Therapeutic Implication of miRNAs as an Active Regulatory Player in the Management of Pain: A Review
Abstract
1. Introduction
2. Role of miRs in Pain
2.1. miR-124a
2.2. miR-103
2.3. hsa-mir-548
2.4. miR-143
2.5. miR-146a
2.6. miR-let-7b
2.7. miR-21
2.8. miR-30c
3. miRs and Pain Signaling Pathways
3.1. miRs and Pain Mechanism
3.2. miRs in Visceral Pain
3.3. Polymorphisms of miRs and Pain
4. miRNAs as Therapeutic Targets
miRs as Prognostic Biomarkers of Pain
5. miRs-Gene Association Networks in Pain
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendell, L.M.; Albers, K.M.; Davis, B.M. Neurotrophins, nociceptors, and pain. Microsc. Res. Tech. 1999, 45, 252–261. [Google Scholar] [CrossRef]
- Wang, M.; Thyagarajan, B. Pain pathways and potential new targets for pain relief. Biotechnol. Appl. Biochem. 2022, 69, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Pattison, L.A.; Callejo, G.; St John Smith, E. Evolution of acid nociception: Ion channels and receptors for detecting acid. Philos. Trans. R. Soc. B 2019, 374, 20190291. [Google Scholar] [CrossRef] [PubMed]
- Steeds, C.E. The anatomy and physiology of pain. Surgery 2009, 27, 507–511. [Google Scholar]
- D’Mello, R.; Dickenson, A.H. Spinal cord mechanisms of pain. Br. J. Anaesth. 2008, 101, 8–16. [Google Scholar] [CrossRef]
- Melzack, R. Pain: Past, present and future. Can. J. Exp. Psychol./Rev. Can. Psychol. Expérimentale 1993, 47, 615. [Google Scholar] [CrossRef] [PubMed]
- Świeboda, P.; Filip, R.; Prystupa, A.; Drozd, M. Assessment of pain: Types, mechanism and treatment. Pain 2013, 2, 2–7. [Google Scholar]
- Tracey, W.D. Nociception. Curr. Biol. 2017, 27, R129–R133. [Google Scholar] [CrossRef] [PubMed]
- Tigerholm, J.; Poulsen, A.H.; Andersen, O.K.; Mørch, C.D. From perception threshold to ion channels—A computational study. Biophys. J. 2019, 117, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Schliessbach, J.; Maurer, K. Pharmacology of pain transmission and modulation. Pain Med. Essent. Rev. 2017, 7–9. [Google Scholar]
- Zhou, H.-Y.; Zhang, H.-M.; Chen, S.-R.; Pan, H.-L. Increased C-fiber nociceptive input potentiates inhibitory glycinergic transmission in the spinal dorsal horn. J. Pharmacol. Exp. Ther. 2008, 324, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Koga, K.; Chen, T.; Zhuo, M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J. Neurochem. 2017, 141, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. Bmj 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Rosenbloom, B.N.; Fashler, S. Chronic pain, psychopathology, and DSM-5 somatic symptom disorder. Can. J. Psychiatry 2015, 60, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Gottrup, H.; Sindrup, S.H.; Bach, F.W. The clinical picture of neuropathic pain. Eur. J. Pharmacol. 2001, 429, 1–11. [Google Scholar] [CrossRef] [PubMed]
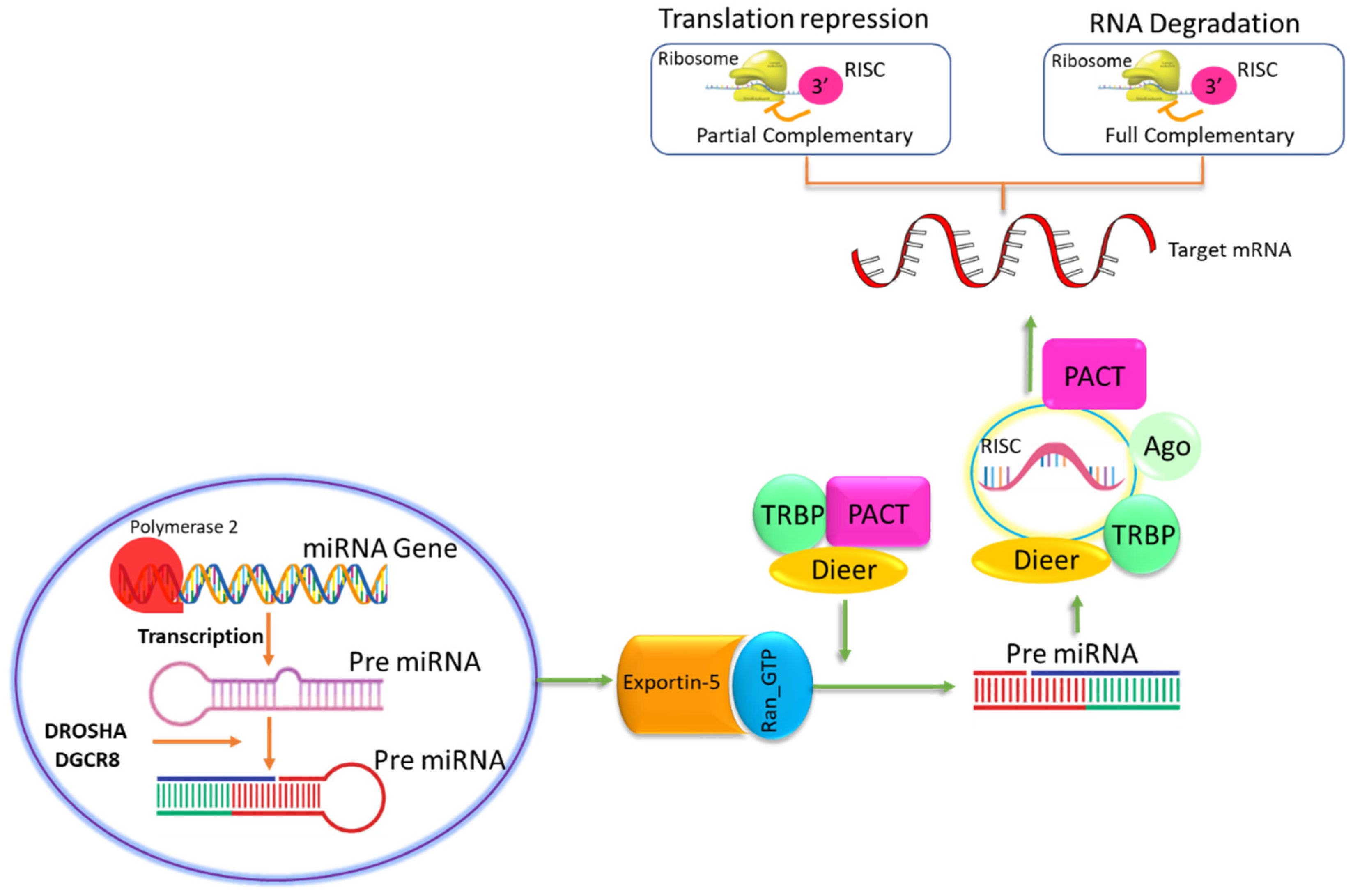
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-Y.; Chang, D.C.; Lin, S.-L. The microRNA (miRNA): Overview of the RNA genes that modulate gene function. Mol. Biotechnol. 2008, 38, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N. MicroRNA biogenesis: Coordinated cropping and dicing. Nature Rev. Mol. Cell Biol. 2005, 6, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Calin, G.A.; Croce, C.M. MicroRNAs: Fundamental facts and involvement in human diseases. Birth Defects Res. Part C Embryo Today Rev. 2006, 78, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Wallace, M.S. Opioids, analgesia, and pain management. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Academia: San Francisco, CA, USA, 2011; pp. 481–526. [Google Scholar]
- Barkin, R.L. Acetaminophen, aspirin, or ibuprofen in combination analgesic products. Am. J. Ther. 2001, 8, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Green, G.A. Understanding NSAIDs: From aspirin to COX-2. Clin. Cornerstone 2001, 3, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Russell, R. Non-steroidal anti-inflammatory drugs and gastrointestinal damage—Problems and solutions. Postgrad. Med. J. 2001, 77, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Varas-Lorenzo, C.; Riera-Guardia, N.; Calingaert, B.; Castellsague, J.; Pariente, A.; Scotti, L.; Sturkenboom, M.; Perez-Gutthann, S. Stroke risk and NSAIDs: A systematic review of observational studies. Pharmacoepidemiol. Drug Saf. 2011, 20, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, P.; Bhojani, K.; Joshi, V. NSAIDs and kidney. Japi 2004, 52, 371. [Google Scholar]
