Identification and Comprehensive Analysis of circRNA-miRNA-mRNA Regulatory Networks in A2780 Cells Treated with Resveratrol
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Proliferation (Cell Counting Kit-8 Assay)
2.3. RNA Sample Detection, Library Construction, and Sequencing
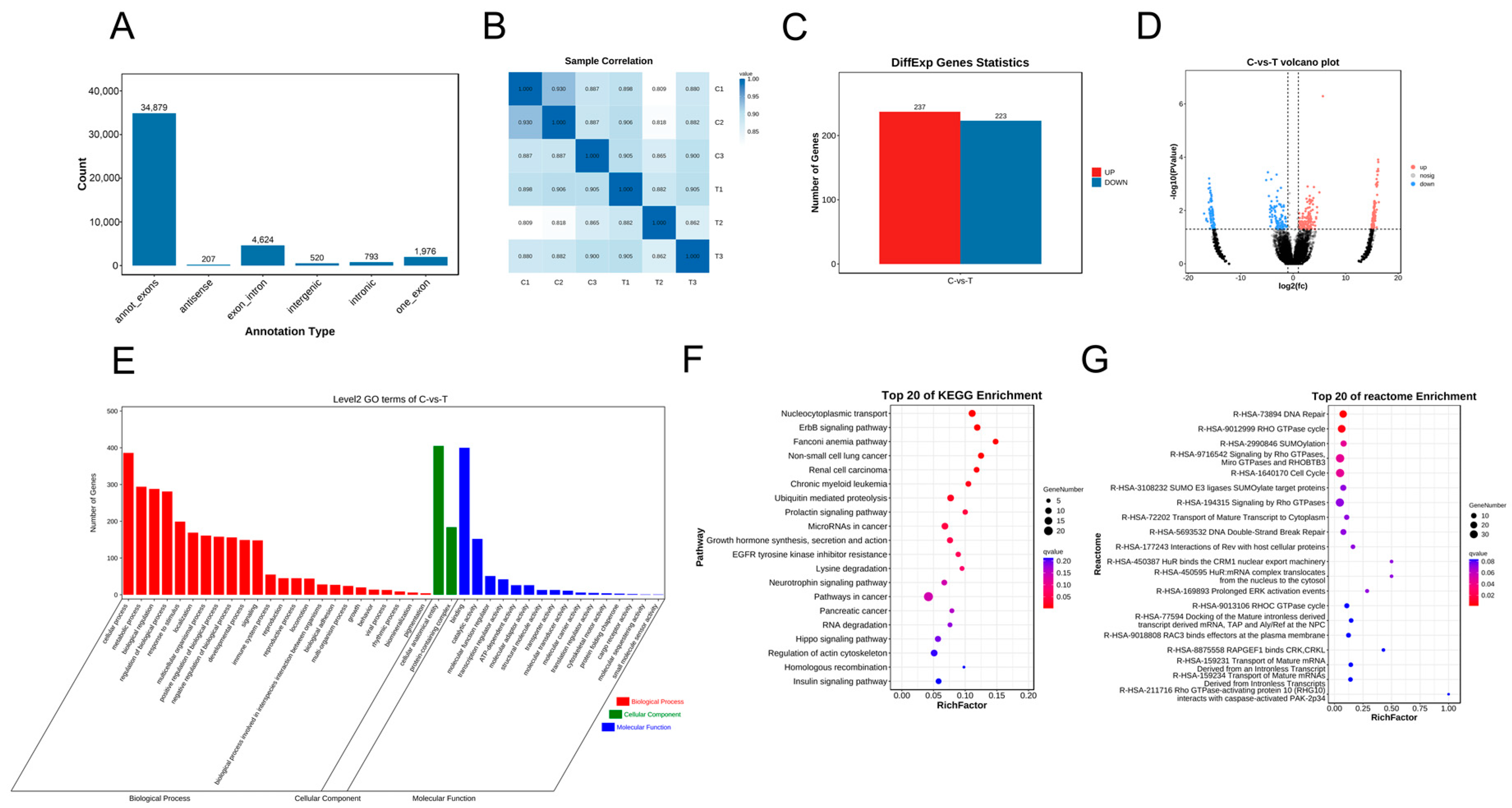
2.4. Identification and Quantification of CircRNAs
2.5. Identification and Quantification of mRNA Expression
2.6. Analysis of miRNA Expression
2.7. Bioinformatic Analyses
2.8. Real-Time Polymerase Chain Reaction
2.9. Statistical Data Analysis
3. Results
3.1. Proliferation of A2780 Cells Treated with Resveratrol
3.2. miRNA Sequencing (miRNA-seq) and Data Analysis
3.3. CircRNA Sequencing (circRNA-seq) Analysis
3.4. mRNA Sequencing (mRNA-seq) and Analysis
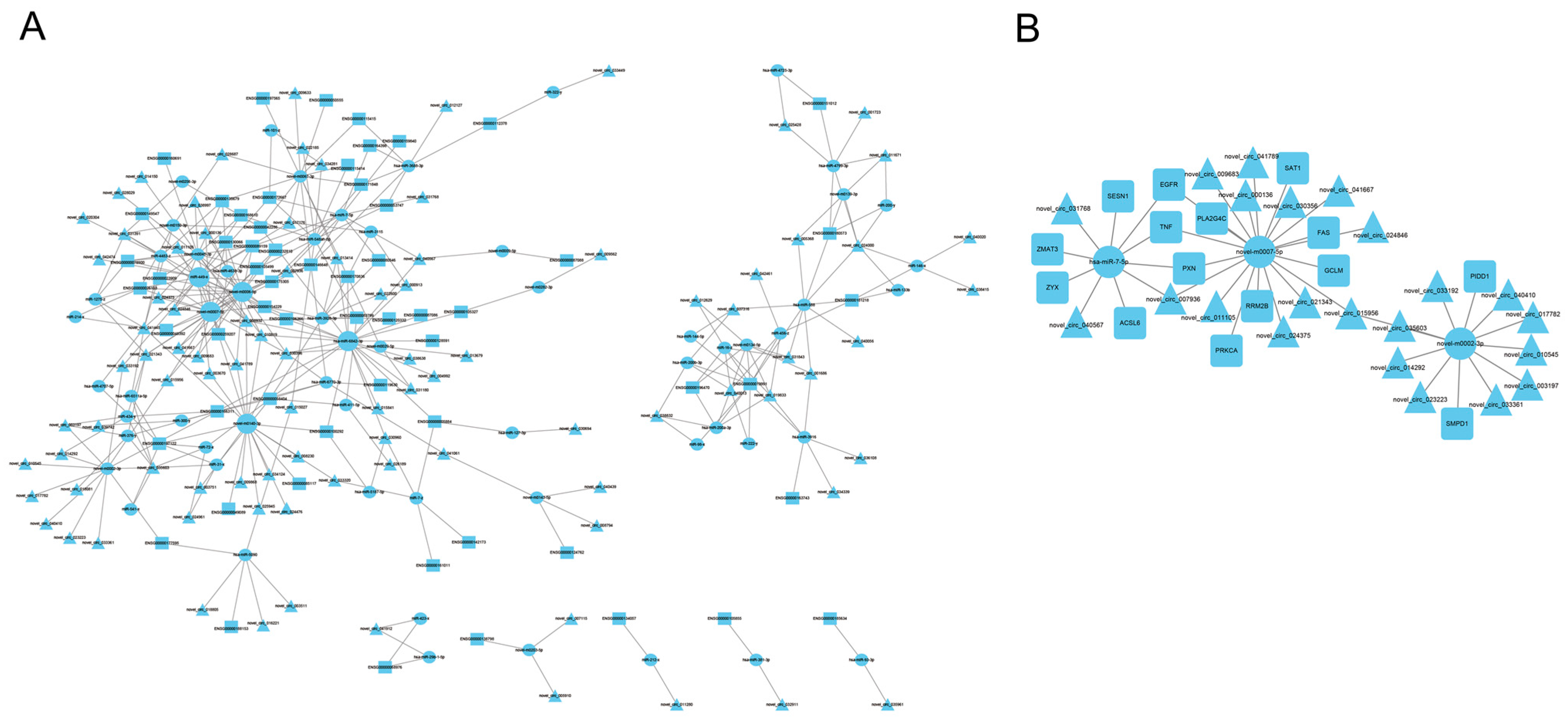
3.5. Construction of circRNA-miRNA-mRNA Regulatory Networks
3.6. RT-qPCR Verification of Differentially Expressed miRNAs in A2780
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viscarra, T.; Buchegger, K.; Jofre, I.; Riquelme, I.; Zanella, L.; Abanto, M.; Parker, A.C.; Piccolo, S.R.; Roa, J.C.; Ili, C.; et al. Functional and transcriptomic characterization of carboplatin-resistant A2780 ovarian cancer cell line. Biol. Res. 2019, 52, 13. [Google Scholar] [CrossRef] [PubMed]
- Shaik, B.; Zafar, T.; Balasubramanian, K.; Gupta, S.P. An Overview of Ovarian Cancer: Molecular Processes Involved and Development of Target-based Chemotherapeutics. Curr. Top. Med. Chem. 2021, 21, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian cancer: An integrated review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, L.; Liu, Q.; Zheng, J.; Zheng, Q.; Chen, Y.; Xia, H.; Wu, Q.; Sun, Y. Identification of upregulated exosomal miRNAs between A2780 and A2780/DDP human ovarian cancer cells by high-throughput sequencing. J. Ovarian Res. 2023, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, H.J.; Li, Y.Y.; Wang, X.; Liu, X.X.; Mai, J. Molecular mechanisms of platinum-based chemotherapy resistance in ovarian cancer (Review). Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.Y.; Ding, D.N.; Xie, Y.Y.; Shen, F.; Li, J.; Liu, F.Y.; Han, F.J. Advances in mechanism of traditional Chinese medicine in inhibiting angiogenesis in ovarian cancer. Zhongguo Zhong Yao Za Zhi 2023, 48, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, L.; Liu, F.; Ding, D.; Wei, W.; Han, F. Research progress on traditional Chinese medicine-induced apoptosis signaling pathways in ovarian cancer cells. J. Ethnopharmacol. 2024, 319, 117299. [Google Scholar] [CrossRef]
