exposure Assessment. Food Chem. Toxicol. 2024, 191, 114848. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Etoh, K.; Nakao, M. A Web-Based Integrative Transcriptome Analysis, RNAseqChef, Uncovers the Cell/Tissue Type-Dependent Action of Sulforaphane. J. Biol. Chem. 2023, 299, 104810. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Periasamy, K.; Rodriguez-zas, S.L.; Hurley, W.L.; Juan, J. A Novel Dynamic Impact Approach (DIA) for Functional Analysis of Time-Course Omics Studies: Validation Using the Bovine Mammary Transcriptome. PLoS ONE 2012, 7, e32455. [Google Scholar] [CrossRef] [PubMed]
- Robaina Cabrera, C.L.; Keir-Rudman, S.; Horniman, N.; Clarkson, N.; Page, C. The Anti-Inflammatory Effects of Cannabidiol and Cannabigerol Alone, and in Combination. Pulm. Pharmacol. Ther. 2021, 69, 102047. [Google Scholar] [CrossRef] [PubMed]
- Ford, H.R.; Bionaz, M. The Experimental and In Silico-Based Evaluation of NRF2 Modulators, Sulforaphane and Brusatol, on the Transcriptome of Immortalized Bovine Mammary Alveolar Cells. Int. J. Mol. Sci. 2024, 25, 4264. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; Elsohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)-Review. Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- van Ackern, I.; Wulf, R.; Dannenberger, D.; Tuchscherer, A.; Kuhla, B. Effects of Endocannabinoids on Feed Intake, Stress Response and Whole-Body Energy Metabolism in Dairy Cows. Sci. Rep. 2021, 11, 23657. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.N.; Zachut, M.; Tam, J.; Contreras, G.A. A Proposed Modulatory Role of the Endocannabinoid System on Adipose Tissue Metabolism and Appetite in Periparturient Dairy Cows. J. Anim. Sci. Biotechnol. 2021, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-karpowicz, I.; Skrzydlewskas, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Pandelides, Z.; Aluru, N.; Thornton, C.; Watts, H.E.; Willett, K.L. Transcriptomic Changes and the Roles of Cannabinoid Receptors and PPARγin Developmental Toxicities Following Exposure to Δ9-Tetrahydrocannabinol and Cannabidiol. Toxicol. Sci. 2021, 182, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two Non-Psychoactive Cannabinoids Reduce Intracellular Lipid Levels and Inhibit Hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Assa-Glazer, T.; Gorelick, J.; Sela, N.; Nyska, A.; Bernstein, N.; Madar, Z. Cannabis Extracts Affected Metabolic Syndrome Parameters in Mice Fed High-Fat/Cholesterol Diet. Cannabis Cannabinoid Res. 2020, 5, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, J.; Assa-Glazer, T.; Zandani, G.; Altberg, A.; Sela, N.; Nyska, A.; Madar, Z. THC and CBD Affect Metabolic Syndrome Parameters Including Microbiome in Mice Fed High Fat-Cholesterol Diet. J. Cannabis Res. 2022, 4, 27. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Mallat, A.; Lotersztajn, S. Endocannabinoids and Liver Disease. I. Endocannabinoids and Their Receptors in the Liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 294, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, M.A.; Yeoman, A.D.; Verma, S.; Smith, A.D.; Longhi, M.S. Autoimmune Hepatitis. Lancet 2013, 382, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Gautam, S.; Aseer, K.R.; Kim, J.; Chandrasekaran, P.; Mazucanti, C.H.; Ghosh, P.; O’Connell, J.F.; Doyle, M.E.; Appleton, A.; et al. Hepatocyte Cannabinoid 1 Receptor Nullification Alleviates Toxin-Induced Liver Damage via NF-ΚB Signaling. Cell Death Dis. 2020, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Ross, R.A. Cannabinoid Receptors and Their Ligands. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Wei, Y.; Zhang, R.; Chang, X.; Meng, L.; Wang, K.; He, Q.; Duan, T. Effects of Prenatal Exposure to THC on Hippocampal Neural Development in Offspring. Toxicol. Lett. 2023, 374, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Ranganat, M.; Chang, S.; Liang, X.; Suhas, G.; Osafo-Addo, A.; Yan, C.; Xinyu, Z.; Bradley, E.A. Single-Cell Transcriptome Mapping Identifies Common and Cell-Type Specific Genes Affected by Acute Delta9-Tetrahydrocannabinol in Humans. Sci. Rep. 2020, 10, 3450. [Google Scholar] [CrossRef]