- Prados-Torres, A.; Calderón-Larrañaga, A.; Hancco-Saavedra, J.; Poblador-Plou, B.; van den Akker, M. Multimorbidity patterns: A systematic review. J. Clin. Epidemiol. 2014, 67, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Owusu Obeng, A.; Hamadeh, I.; Smith, M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S. Opioid analgesics adverse effects: The other side of the coin. Curr. Pharm. Des. 2019, 25, 3197–3202. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.T.; Zuelsdorff, M.; Fleming, M. Adverse effects and cognitive function among primary care patients taking opioids for chronic nonmalignant pain. J. Opioid Manag. 2006, 2, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.C.; Sullivan, L.E.; Tetrault, J.M.; Desai, R.A.; Fiellin, D.A. Non-medical use, abuse and dependence on prescription opioids among US adults: Psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008, 94, 38–47. [Google Scholar] [CrossRef]
- Ayoub, S.S. Paracetamol (acetaminophen): A familiar drug with an unexplained mechanism of action. Temperature 2021, 8, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jakobsson, J. Acetaminophen, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 selective inhibitors: An update. Plast. Reconstr. Surg. 2014, 134, 24S–31S. [Google Scholar] [CrossRef] [PubMed]
- Söderpalm, B. Anticonvulsants: Aspects of their mechanisms of action. Eur. J. Pain 2002, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Swann, A.C. Major system toxicities and side effects of anticonvulsants. J. Clin. Psychiatry 2001, 62, 16–21. [Google Scholar] [PubMed]
- Nagakura, Y. The need for fundamental reforms in the pain research field to develop innovative drugs. Expert Opin. Drug Discov. 2017, 12, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Emanueli, C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef]
- Dai, Z.; Chu, H.; Ma, J.; Yan, Y.; Zhang, X.; Liang, Y. The regulatory mechanisms and therapeutic potential of microRNAs: From chronic pain to morphine tolerance. Front. Mol. Neurosci. 2018, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Nakasa, T.; Tanaka, N.; Ishikawa, M.; Yamada, K.; Yamasaki, K.; Kamei, N.; Izumi, B.; Adachi, N.; Miyaki, S. Responses of microRNAs 124a and 223 following spinal cord injury in mice. Spinal Cord 2010, 48, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Suzuki, H. Emerging roles of microRNAs in chronic pain. Neurochem. Int. 2014, 77, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kynast, K.L.; Russe, O.Q.; Möser, C.V.; Geisslinger, G.; Niederberger, E. Modulation of central nervous system–specific microRNA-124a alters the inflammatory response in the formalin test in mice. Pain 2013, 154, 368–376. [Google Scholar] [CrossRef]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Manners, M.T.; Ertel, A.; Tian, Y.; Ajit, S.K. Genome-wide redistribution of MeCP2 in dorsal root ganglia after peripheral nerve injury. Epigenetics Chromatin 2016, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Géranton, S.M.; Morenilla-Palao, C.; Hunt, S.P. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum-and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J. Neurosci. 2007, 27, 6163–6173. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α–PU. 1 pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, M.; Xu, Q.; Tian, W. Advances with Non-Coding RNAs in Neuropathic Pain. Front. Neurosci. 2021, 15, 1574. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.J.; Dhaka, A. ThermoTRPs and pain. Neuroscientist 2016, 22, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiu, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef] [PubMed]
- Morchio, M.; Sher, E.; Collier, D.A.; Lambert, D.W.; Boissonade, F.M. The Role of miRNAs in Neuropathic Pain. Biomedicines 2023, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Heyn, J.; Luchting, B.; Hinske, L.C.; Hübner, M.; Azad, S.C.; Kreth, S. miR-124a and miR-155 enhance differentiation of regulatory T cells in patients with neuropathic pain. J. Neuroinflamm. 2016, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elramah, S.; López-González, M.J.; Bastide, M.; Dixmérias, F.; Roca-Lapirot, O.; Wielanek-Bachelet, A.-C.; Vital, A.; Leste-Lasserre, T.; Brochard, A.; Landry, M. Spinal miRNA-124 regulates synaptopodin and nociception in an animal model of bone cancer pain. Sci. Rep. 2017, 7, 10949. [Google Scholar] [CrossRef]
- Favereaux, A.; Thoumine, O.; Bouali-Benazzouz, R.; Roques, V.; Papon, M.A.; Salam, S.A.; Drutel, G.; Léger, C.; Calas, A.; Nagy, F. Bidirectional integrative regulation of Cav1. 2 calcium channel by microRNA miR-103: Role in pain. EMBO J. 2011, 30, 3830–3841. [Google Scholar] [CrossRef] [PubMed]
- Fossat, P.; Dobremez, E.; Bouali-Benazzouz, R.; Favereaux, A.; Bertrand, S.S.; Kilk, K.; Léger, C.; Cazalets, J.-R.; Langel, Ü.; Landry, M. Knockdown of L calcium channel subtypes: Differential effects in neuropathic pain. J. Neurosci. 2010, 30, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Shi, J.; Liu, K.; Liu, N.; Wang, Y.; Fu, Z.; Ding, J.; Jia, L.; Yuan, W. Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 2013, 61, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, J.; Xu, M.; Pasternak, G.W.; Pan, Y.-X. Morphine regulates expression of μ-opioid receptor MOR-1A, an intron-retention carboxyl terminal splice variant of the μ-opioid receptor (OPRM1) gene via miR-103/miR-107. Mol. Pharmacol. 2014, 85, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Pharmacological inhibition of voltage-gated Ca2+ channels for chronic pain relief. Curr. Neuropharmacol. 2013, 11, 606–620. [Google Scholar] [PubMed]