- Li, H.; Luo, F.; Jiang, X.; Zhang, W.; Xiang, T.; Pan, Q.; Cai, L.; Zhao, J.; Weng, D.; Li, Y.; et al. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype. J. Immunother. Cancer 2022, 10, e004029. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anticancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Vernousfaderani, E.K.; Akhtari, N.; Rezaei, S.; Rezaee, Y.; Shiranirad, S.; Mashhadi, M.; Hashemi, A.; Khankandi, H.P.; Behzad, S. Resveratrol and colorectal cancer: A molecular approach to clinical researches. Curr. Top. Med. Chem. 2021, 21, 2634–2646. [Google Scholar] [CrossRef]
- Almeida, T.C.; da Silva, G.N.; de Souza, D.V.; de Moraes Malinverni, A.C.; Aguiar, O.; Estadella, D.; Ribeiro, D.A. Resveratrol effects in oral cancer cells: A comprehensive review. Med. Oncol. 2021, 38, 97. [Google Scholar] [CrossRef] [PubMed]
- Hankittichai, P.; Thaklaewphan, P.; Wikan, N.; Ruttanapattanakul, J.; Potikanond, S.; Smith, D.R.; Nimlamool, W. Resveratrol enhances cytotoxic effects of cisplatin by inducing cell cycle arrest and apoptosis in ovarian adenocarcinoma SKOV-3 cells through activating the p38 MAPK and suppressing AKT. Pharmaceuticals 2023, 16, 755. [Google Scholar] [CrossRef] [PubMed]
- Najafiyan, B.; Bokaii Hosseini, Z.; Esmaelian, S.; Firuzpour, F.; Rahimipour Anaraki, S.; Kalantari, L.; Hheidari, A.; Mesgari, H.; Nabi-Afjadi, M. Unveiling the potential effects of resveratrol in lung cancer treatment: Mechanisms and nanoparticle-based drug delivery strategies. Biomed. Pharmacother. 2024, 172, 116207. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, M.; Tang, C.; Huang, Z.; Najafi, M. Targeting of cancer cell death mechanisms by resveratrol: A review. Apoptosis 2021, 26, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, M.; Schneider, H.; Unger, K.; Sander, P.; Schneider, E.M.; Fischer-Posovszky, P.; Handrick, R.; Otte, K. MiR-744–5p inducing cell death by directly targeting HNRNPC and NFIX in ovarian cancer cells. Sci. Rep. 2018, 8, 9020. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhou, X.; Bao, M.; Jia, R.; Chu, Y.; Lin, Y. miRNA-612 suppresses ovarian cancer cell tumorigenicity by downregulating NOB1. Am. J. Transl. Res. 2022, 14, 3904–3914. [Google Scholar] [PubMed]