- Truong, V.B.; Davis, O.S.; Gracey, J.; Neal, M.S.; Khokhar, J.Y.; Favetta, L.A. Sperm Capacitation and Transcripts Levels Are Altered by in Vitro THC Exposure. BMC Mol. Cell Biol. 2023, 24, 6. [Google Scholar] [CrossRef]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 2019, 24, 1694. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro-and Anti-Inflammatory Cytokines: A Systematic Review of in Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177–195. [Google Scholar] [CrossRef]
- Zachut, M.; Kra, G.; Moallem, U.; Livshitz, L.; Levin, Y.; Udi, S.; Nemirovski, A.; Tam, J. Characterization of the Endocannabinoid System in Subcutaneous Adipose Tissue in Periparturient Dairy Cows and Its Association to Metabolic Profiles. PLoS ONE 2018, 13, e0205996. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Gaffen, S.L. Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications. Cold Spring Harb. Perspect. Biol. 2018, 10, a028522. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Tacke, F. Immunology in the Liver-from Homeostasis to Disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef] [PubMed]
- Kadegowda, A.K.G.; Bionaz, M.; Piperova, L.S.; Erdman, R.A.; Loor, J.J. Peroxisome Proliferator-Activated Receptor-γ Activation and Long-Chain Fatty Acids Alter Lipogenic Gene Networks in Bovine Mammary Epithelial Cells to Various Extents. J. Dairy Sci. 2009, 92, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J.J. Functional Role of PPARs in Ruminants: Potential Targets for Fine-Tuning Metabolism during Growth and Lactation. PPAR Res. 2013, 2013, 684159. [Google Scholar] [CrossRef]
- Bionaz, M.; Vargas-Bello-Pérez, E.; Busato, S. Advances in Fatty Acids Nutrition in Dairy Cows: From Gut to Cells and Effects on Performance. J. Anim. Sci. Biotechnol. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. Cannabinoid Activation of Peroxisome Proliferator-Activated Receptors: An Update and Review of the Physiological Relevance. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2013, 2, 17–25. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Doohan, P.T.; Oldfield, L.D.; Arnold, J.C.; Anderson, L.L. Cannabinoid Interactions with Cytochrome P450 Drug Metabolism: A Full-Spectrum Characterization. AAPS J. 2021, 23, 91. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Gao, J.L.; Liao, X.D.; Huang, T.H.; Zhang, M.N.; Wang, M.Q.; Tian, Y.; Bai, J.; Zhou, C.H. MiR-454 Regulates Triglyceride Synthesis in Bovine Mammary Epithelial Cells by Targeting PPAR-γ. Gene 2019, 691, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol Is a Potent Inhibitor of the Catalytic Activity of Cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of Cytochrome P450 Enzymes Responsible for Metabolism of Cannabidiol by Human Liver Microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Ebisawa, J.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Potent Inhibition of Human Cytochrome P450 3A Isoforms by Cannabidiol: Role of Phenolic Hydroxyl Groups in the Resorcinol Moiety. Life Sci. 2011, 88, 730–736. [Google Scholar] [CrossRef]
- Yamaori, S.; Maeda, C.; Yamamoto, I.; Watanabe, K. Differential Inhibition of Human Cytochrome P450 2A6 and 2B6 by Major Phytocannabinoids. Forensic Toxicol. 2011, 29, 117–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irawan, A.; Bionaz, M. Liver Transcriptomic Profiles of Ruminant Species Fed Spent Hemp Biomass Containing Cannabinoids. Genes 2024, 15, 963. https://doi.org/10.3390/genes15070963
Irawan A, Bionaz M. Liver Transcriptomic Profiles of Ruminant Species Fed Spent Hemp Biomass Containing Cannabinoids. Genes. 2024; 15(7):963. https://doi.org/10.3390/genes15070963
Chicago/Turabian StyleIrawan, Agung, and Massimo Bionaz. 2024. "Liver Transcriptomic Profiles of Ruminant Species Fed Spent Hemp Biomass Containing Cannabinoids" Genes 15, no. 7: 963. https://doi.org/10.3390/genes15070963