- Liang, T.; Guo, L.; Liu, C. Genome-wide analysis of mir-548 gene family reveals evolutionary and functional implications. BioMed Res. Int. 2012, 2012, 679563. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, M.A.; Cui, Y.; Elliott, E.E.; Mo, X.; Otero, J.J.; Winter, J.O. MicroRNA-mRNA interactions at low levels of compressive solid stress implicate mir-548 in increased glioblastoma cell motility. Sci. Rep. 2020, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, M.; Cheng, Q.; Zhu, M.; Shi, G. Identification of microRNA mediating type I interferon pathway in chronic hepatitis B patients. Chin. J. Viral Dis 2012, 2, 107–112. [Google Scholar]
- Xing, T.; Xu, H.; Yu, W.; Wang, B.; Zhang, J. Expression profile and clinical significance of miRNAs at different stages of chronic hepatitis B virus infection. Int. J. Clin. Exp. Med. 2015, 8, 5611. [Google Scholar] [PubMed]
- Ramos-Sanchez, E.M.; Reis, L.C.; Souza MD, A.; Muxel, S.M.; Santos, K.R.; Lagos, D.; Goto, H. miR-548d-3p is up-regulated in human visceral leishmaniasis and suppresses parasite growth in macrophages. Front. Cell. Infect. Microbiol. 2022, 12, 826039. [Google Scholar]
- Xing, T.-J.; Xu, H.-T.; Yu, W.-Q.; Wang, B.; Zhang, J. MiRNA-548ah, a potential molecule associated with transition from immune tolerance to immune activation of chronic hepatitis B. Int. J. Mol. Sci. 2014, 15, 14411–14426. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, J.; Xu, X.; Wang, J.; Ao, F.; Wan, Y.; Zhu, Y. MicroRNA-548 down-regulates host antiviral response via direct targeting of IFN-λ1. Protein Cell 2013, 4, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Tam Tam, S.; Bastian, I.; Zhou, X.-F.; Vander Hoek, M.; Michael, M.Z.; Gibbins, I.L.; Haberberger, R. MicroRNA-143 expression in dorsal root ganglion neurons. Cell Tissue Res. 2011, 346, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Dong, W.; Huang, J.; Pan, Q.; Fan, X.; Zhang, C.; Huang, L. MicroRNA-143 as a tumor suppressor for bladder cancer. J. Urol. 2009, 181, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, L.; Peng, X.; Geng, S.; Zhou, X. Study of Qingluo Tongbi Compound treating rheumatoid arthritis based on miRNA network. Chin. J. Immunol. 2016, 12, 495–499. [Google Scholar]
- Yuan, C.; Geng, S.; Zhu, Y.; Peng, X.; Zhou, X.; Zhou, L. Comparison of abnormal expression of miRNAs in peripheral blood of rheumatoid arthritis patients and osteoclasts in rat and analysis of any miRNAs. Chin. J. Immunol. 2017, 12, 715–720. [Google Scholar]
- Cerdá-Olmedo, G.; Mena-Durán, A.V.; Monsalve, V.; Oltra, E. Identification of a microRNA signature for the diagnosis of fibromyalgia. PLoS ONE 2015, 10, e0121903. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Rodriguez, C.E.; Donald, G.W.; Hertzer, K.M.; Jung, X.S.; Chang, H.-H.; Moro, A.; Reber, H.A.; Hines, O.J.; Eibl, G. miR-143 decreases COX-2 mRNA stability and expression in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2013, 439, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.L.; Zhu, Y.M.; Qian, F.Y.; Yuan, C.C.; Yuan, D.P.; Zhou, X.P. MicroRNA-143-3p contributes to the regulation of pain responses in collagen-induced arthritis. Mol. Med. Rep. 2018, 18, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Zurolo, E.; Prabowo, A.; Fluiter, K.; Spliet, W.G.; van Rijen, P.C.; Gorter, J.A.; Aronica, E. MicroRNA-146a: A key regulator of astrocyte-mediated inflammatory response. PLoS ONE 2012, e44789. [Google Scholar] [CrossRef] [PubMed]
- Meisgen, F.; Landén, N.X.; Wang, A.; Réthi, B.; Bouez, C.; Zuccolo, M.; Gueniche, A.; Ståhle, M.; Sonkoly, E.; Breton, L. MiR-146a negatively regulates TLR2-induced inflammatory responses in keratinocytes. J. Investig. Dermatol. 2014, 134, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Enlund, E.; Funcke, J.-B.; Tews, D.; Holzmann, K.; Debatin, K.-M.; Wabitsch, M.; Fischer-Posovszky, P. miR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci. Rep. 2016, 6, 38339. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gibson, G.; Kim, J.-S.; Kroin, J.; Xu, S.; Van Wijnen, A.J.; Im, H.-J. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene 2011, 480, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi-Jahromi, S.S.; Ahmadzadeh, A.; Rezaieyazdi, Z.; Aslani, M.; Omidian, S.; Mirshafiey, A. The role of β-d-mannuronic acid, as a new non-steroidal anti-inflammatory drug on expression of miR-146a, IRAK1, TRAF6, NF-κB and pro-inflammatory cytokines following a clinical trial in rheumatoid arthritis patients. Immunopharmacol. Immunotoxicol. 2020, 42, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Chondrocyte innate immune myeloid differentiation factor 88–dependent signaling drives procatabolic effects of the endogenous toll-like receptor 2/toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010, 62, 2004–2012. [Google Scholar]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, D.; Scott, G.; Schokrpur, S.; Patil, C.; Campisi, J.; Benz, C. Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene 2008, 27, 5643–5647. [Google Scholar] [CrossRef]