- Feng, S.; Luo, S.; Ji, C.; Shi, J. miR-29c-3p regulates proliferation and migration in ovarian cancer by targeting KIF4A. World J. Surg. Oncol. 2020, 18, 315. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Ding, W.; Sun, B.; Zhu, L.; Gao, Y.; Chen, L. Upregulated circRNA_102231 promotes gastric cancer progression and its clinical significance. Bioengineered 2021, 12, 4936–4945. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Liu, H.L.; Yang, L.L.; Kang, F.H.; Xin, L.P.; Huang, L.R.; Guo, Q.F.; Wang, Y.L. Circular RNA circRNA_0000285 promotes cervical cancer development by regulating FUS. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8771–8778. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Jakobsen, T.; Hager, H.; Kjems, J. The emerging roles of circRNAs in cancer and oncology. Nat. Rev. Clin. Oncol. 2022, 19, 188–206. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Zeng, X.; Liu, J.; Liu, F.; Zhang, Z. circRNA-miRNA-mRNA in breast cancer. Clin. Chim. Acta 2021, 523, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, Y.; Shen, Q.; Chen, Q.; Zhu, X.J.; Jiang, S.S.; Zhang, Q. CircRNA_MYLK promotes malignant progression of ovarian cancer through regulating microRNA-652. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5281–5291. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Lv, H.; Yang, S.; Yang, X. CircRNA PLOD2 enhances ovarian cancer propagation by controlling miR-378. Saudi J. Biol. Sci. 2021, 28, 6260–6265. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.; Wang, H.; Shi, C.; Zhang, C.; Wang, M. CircRNA hsa_circ_0013958 may contribute to the development of ovarian cancer by affecting epithelial-mesenchymal transition and apoptotic signaling pathways. J. Clin. Lab. Anal. 2020, 34, e23292. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gao, Y.Q.; Sun, X.F. Circular RNA ITCH suppresses proliferation and promotes apoptosis in human epithelial ovarian cancer cells by sponging miR-10a-α. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8119–8126. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol—A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, F.; He, M.; Long, F.; Hu, D.; Chen, J.; Fang, M.; Wang, Z. Molecular mechanism of resveratrol promoting differentiation of preosteoblastic MC3T3-E1 cells based on network pharmacology and experimental validation. BMC Complement. Med. Ther. 2024, 24, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, F.; Li, P.; Gu, J.; Han, J.; Ni, Z.; Liu, F. Resveratrol inhibits HeLa cell proliferation by regulating mitochondrial function. Ecotoxicol. Environ. Saf. 2022, 241, 113788. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, J.; Qin, S.; Zhou, Z.; Zeng, Q.; Long, R.; Mao, Z.; Dong, X.; Zhao, R.; Zhang, R.; et al. Resveratrol induces autophagy impeding BAFF-stimulated B-cell proliferation and survival by inhibiting the Akt/mTOR pathway. Biochem. Pharmacol. 2022, 202, 115139. [Google Scholar] [CrossRef]