- Lu, Y.; Cao, D.-L.; Jiang, B.-C.; Yang, T.; Gao, Y.-J. MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav. Immun. 2015, 49, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R. Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm. Pharmacol. Ther. 2015, 35, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Cardinali, C.; Morelli, M.B.; Santoni, M.; Nabissi, M.; Amantini, C. Danger-and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J. Neuroinflamm. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Banerjee, B. Role of microRNA in visceral pain. J. Neurogastroenterol. Motil. 2015, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Adlakha, Y.K. MicroRNAs: The tiny robust players unraveling the multifaceted channels of pain. Benth. Sci. 2016, 26, 126–160. [Google Scholar]
- Park, C.-K.; Xu, Z.-Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.-J.; Ji, R.-R. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglioli, A.; Efe, I.E.; Guneykaya, D.; Ivanov, A.; Huang, Y.; Orlowski, E.; Krüger, C.; Deisz, R.A.; Markovic, D.; Flüh, C. let-7 MicroRNAs regulate microglial function and suppress glioma growth through toll-like receptor 7. Cell Rep. 2019, 29, 3460–3471.e3467. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xiao, W.; Wang, F.; Liu, J.; Zhi, L.-J. miR-21-5p inhibits neuropathic pain development via directly targeting C-C motif ligand 1 and tissue inhibitor of metalloproteinase-3. J. Cell. Biochem. 2019, 120, 16614–16623. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Suzuki, H. Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem. Biophys. Res. Commun. 2013, 435, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Leinders, M.; Üçeyler, N.; Thomann, A.; Sommer, C. Aberrant microRNA expression in patients with painful peripheral neuropathies. J. Neurol. Sci. 2017, 380, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Xu, Z.-Z.; Wang, X.; Park, J.Y.; Zhuang, Z.-Y.; Tan, P.-H.; Gao, Y.-J.; Roy, K.; Corfas, G.; Lo, E.H. Distinct roles of matrix metalloproteases in the early-and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hackel, D.; Krug, S.M.; Sauer, R.-S.; Mousa, S.A.; Böcker, A.; Pflücke, D.; Wrede, E.-J.; Kistner, K.; Hoffmann, T.; Niedermirtl, B. Transient opening of the perineurial barrier for analgesic drug delivery. Proc. Natl. Acad. Sci. USA 2012, 109, E2018–E2027. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pascal, L.E.; Li, F.; Chen, W.; Dhir, R.; Balasubramani, G.K.; DeFranco, D.B.; Yoshimura, N.; He, D.; Wang, Z. Tight junction protein claudin-1 is downregulated by TGF-β1 via MEK signaling in benign prostatic epithelial cells. Prostate 2020, 80, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Takano, K.i.; Yamamoto, T.; Murata, M.; Son, S.; Imamura, M.; Yamaguchi, H.; Osanai, M.; Chiba, H.; Himi, T. Transforming growth factor-β induces epithelial to mesenchymal transition by down-regulation of claudin-1 expression and the fence function in adult rat hepatocytes. Liver Int. 2008, 28, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, A.K.; Krug, S.M.; Salvador, E.; Sauer, R.S.; Karl-Schöller, F.; Malcangio, M.; Sommer, C.; Rittner, H.L. MicroRNA-21-5p functions via RECK/MMP9 as a proalgesic regulator of the blood nerve barrier in nerve injury. Ann. N. Y. Acad. Sci. 2022, 1515, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Lantero, A.; Tramullas, M.; Díaz, A.; Hurlé, M.A. Transforming growth factor-β in normal nociceptive processing and pathological pain models. Mol. Neurobiol. 2012, 45, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Tramullas, M.; Francés, R.; de la Fuente, R.; Velategui, S.; Carcelén, M.; García, R.; Hurlé, M.A. MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci. Transl. Med. 2018, 10, eaao6299. [Google Scholar] [CrossRef] [PubMed]
- Fidilio, A.; Grasso, M.; Turnaturi, R.; Caruso, G.; Spitale, F.M.; Vicario, N.; Parenti, R.; Spoto, S.; Musso, N.; Marrazzo, A. The multimodal MOPr/DOPr agonist LP2 reduces allodynia in chronic constriction injured rats by rescue of TGF-β1 signalling. Front. Pharmacol. 2021, 12, 749365. [Google Scholar] [CrossRef] [PubMed]
- Francés, R.; Mata-Garrido, J.; de la Fuente, R.; Carcelén, M.; Lafarga, M.; Berciano, M.T.; Tramullas, M. Identification of epigenetic interactions between MicroRNA-30c-5p and DNA methyltransferases in neuropathic pain. Int. J. Mol. Sci. 2022, 23, 13994. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.-Y.; Xue, Y.; Li, J.-F.; Traub, R.J.; Cao, D.-Y. Do microRNAs modulate visceral pain? BioMed Res. Int. 2018, 2018, 5406973. [Google Scholar] [CrossRef] [PubMed]
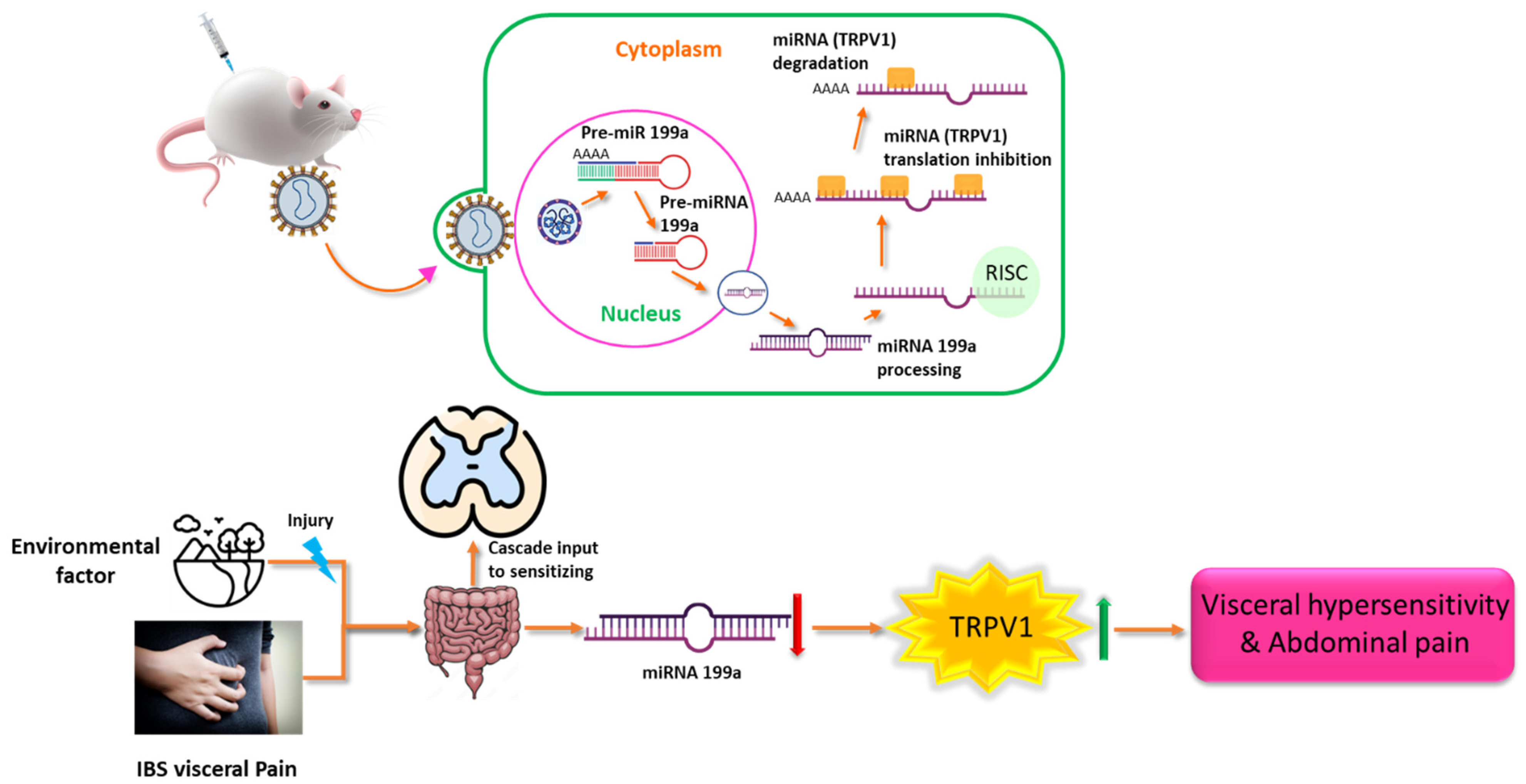
- Zhou, Q.; Yang, L.; Larson, S.; Basra, S.; Merwat, S.; Tan, A.; Croce, C.; Verne, G.N. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 2015, 65, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-J.; Mao, W.-M.; Wang, Q.; Yang, G.-G.; Wu, W.-J.; Shao, S.-X. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem. Biophys. Res. Commun. 2016, 469, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Bradesi, S.; Karagiannides, I.; Bakirtzi, K.; Joshi, S.M.; Koukos, G.; Iliopoulos, D.; Pothoulakis, C.; Mayer, E.A. Identification of spinal cord MicroRNA and gene signatures in a model of chronic stress-induced visceral hyperalgesia in rat. PLoS ONE 2015, 10, e0130938. [Google Scholar] [CrossRef] [PubMed]
- Fourie, N.H.; Peace, R.M.; Abey, S.K.; Sherwin, L.B.; Rahim-Williams, B.; Smyser, P.A.; Wiley, J.W.; Henderson, W.A. Elevated circulating miR-150 and miR-342-3p in patients with irritable bowel syndrome. Exp. Mol. Pathol. 2014, 96, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Souba, W.W.; Croce, C.M.; Verne, G.N. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2009, 59, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Huang, Y.; Zhu, S.; Li, P.; Chen, X.; Hou, Z.; Liu, F. MiR-144 increases intestinal permeability in IBS-D rats by targeting OCLN and ZO1. Cell. Physiol. Biochem. 2018, 44, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.; Hamilton, A.; Aghajanova, L.; Vo, K.; Nezhat, C.; Lessey, B.; Giudice, L. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 2009, 15, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Kästingschäfer, C.S.; Schäfer, S.D.; Kiesel, L.; Götte, M. miR-142-3p is a novel regulator of cell viability and proinflammatory signalling in endometrial stroma cells. Reprod. BioMedicine Online 2015, 30, 553–556. [Google Scholar] [CrossRef]
- Wright, K.R.; Mitchell, B.; Santanam, N. Redox regulation of microRNAs in endometriosis-associated pain. Redox Biol. 2017, 12, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Deeb, W.S.; El Amir, A.; Zaki, S.S.; El Sawah, H.; Al Mohamady, M.; Metwally, A.A.; Katta, M.A. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int. J. Gynecol. Obstet. 2018, 141, 14–19. [Google Scholar] [CrossRef]
- Freire, V.S.; Burkhard, F.C.; Kessler, T.M.; Kuhn, A.; Draeger, A.; Monastyrskaya, K. MicroRNAs may mediate the down-regulation of neurokinin-1 receptor in chronic bladder pain syndrome. Am. J. Pathol. 2010, 176, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Monastyrskaya, K.; Sánchez-Freire, V.; Gheinani, A.H.; Klumpp, D.J.; Babiychuk, E.B.; Draeger, A.; Burkhard, F.C. miR-199a-5p regulates urothelial permeability and may play a role in bladder pain syndrome. Am. J. Pathol. 2013, 182, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ma, Z.; Zhu, J.; Zeng, M.; Liu, H.; Dong, Z. miR-214 represses mitofusin-2 to promote renal tubular apoptosis in ischemic acute kidney injury. Am. J. Physiol.-Ren. Physiol. 2020, 318, F878–F887. [Google Scholar] [CrossRef]
- Wu, C.; Rakhshandehroo, T.; Wettersten, H.I.; Campos, A.; Von Schalscha, T.; Jain, S.; Yu, Z.; Tan, J.; Mose, E.; Childers, B.G. Pancreatic cancer cells upregulate LPAR4 in response to isolation stress to promote an ECM-enriched niche and support tumour initiation. Nat. Cell Biol. 2023, 25, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.N.; Pochiraju, S.; Kannampalli, P.; Bruckert, M.; Addya, S.; Yadav, P.; Miranda, A.; Shaker, R.; Banerjee, B. MicroRNA-mediated GABAAα-1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. Pain 2013, 154, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, J.; Kannampalli, P.; Nie, L.; Meng, H.; Medda, B.K.; Shaker, R.; Sengupta, J.N.; Banerjee, B. MicroRNA–mediated downregulation of potassium-chloride-cotransporter and vesicular γ-aminobutyric acid transporter expression in spinal cord contributes to neonatal cystitis–induced visceral pain in rats. Pain 2017, 158, 2461–2474. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, A. It’s not cool to reduce the skin temperature and activate the TRPM8 ion channel after spinal injury. Scand. J. Pain 2013, 4, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Haggard, P.; Iannetti, G.D.; Longo, M.R. Spatial sensory organization and body representation in pain perception. Current Biology 2013, 23, R164–R176. [Google Scholar] [CrossRef] [PubMed]
- Theodosis, D.T.; Poulain, D.A.; Oliet, S.H. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol. Rev. 2008, 88, 983–1008. [Google Scholar] [CrossRef] [PubMed]