- Si, L.; Zhang, M.; Guan, E.; Han, Q.; Liu, Y.; Long, X.; Long, F.; Zhao, R.C.; Huang, J.; Liu, Z.; et al. Resveratrol inhibits proliferation and promotes apoptosis of keloid fibroblasts by targeting HIF-1α. J. Plast. Surg. Hand Surg. 2020, 54, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Song, J.; Lee, J.; Moon, S.K.; Moon, B. Resveratrol Attenuates the Proliferation of Prostatic Stromal Cells in Benign Prostatic Hyperplasia by Regulating Cell Cycle Progression, Apoptosis, Signaling Pathways, BPH Markers, and NF-κB Activity. Int. J. Mol. Sci. 2021, 22, 5969. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Yang, Y.; Liu, T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect. Agents Cancer 2019, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Brodaczewska, K.; Wcisło, G.; Majewska, A.; Borkowska, A.; Filipiak-Duliban, A.; Gawrylak, A.; Wilkus, K.; Piwocka, K.; Kominek, A.; et al. Hypoxia, but Not Normoxia, Reduces Effects of Resveratrol on Cisplatin Treatment in A2780 Ovarian Cancer Cells: A Challenge for Resveratrol Use in Anticancer Adjuvant Cisplatin Therapy. Int. J. Mol. Sci. 2023, 24, 5715. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Maurya, P.K. CircRNA-miRNA-mRNA interactome analysis in endometrial cancer. J. Biomol. Struct. Dyn. 2023, 162, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sakshi, S.; Jayasuriya, R.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Role of circRNA-miRNA-mRNA interaction network in diabetes and its associated complications. Mol. Ther. Nucleic Acids 2021, 26, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Ghazimoradi, M.H.; Babashah, S. The role of CircRNA/miRNA/mRNA axis in breast cancer drug resistance. Front. Oncol. 2022, 12, 966083. [Google Scholar] [CrossRef]
- Yan, J.; Ye, G.; Jin, Y.; Miao, M.; Li, Q.; Zhou, H. Identification of novel prognostic circRNA biomarkers in circRNA-miRNA-mRNA regulatory network in gastric cancer and immune infiltration analysis. BMC Genom. 2023, 24, 323. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, H.; Peng, X.; Chai, Y.; Wang, S.; Wen, G. Identification and comprehensive analysis of circRNA-miRNA-mRNA regulatory networks in osteoarthritis. Front. Immunol. 2022, 13, 1050743. [Google Scholar] [CrossRef]