- Craig, K.D.; Versloot, J.; Goubert, L.; Vervoort, T.; Crombez, G. Perceiving pain in others: Automatic and controlled mechanisms. J. Pain 2010, 11, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Chen, S.-R.; Pan, H.-L. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev. Clin. Pharmacol. 2011, 4, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-T.; Ji, L.-J.; Wang, Z.; Wu, X.; Wang, Q.; Sun, S.; Lu, J.-M.; Zhang, Y. MicroRNA-93 alleviates neuropathic pain through targeting signal transducer and activator of transcription 3. Int. Immunopharmacol. 2017, 46, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.-Y.; Peng, C.-T.; Wang, H.-J. MicroRNA-146a-5p mediates high glucose-induced endothelial inflammation via targeting interleukin-1 receptor-associated kinase 1 expression. Front. Physiol. 2017, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Elramah, S.; Landry, M.; Favereaux, A. MicroRNAs regulate neuronal plasticity and are involved in pain mechanisms. Front. Cell. Neurosci. 2014, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, E.; Kynast, K.; Lötsch, J.; Geisslinger, G. MicroRNAs as new players in the pain game. Pain 2011, 152, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.-T.; Li, J.; Liu, B.-B.; Luo, L.; Liu, Q.; Geng, D. BDNF–ERK–CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J. Psychiatry Neurosci. 2014, 39, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D.A.; Srivastava, T.; Soderling, T.R. Structural modulation of dendritic spines during synaptic plasticity. The Neuroscientist 2012, 18, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, Z. miRNAs in synapse development and synaptic plasticity. Curr. Opin. Neurobiol. 2017, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Reza-Zaldivar, E.E.; Hernández-Sápiens, M.A.; Minjarez, B.; Gómez-Pinedo, U.; Sánchez-González, V.J.; Márquez-Aguirre, A.L.; Canales-Aguirre, A.A. Dendritic spine and synaptic plasticity in Alzheimer’s disease: A focus on MicroRNA. Front. Cell Dev. Biol. 2020, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- López-González, M.J.; Landry, M.; Favereaux, A. MicroRNA and chronic pain: From mechanisms to therapeutic potential. Pharmacol. Ther. 2017, 180, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.M.; Bekker, A.; Tao, Y.-X. Noncoding RNAs: New players in chronic pain. Anesthesiology 2014, 121, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Ambalavanar, R.; Wei, D.; Dessem, D. Downregulation of selective microRNAs in trigeminal ganglion neurons following inflammatory muscle pain. Mol. Pain 2007, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Recchiuti, A.; Krishnamoorthy, S.; Fredman, G.; Chiang, N.; Serhan, C.N. MicroRNAs in resolution of acute inflammation: Identification of novel resolvin D1-miRNA circuits. FASEB J. 2011, 25, 544. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.-R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Wesselmann, U.; Baranowski, A.P.; Börjesson, M.; Curran, N.C.; Czakanski, P.P.; Giamberardino, M.A.; Ness, T.J.; Robbins, M.T.; Traub, R.J. Emerging therapies and novel approaches to visceral pain. Drug Discov. Today Ther. Strateg. 2009, 6, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Kohno, T.; Moore, K.A.; Woolf, C.J. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003, 26, 696–705. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Medina-Ríos, I.; Márquez-Gallardo, L.D.; Reyes-Muñoz, J.; Serrano-Cano, F.I.; Pathak, S.; Paul, S. Functional implications and clinical potential of MicroRNAs in irritable bowel syndrome: A concise review. Dig. Dis. Sci. 2023, 68, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hu, H.; Liu, B.; Chen, Y.; Tao, Y.; Zhou, X.; Li, M. Biomaterial-assisted drug delivery for interstitial cystitis/bladder pain syndrome treatment. J. Mater. Chem. B 2021, 9, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, A.; O, D.; De Moor, B.; Waelkens, E.; Meuleman, C.; Tomassetti, C.; Peeraer, K.; D’Hooghe, T. Biomarkers of endometriosis. Fertil Steril. 2013, 99, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, S.; Taylor, H.S. MicroRNAs in endometriosis: Biological function and emerging biomarker candidates. Biol. Reprod. 2019, 101, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.S.; Hines, R.M. Regulation of GABAA receptor subunit expression in substance use disorders. Int. J. Mol. Sci. 2020, 21, 4445. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip. Rev. RNA 2012, 3, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Kress, M.; Hüttenhofer, A.; Landry, M.; Kuner, R.; Favereaux, A.; Greenberg, D.S.; Bednarik, J.; Heppenstall, P.; Kronenberg, F.; Malcangio, M. microRNAs in nociceptive circuits as predictors of future clinical applications. Front. Mol. Neurosci. 2013, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, H.; Chen, L.; Wang, Y.; Yao, X.; Jiang, X. Association of microRNAs genes polymorphisms with arthritis: A systematic review and meta-analysis. Biosci. Rep. 2019, 39, BSR20190298. [Google Scholar] [CrossRef]
- Jiangpan, P.; Qingsheng, M.; Zhiwen, Y.; Tao, Z. Emerging role of microRNA in neuropathic pain. Curr. Drug Metab. 2016, 17, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Bali, K.K.; Kuner, R. Noncoding RNAs: Key molecules in understanding and treating pain. Trends Mol. Med. 2014, 20, 437–448. [Google Scholar] [CrossRef]
- Tan, P.-H.; Pao, Y.-Y.; Cheng, J.-K.; Hung, K.-C.; Liu, C.-C. MicroRNA-based therapy in pain medicine: Current progress and future prospects. Acta Anaesthesiol. Taiwanica 2013, 51, 171–176. [Google Scholar] [CrossRef]
- Garzon, R.; Marcucci, G.; Croce, C.M. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat. Rev. Drug Discov. 2010, 9, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, J.-L. Lentiviral vector-mediated gene transfer and RNA silencing technology in neuronal dysfunctions. In Lentivirus Gene Engineering Protocols, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–35. [Google Scholar]
- Sun, B.; Gan, L. Manipulation of gene expression in the central nervous system with lentiviral vectors. In Alzheimer’s Disease and Frontotemporal Dementia: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2011; pp. 155–168. [Google Scholar]
- Bäumer, N.; Berdel, W.E.; Bäumer, S. Immunoprotein-mediated siRNA delivery. Mol. Pharm. 2017, 14, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Campos Pereira, T.; Lopes-Cendes, I. Emerging RNA-based drugs: siRNAs, microRNAs and derivates. Cent. Nerv. Syst. Agents Med. Chem. (Former. Curr. Med. Chem.-Cent. Nerv. Syst. Agents) 2012, 12, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Ajit, S.K. MicroRNA-based biomarkers in pain. Adv. Pharmacol. 2016, 75, 35–62. [Google Scholar] [PubMed]
- Al-Rawaf, H.A.; Alghadir, A.H.; Gabr, S.A. MicroRNAs as biomarkers of pain intensity in patients with chronic fatigue syndrome. Pain Pract. 2019, 19, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Suzuki, H. microRNA and Pain. In microRNA: Medical Evidence: From Molecular Biology to Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2015; pp. 17–39. [Google Scholar]
- Kusuda, R.; Cadetti, F.; Ravanelli, M.I.; Sousa, T.A.; Zanon, S.; De Lucca, F.L.; Lucas, G. Differential expression of microRNAs in mouse pain models. Mol. Pain 2011, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kroin, J.S.; Kc, R.; Gibson, G.; Chen, D.; Corbett, G.T.; Pahan, K.; Fayyaz, S.; Kim, J.S.; Van Wijnen, A.J. Altered spinal microRNA-146a and the microRNA-183 cluster contribute to osteoarthritic pain in knee joints. J. Bone Miner. Res. 2013, 28, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Kynast, K.L.; Russe, O.Q.; Geisslinger, G.; Niederberger, E. Novel findings in pain processing pathways: Implications for miRNAs as future therapeutic targets. Expert Rev. Neurother. 2013, 13, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Ciszek, B.P.; Khan, A.A.; Dang, H.; Slade, G.D.; Smith, S.; Bair, E.; Maixner, W.; Zolnoun, D.; Nackley, A.G. MicroRNA expression profiles differentiate chronic pain condition subtypes. Transl. Res. 2015, 166, 706–720.e711. [Google Scholar] [CrossRef] [PubMed]
- Maharshak, N.; Shenhar-Tsarfaty, S.; Aroyo, N.; Orpaz, N.; Guberman, I.; Canaani, J.; Halpern, Z.; Dotan, I.; Berliner, S.; Soreq, H. MicroRNA-132 modulates cholinergic signaling and inflammation in human inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 1346–1353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaknine, S.; Soreq, H. Central and peripheral anti-inflammatory effects of acetylcholinesterase inhibitors. Neuropharmacology 2020, 168, 108020. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhou, S.; Yi, S.; Gu, X. The regulatory roles of non-coding RNAs in nerve injury and regeneration. Prog. Neurobiol. 2015, 134, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Burrows, K.; Figueroa-Hall, L.K.; Alarbi, A.M.; Stewart, J.L.; Kuplicki, R.; Tan, C.; Hannafon, B.N.; Ramesh, R.; Savitz, J.; Khalsa, S. Association between inflammation, reward processing, and ibuprofen-induced increases of miR-23b in astrocyte-enriched extracellular vesicles: A randomized, placebo-controlled, double-blind, exploratory trial in healthy individuals. Brain Behav. Immun.-Health 2023, 27, 100582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, Z.; Wang, J. MicroRNA-124: A key player in microglia-mediated inflammation in neurological diseases. Front. Cell. Neurosci. 2021, 15, 771898. [Google Scholar] [CrossRef] [PubMed]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An important regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zheng, R.; Shao, G. Mechanisms and application strategies of miRNA-146a regulating inflammation and fibrosis at molecular and cellular levels. Int. J. Mol. Med. 2023, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M.; Barbier, E.; Johnstone, A.; Tapocik, J.; Meinhardt, M.; Pfarr, S.; Wahlestedt, C.; Sommer, W. Reprogramming of mPFC transcriptome and function in alcohol dependence. Genes Brain Behav. 2017, 16, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Torrellas, C. Epigenetics of aging and Alzheimer’s disease: Implications for pharmacogenomics and drug response. Int. J. Mol. Sci. 2015, 16, 30483–30543. [Google Scholar] [CrossRef] [PubMed]