- Sun, S.; Fang, H. Curcumin inhibits ovarian cancer progression by regulating circ-PLEKHM3/miR-320a/SMG1 axis. J. Ovarian Res. 2021, 14, 158. [Google Scholar] [CrossRef]
- Ma, H.; Qu, S.; Zhai, Y.; Yang, X. Circ_0025033 promotes ovarian cancer development via regulating the hsa_miR-370-3p/SLC1A5 axis. Cell. Mol. Biol. Lett. 2022, 27, 94. [Google Scholar] [CrossRef]
- Yu, G.; Li, N.; Zhao, Y.; Wang, W.; Feng, X.L. Salidroside induces apoptosis in human ovarian cancer SKOV3 and A2780 cells through the p53 signaling pathway. Oncol. Lett. 2018, 15, 6513–6518. [Google Scholar] [CrossRef]
- Huang, J. Current developments of targeting the p53 signaling pathway for cancer treatment. Pharmacol. Ther. 2021, 220, 107720. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, J.; Shi, K.; Li, H.; Du, J.; Hu, D.; Liu, Z.; Wang, W. Germacrone Induces Lung Cancer Cell Apoptosis and Cell Cycle Arrest via the Akt/MDM2/p53 Signaling Pathway. Mol. Med. Rep. 2021, 23, 452. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, W.H.; Chen, B.Y.; Nie, J.; Su, Z.R.; Zheng, J.N.; Gong, S.T.; Chen, J.N.; Jiang, D.; Li, Y. miR-29b suppresses proliferation and induces apoptosis of hepatocellular carcinoma ascites H22 cells via regulating TGF-β1 and p53 signaling pathway. Int. J. Mol. Med. 2021, 48, 157. [Google Scholar] [CrossRef]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting cell death pathways for cancer therapy: Recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Zhang, Q.; Zhang, T.; Xie, J.; Wei, W.; Wang, Y.; Yu, H.; Zhou, H. A necroptosis related prognostic model of pancreatic cancer based on single cell sequencing analysis and transcriptome analysis. Front. Immunol. 2022, 13, 1022420. [Google Scholar] [CrossRef]
- Long, K.; Gu, L.; Li, L.; Zhang, Z.; Li, E.; Zhang, Y.; He, L.; Pan, F.; Guo, Z.; Hu, Z. Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer. Cell Death Dis. 2021, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yin, F.; Zhang, W.; Li, L. STROBE-compliant integrin through focal adhesion involve in cancer stem cell and multidrug resistance of ovarian cancer. Medicine 2017, 96, e6345. [Google Scholar] [CrossRef]
- Yang, L.; Gu, Y. SPTBN2 regulates endometroid ovarian cancer cell proliferation, invasion and migration via ITGB4-mediated focal adhesion and ECM receptor signalling pathway. Exp. Ther. Med. 2023, 25, 277. [Google Scholar] [CrossRef]