| Diseases | miRs | Tissues | Targets | Ref |
|---|---|---|---|---|
| IBS | miR-199 ↓ | Human colon; Rat colon/DRG | TRPV1 ↑ | [99] |
| miR-24 ↑ | Human/mouse intestinal mucosa | SERT ↓ | [100] | |
| miR-17-5p ↑ | Lumbar spinal cord | STAT3 ↓; gp130 ↑ | [101] | |
| miR-150 ↑ miR-342-3p ↑ | Human whole blood | - | [102] | |
| miR-29a ↑ | Human small bowel and colon; human blood macrovesicles | Glutamate ammonia ligase ↓ | [103] | |
| miR-144 ↑ | Rat distal colonic epithelial cells | Occludin ↓; ZO1 ↓ | [104] | |
| Endometriosis | miR-9 ↓; miR-34 ↓ | Human endometrial tissues | - | [105] |
| miR-142-3p ↑ | Endometrial stroma cells | Steroid sulfatase ↓; gp130 ↓ | [106] | |
| miR-29 ↑; miR-181 ↑; let-7 ↑ | ox-LDL-treated human endometrial cell lines | NGF ↑; IL-6 ↑; PTGES3 ↑ | [107] | |
| miR-122 ↑; miR-199a ↑ | Serum; peritoneal fluid | - | [108] | |
| BPS/IC | miR-449b ↑ miR-500 ↑ | Bladder smooth muscle cells | NK1 receptor↓ | [109] |
| miR-199a-5p ↑ | Bladder smooth muscle; Mature bladder urothelium; Primary urothelial culture | LIN7C ↓; ARHGAP12 ↓; PALS1 ↓; RND1↓; PVRL1 ↓ | [110] | |
| miR-214 ↓ | Postmenopausal women’s bladder tissue; Ovariectomized rats’ APMSCs | Mfn2 ↑ | [111] | |
| miR-139-5p ↓ | Postmenopausal women’s bladder tissue | LPAR4 ↑ | [112] | |
| miR-181a ↑ | Rat spinal cord | GABAA ↓ | [113] | |
| miR-92b-3p ↑ | Rat spinal cord | KCC2 ↓; VGAT ↓ | [114] |
| miRs | Targets | Role | Ref |
|---|---|---|---|
| miR-1 | Sodium voltage-gated channel alpha subunit 1 (SCN1A) | Involved in controlling neuronal excitability, and neuropathic pain has been linked to its dysfunction | [49] |
| miR-21 | Programmed cell death 4 (PDCD4), Sprouty homolog 2 (SPRY2), and others | Implicated in neuroinflammation and it has been found to be upregulated in models of neuropathic pain | [161] |
| miR-23b | Prostaglandin-endoperoxide synthase 2 (PTGS2/COX-2) | Involved in controlling inflammatory pathways; a malfunction in this regard could be the cause of inflammatory pain | [162] |
| miR-124 | Signal transducer and activator of transcription 3 (STAT3) | Linked to neuroinflammation and microglial activation; dysregulation may be a factor in neuropathic pain | [163] |
| miR-155 | Suppressor of cytokine signaling 1 (SOCS1), SH2-containing inositol phosphatase 1 (SHIP1), and others | Involved in immune response and neuroinflammatory modulation; elevated in chronic pain models | [164] |
| miR-146a | Interleukin-1 receptor-associated kinase 1 (IRAK1) and tumor necrosis factor receptor-associated factor 6 (TRAF6) | Involved in the regulation of immune responses and inflammation; dysregulation may contribute to chronic pain conditions | [165] |
| miR-29 | Collagens, involved in extracellular matrix regulation | Linked to the control of extracellular matrix components and fibromyalgia; dysregulation may be a factor in the abnormalities of connective tissue in chronic pain | [124] |
| miR-30a | Serine/threonine-protein kinase WNK1 | Connected to WNK1 expression variation, which may affect sensitivity to pain | [166] |
| miR-128 | Voltage-gated sodium channel alpha subunit 2 (SCN2A) | Involved in controlling the excitability of neurons; dysregulation could lead to neuropathic pain | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.; Shahzadi, S.; Yasir, M.; Chun, W.; Kloczkowski, A. Therapeutic Implication of miRNAs as an Active Regulatory Player in the Management of Pain: A Review. Genes 2024, 15, 1003. https://doi.org/10.3390/genes15081003
Hassan M, Shahzadi S, Yasir M, Chun W, Kloczkowski A. Therapeutic Implication of miRNAs as an Active Regulatory Player in the Management of Pain: A Review. Genes. 2024; 15(8):1003. https://doi.org/10.3390/genes15081003
Chicago/Turabian StyleHassan, Mubashir, Saba Shahzadi, Muhammad Yasir, Wanjoo Chun, and Andrzej Kloczkowski. 2024. "Therapeutic Implication of miRNAs as an Active Regulatory Player in the Management of Pain: A Review" Genes 15, no. 8: 1003. https://doi.org/10.3390/genes15081003
APA StyleHassan, M., Shahzadi, S., Yasir, M., Chun, W., & Kloczkowski, A. (2024). Therapeutic Implication of miRNAs as an Active Regulatory Player in the Management of Pain: A Review. Genes, 15(8), 1003. https://doi.org/10.3390/genes15081003