- El-Kott, A.F.; Shati, A.A.; Ali Al-Kahtani, M.; Alharbi, S.A. The apoptotic effect of resveratrol in ovarian cancer cells is associated with downregulation of galectin-3 and stimulating miR-424-3p transcription. J. Food Biochem. 2019, 43, e13072. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wen, Y.; Yuan, X.; He, X. MicroRNA-mediated reprogramming of glucose, fatty acid and amino acid metabolism in cancer. Genome Instab. Dis. 2022, 4, 47–69. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zhang, G.; Luo, C.; Zhang, S.; Luo, R.; Deng, B. hsa-miR-7-5p suppresses proliferation, migration and promotes apoptosis in hepatocellular carcinoma cell lines by inhibiting SPC24 expression. Biochem. Biophys. Res. Commun. 2021, 561, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Wang, S.; Tao, L.; Pang, L.; Fu, R.; Fu, Y.; Liang, W.; Li, F.; Jia, W. MiR-212-3p suppresses high-grade serous ovarian cancer progression by directly targeting MAP3K3. Am. J. Transl. Res. 2020, 12, 875–888. [Google Scholar] [PubMed]
- Yue, H.; Liu, L.; Song, Z. miR-212 regulated by HIF-1α promotes the progression of pancreatic cancer. Exp. Ther. Med. 2019, 17, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, N. miR-212-5p inhibits nasopharyngeal carcinoma progression by targeting METTL3. Open Med. 2022, 17, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, X.; Pan, X.; Xu, J.; Jiang, R.; Li, Y. LINC02532 by Mediating miR-541-3p/HMGA1 Axis Exerts a Tumor Promoter in Breast cancer. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.P.; Su, F.; Zhang, S.P.; Chen, H.K.; Li, Z.J.; Xing, G.Q.; Liu, H.J.; Li, Y.Y. miR-212 as potential biomarker suppresses the proliferation of gastric cancer via targeting SOX4. J. Clin. Lab. Anal. 2020, 34, e23511. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, H.; Zhang, G.; Wu, J.; Zhu, W.; Gu, Y.; He, Y.I. MiR-212-3p inhibits cell proliferation and promotes apoptosis by targeting nuclear factor IA in bladder cancer. J. Biosci. 2019, 44, 80. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, L.; Liu, L. Circ_0085616 contributes to the radioresistance and progression of cervical cancer by targeting miR-541-3p/ARL2 signaling. Histol. Histopathol. 2023, 38, 571–584. [Google Scholar] [CrossRef]
- Tang, J.; Wang, R.; Tang, R.; Gu, P.; Han, J.; Huang, W. CircRTN4IP1 regulates the malignant progression of intrahepatic cholangiocarcinoma by sponging miR-541-5p to induce HIF1A production. Pathol. Res. Pract. 2022, 230, 153732. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Yang, J.; Wang, J.; Zhang, R.; Li, J.; Zhang, R. CircMTO1 suppresses hepatocellular carcinoma progression via the miR-541-5p/ZIC1 axis by regulating Wnt/β-catenin signaling pathway and epithelial-to-mesenchymal transition. Cell Death Dis. 2021, 13, 12. [Google Scholar] [CrossRef] [PubMed]




| Transcripts | Primers | |
|---|---|---|
| miR-212-x | F | AGACCTTGGCTCTAGACTGC |
| RT | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACACAGTAA | |
| hsa-miR-7-5p | F | CCCGTGGAAGACTAGTGATTT |
| RT | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAACAACA | |
| novel-m0007-5p | F | GTGCGCTTGTTGTGATTCCT |
| RT | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAAAATGG | |
| novel-m0002-3p | F | TGAGGCTGTGATGCTCTCCT |
| RT | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAGGGCTC | |
| miR-541-X | F | CCCGAAAGGGATTCTGATGT |
| RT | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTGACCA | |
| U6 | F | CTCGCTTCGGCAGCACA |
| RT | AACGCTTCACGAATTTGCGT | |
| sRNA | log2FoldChange | p-Value | FDR | Regulation |
|---|---|---|---|---|
| hsa-miR-7-5p | −1.48 | 2.98 × 10−18 | 2.96 × 10−15 | Down |
| novel-m0002-3p | −1.74 | 3.90 × 10−13 | 1.11 × 10−10 | Down |
| miR-541-x | −6.01 | 0.005796 | 0.06622 | Down |
| miR-212-x | 1.88 | 5.11 × 10−13 | 1.27 × 10−10 | Up |
| novel-m0007-5p | −1.35 | 4.79 × 10−10 | 5.01× 10−8 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhang, Y.; Zhou, Q.; Zhen, C.; Huang, H.; Liu, X. Identification and Comprehensive Analysis of circRNA-miRNA-mRNA Regulatory Networks in A2780 Cells Treated with Resveratrol. Genes 2024, 15, 965. https://doi.org/10.3390/genes15070965
Zhu W, Zhang Y, Zhou Q, Zhen C, Huang H, Liu X. Identification and Comprehensive Analysis of circRNA-miRNA-mRNA Regulatory Networks in A2780 Cells Treated with Resveratrol. Genes. 2024; 15(7):965. https://doi.org/10.3390/genes15070965
Chicago/Turabian StyleZhu, Weihua, Yuanting Zhang, Qianqian Zhou, Cheng Zhen, Herong Huang, and Xiaoying Liu. 2024. "Identification and Comprehensive Analysis of circRNA-miRNA-mRNA Regulatory Networks in A2780 Cells Treated with Resveratrol" Genes 15, no. 7: 965. https://doi.org/10.3390/genes15070965
APA StyleZhu, W., Zhang, Y., Zhou, Q., Zhen, C., Huang, H., & Liu, X. (2024). Identification and Comprehensive Analysis of circRNA-miRNA-mRNA Regulatory Networks in A2780 Cells Treated with Resveratrol. Genes, 15(7), 965. https://doi.org/10.3390/genes15070965






